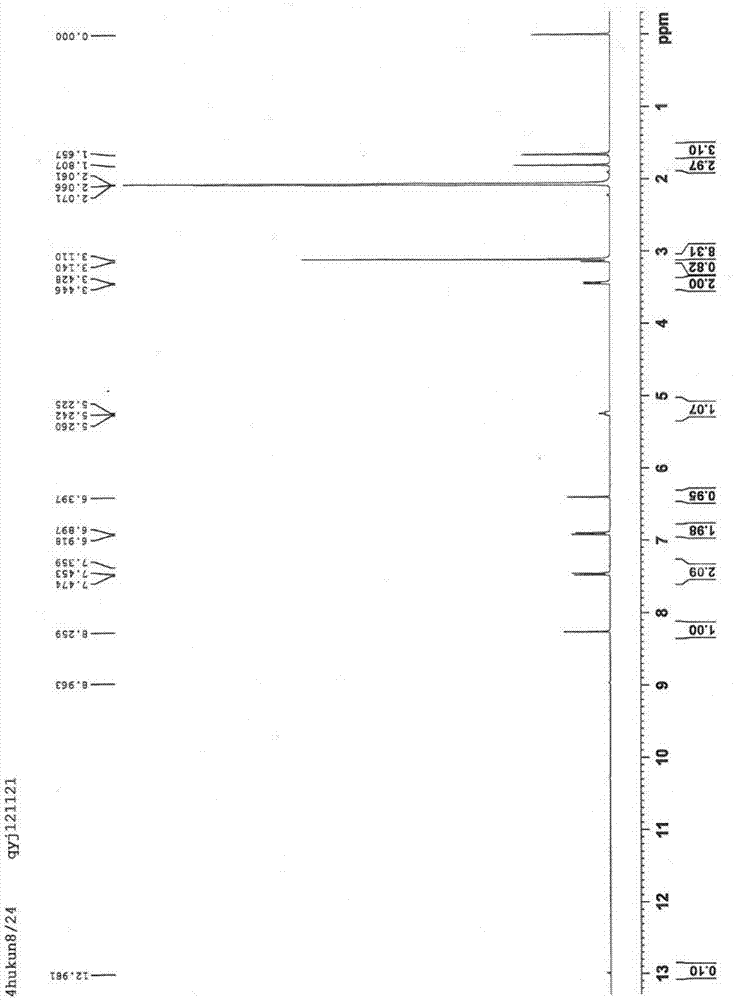
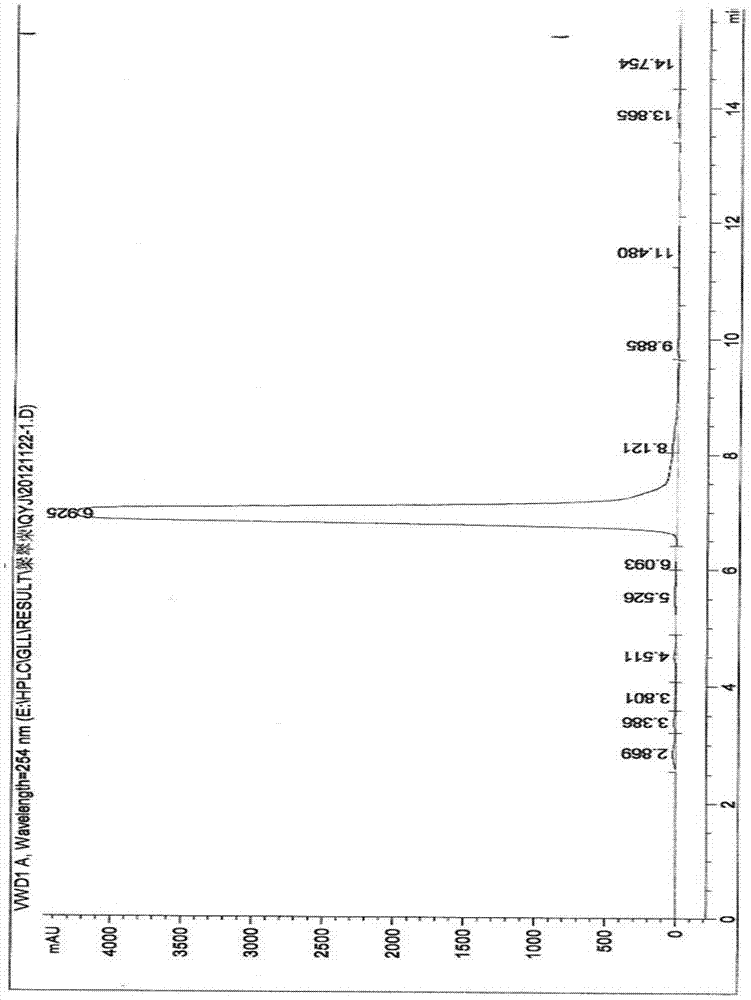
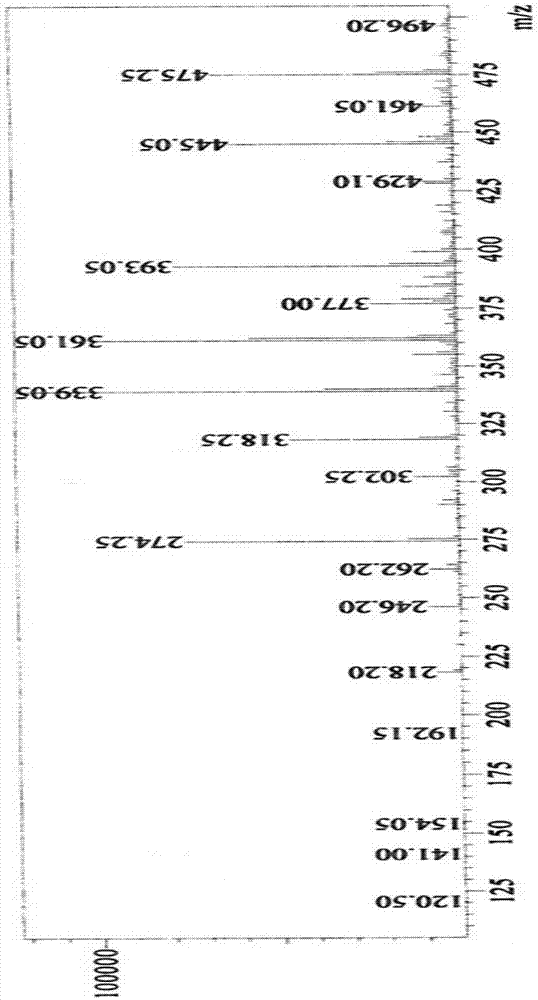
Synthesis method of Lupinus luteus wighteone
A synthetic method, the technology of genistein, which is applied in the field of chemical synthesis, can solve the problem of high cost, and achieve the effects of convenient post-processing, cost reduction, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Dissolve 8 grams of genistein in 150 milliliters of DMF, add 7 milliliters of acetic anhydride, reflux for 2-3 hours, and then detect with TLC. After the reaction, filter with suction to obtain a white solid, wash the solid with water, and then dry it get the product a;
[0032] (2) Dissolve 1 gram of product a in 50 ml of dry DMF, then add 3.5 g of potassium carbonate and 1.6 ml of bromoisoprene, react at room temperature for 1 h, then detect with TLC, evaporate under reduced pressure after the reaction Remove the solvent, wash with petroleum ether, then recrystallize 2-3 times with acetone, and obtain product b after drying;
[0033] (3) 0.5 g of product b was dissolved in 40 ml of N,N-diethylaniline, refluxed for 0.5-1 h, and then detected by TLC. After the reaction, the reaction solution was extracted with ethyl acetate, and then washed with saturated ammonium chloride solution To neutrality, wash with water, dry, pour out the solution, evaporate the solvent un...
Embodiment 2
[0041] (1) Dissolve 12 grams of genistein in 150 ml of DMF, add 7 ml of acetic anhydride, reflux for 2-3 hours, and then detect with TLC. After the reaction, filter the white solid, wash the solid with water, and then dry it get the product a;
[0042] (2) Dissolve 5 g of product a in 50 ml of dry DMF, add 3.5 g of potassium carbonate and 1.6 ml of brominated isoprene, react at room temperature for 1 h, then detect with TLC, evaporate under reduced pressure after the reaction Remove the solvent, wash with petroleum ether, then recrystallize 2-3 times with acetone, and obtain product b after drying;
[0043] (3) 1.5 g of product b was dissolved in 40 ml of N,N-diethylaniline, refluxed for 0.5-1 h, and then detected by TLC. After the reaction, the reaction solution was extracted with ethyl acetate, and then washed with saturated ammonium chloride solution To neutrality, wash with water, dry, pour out the solution, evaporate the solvent under reduced pressure to obtain a white s...
Embodiment 3
[0050] (1) Dissolve 10 grams of genistein in 150 milliliters of DMF, add 7 milliliters of acetic anhydride, reflux for 2-3 hours, and then detect with TLC. After the reaction, filter with suction to obtain a white solid, wash the solid with water, and then dry it get the product a;
[0051] (2) Dissolve 3 grams of product a in 50 ml of dry DMF, add 3.5 g of potassium carbonate and 1.6 ml of brominated isoprene, react at room temperature for 1 h, then detect with TLC, evaporate under reduced pressure after the reaction Remove the solvent, wash with petroleum ether, then recrystallize 2-3 times with acetone, and obtain product b after drying;
[0052] (3) 1 g of product b was dissolved in 40 ml of N,N-diethylaniline, refluxed for 0.5-1 h, and then detected by TLC. After the reaction, the reaction solution was extracted with ethyl acetate, and then washed with saturated ammonium chloride solution To neutrality, wash with water, dry, pour out the solution, evaporate the solvent u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com