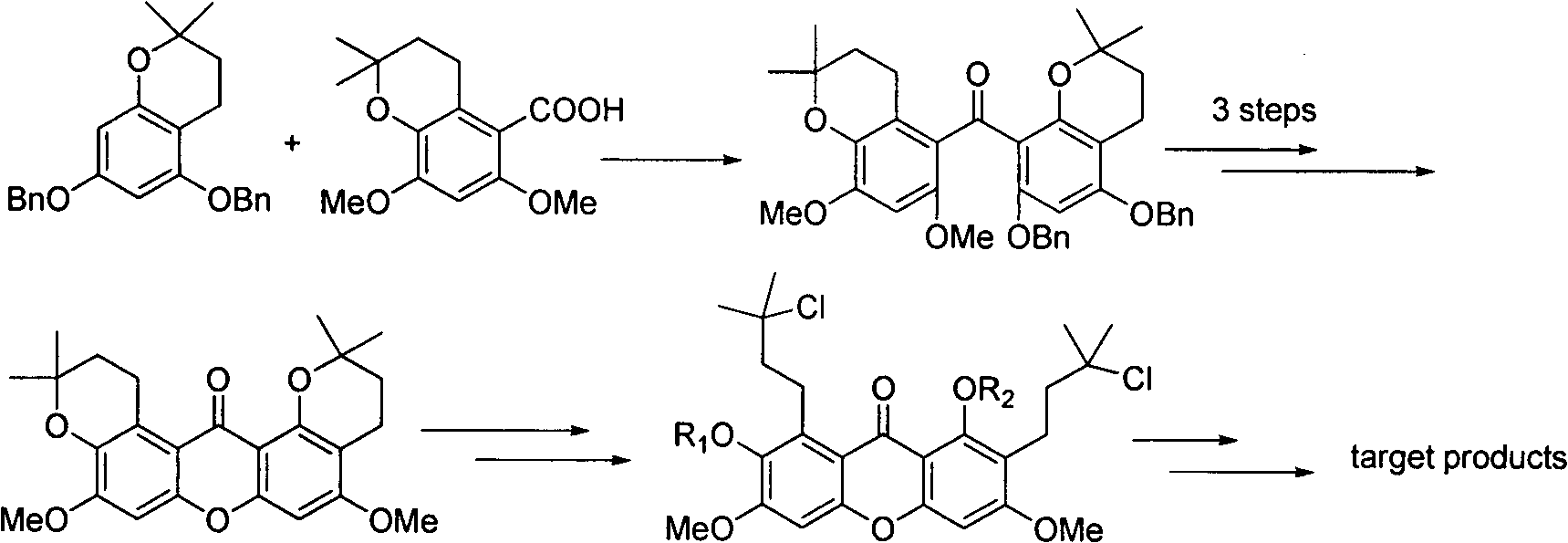
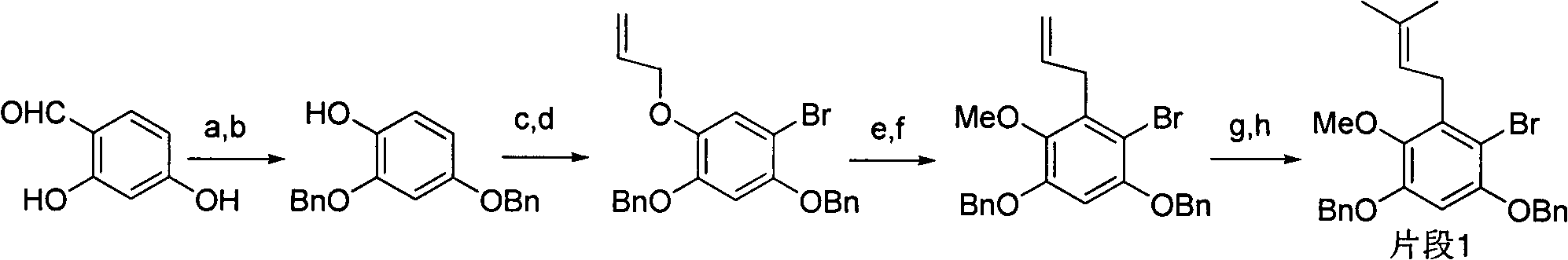
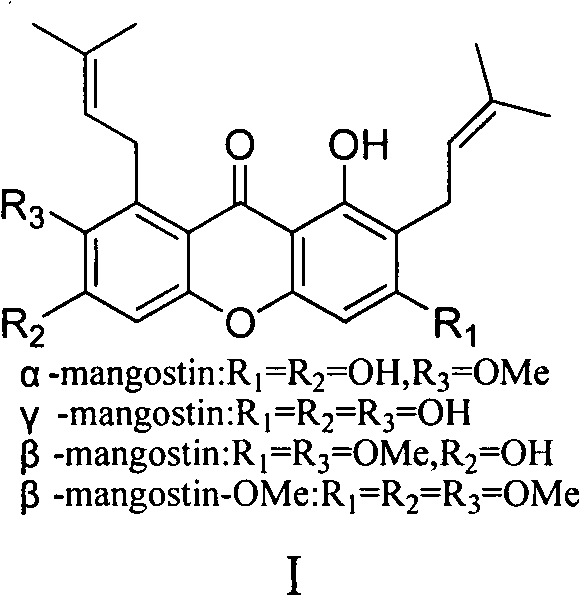Total synthesis method of mangostin
A synthesis method and compound technology, applied in the field of synthesis of natural product mangostin, can solve the problems of long cycle, harsh reaction conditions, limited sources, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] The preparation of 2,4,5-trimethoxybenzoyl chloride (V):
[0068] Dissolve 4.24g (0.02mol) of 2,4,5-trimethoxybenzoic acid in 50mL of dichloromethane, add 6mL of oxalyl chloride, stir at room temperature, evaporate the solvent under reduced pressure, and directly put it into the next reaction.
Embodiment 2
[0070] 2-Hydroxy-2',4,4',5',6-pentamethoxybenzophenone (VIb) and 2-Hydroxy-2',4,4',5',6-pentamethoxy Preparation of benzophenone (VIa):
[0071] Add 60 mL of diethyl ether to the solid 2,4,5-trimethoxybenzoyl chloride (V) prepared in Example 1, slowly add 3.30 g (0.018 mol) of 1,3,5-trimethoxybenzene, batch by batch 9.35 g (0.07 mol) of aluminum trichloride was added, and the reaction solution turned blood red, and massive viscous solids appeared. The ice bath was removed and refluxed for 36 hours. Extracted with ethyl acetate and purified by silica gel column chromatography (petroleum ether / ethyl acetate=4:1) to obtain 2-hydroxy-2',4,4',5,6'-pentamethoxy as a yellow granular solid Benzophenone (VIa) 1.3g, yield 20%, compound 2-hydroxy-2',4,4',5',6-pentamethoxybenzophenone (VIb) 1.5g, yield 22 %.
[0072] Compound VIa
[0073] 1 H-NMR (300MHz, CDCl 3 ): δ3.72(s, 6H, C 4 -OCH 3 , C 5 -OCH 3 ), δ3.86(s, 3H, C 4’ -OCH 3 ), δ3.88(s, 6H, C 2’ -OCH 3 , C 6’ -OCH 3 )...
Embodiment 3
[0079]Preparation of 1,3,6,7-tetramethoxyxanthone (VII):
[0080] 2-Hydroxy-2',4,4',5,6'-pentamethoxybenzophenone (VIa) or 2-hydroxy-2',4,4',5',6-pentamethoxy A total of 3.48g (0.01mol) of benzophenone (VIb) was dissolved in 60mL of methanol, 90mL of water and 6.0g (0.15mol) of sodium hydroxide were added, refluxed at 80°C for 24 hours, cooled to room temperature, a large number of light yellow flocs appeared After suction filtration and drying, methanol was recrystallized to obtain 2.76 g of off-white amorphous solid 1,3,6,7-tetramethoxyxanthone (VII), with a yield of 87.3%.
[0081] 1 H-NMR (300MHz, CDCl 3 ): δ3.90(s, 3H, C 3 -OCH 3 ), δ3.97(s, 6H, C 6 -OCH 3 , C 7 -OCH 3 ), δ3.98(s, 3H, C 1 -OCH 3 ), δ6.34(s, 1H, C 4 -H), δ6.46(s, 1H, C 5 -H), δ6.81(s, 1H, C 2 -H), δ7.65(s, 1H, C 8 -H);
[0082] EI-MS (m / z): 316 (M + ), 299, 287, 270, 185, 83.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com