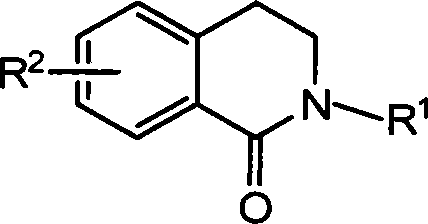
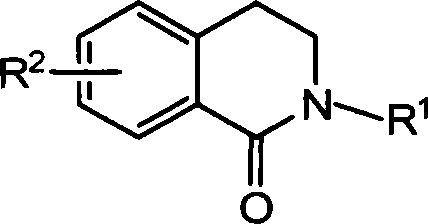
Method for synthesizing 2-substituted-3,4-dihydro-1-isoquinoline ketones and use thereof for preparing cardiovascular agents
A technology of isoquinolinone and synthesis method, which is applied in the direction of cardiovascular system diseases, drug combinations, medical preparations containing active ingredients, etc., and can solve the problem that the yield is only 16%.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: Preparation of 3-allyloxy-4-methoxybenzoic acid methyl ester
[0048] Take 2.0g (11mmol) methyl isovanillate in a 250ml round bottom flask, add 30ml acetone, 4.6g (33mmol) anhydrous potassium carbonate, stir at room temperature for 30min, add 1.5ml (16.5mmol) allyl bromide, heat to reflux 2h. Cool to room temperature, filter, evaporate the mother liquor to remove acetone under reduced pressure, add 150ml of ethyl acetate for extraction. Sequentially use 2mol·L -1 Wash with hydrochloric acid, saturated sodium bicarbonate solution, water and brine. The organic phase was dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure, and the residue was recrystallized from ethanol to obtain 2.3 g of white crystals. Yield: 98%, mp: 56.5-58.5°C. 1 HNMR (300MHz, CDCl 3 ),δ H : 7.62(d, 1H, Ar-H), 7.39(s, 1H, Ar-H), 6.82(d, 1H, Ar-H), 6.15(m, 1H, =CH-), 5.33(d, 2H ,=CH 2 ), 4.52 (d, 2H, -OCH 2 -), 3.94(s, 3H, -OCH 3 ), 3.82(s,...
Embodiment 2
[0049] Embodiment 2: Preparation of 2-allyl-3-hydroxyl-4-methoxybenzoic acid methyl ester
[0050] Take 2.0g (9mmol) of methyl 3-allyloxy-4-methoxybenzoate in a 100ml round bottom flask, and add 10ml of freshly steamed N,N-dimethylaniline. Under nitrogen protection, the reaction mixture was heated and stirred at reflux for 4h. Cool to room temperature, add 30ml 3mol·L -1 hydrochloric acid, extracted with ethyl acetate (3 x 40ml), and the organic phases were combined and washed successively with saturated sodium bicarbonate solution, water and brine. The organic phase was dried with anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. The residue was separated by flash column chromatography (eluent: petroleum ether: ethyl acetate = 5:1), and ethyl acetate / petroleum ether was crystallized to obtain 1.8g Colorless needle-like crystals. Yield: 90%, mp: 74.0-74.5°C. IR (cm -1 ): 3455, 1701, 1602, 1578. 1 HNMR (300MHz, CDCl 3 ),δ H : 7.52(d, 1H, A...
Embodiment 3
[0051] Embodiment 3: Preparation of 2-oxyethyl-3-hydroxyl-4-methoxybenzoic acid methyl ester
[0052] Get 1.8g (8mmol) compound 2-allyl-3-hydroxyl-4-methoxybenzoic acid methyl ester in 250ml round bottom flask, add 48ml acetone, 16ml water, 16ml tert-butanol and 0.1g (0.4mmol ) osmium tetroxide, stirred at room temperature for 30 min in the dark, added 4.2 g (20 mmol) of sodium periodate, and stirred at room temperature for 4 h in the dark. Diatomaceous earth was filtered, the mother liquor was evaporated under reduced pressure to remove acetone and tert-butanol, and 100ml of ethyl acetate was added for extraction. Wash successively with saturated sodium bisulfite, water and brine. The organic phase was dried with anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. The residue was separated by flash column chromatography (eluent: petroleum ether: ethyl acetate = 3:1), and crystallized from ethyl acetate / petroleum ether to obtain 1.47g Colorless n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com