Positron emitted computerized tomography imaging agent benzamide derivative synthesizing method
A synthesis method and a formamide-based technology are used in the synthesis field of positron emission computed tomography imaging agent benzamide derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
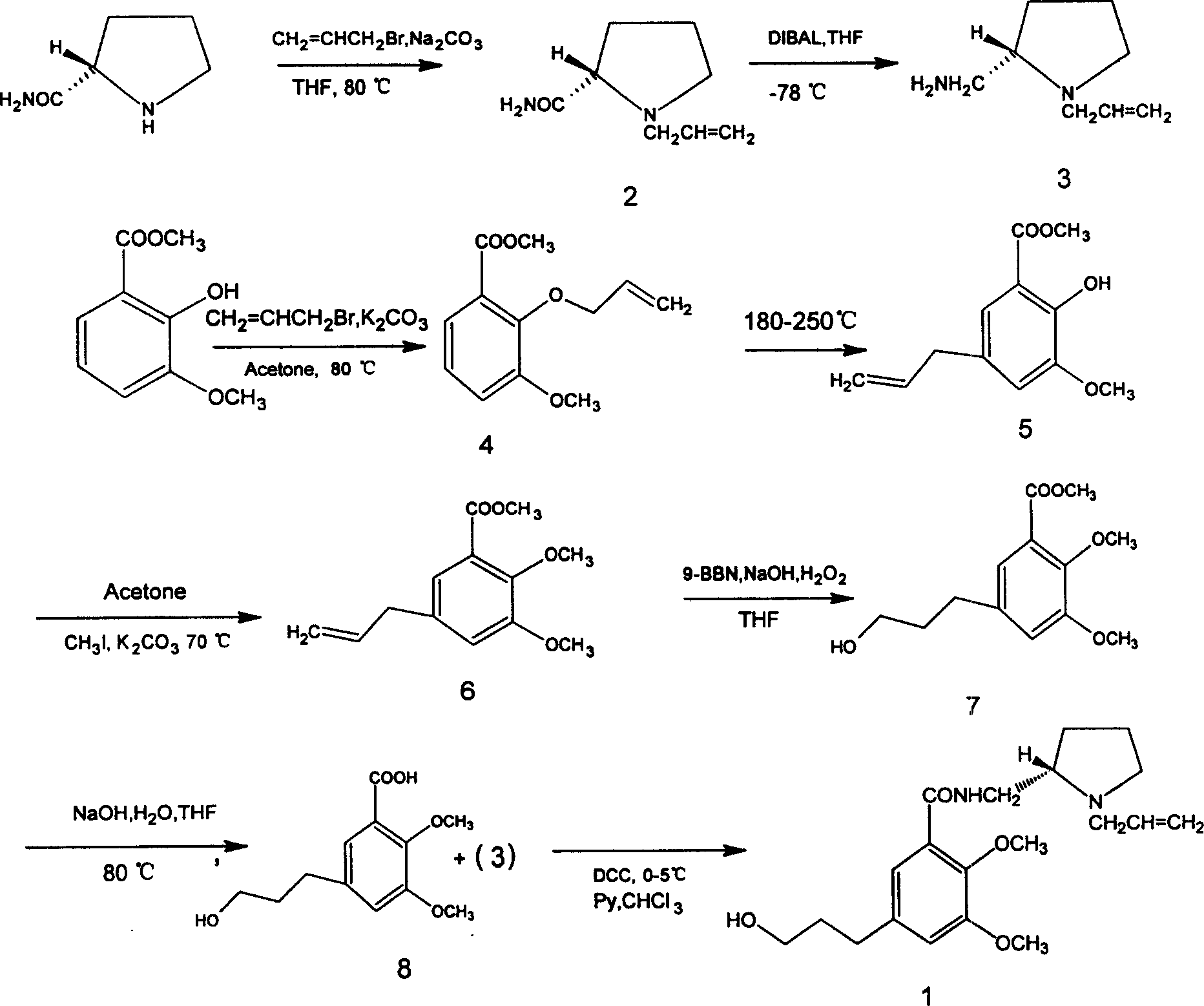
[0018] Preparation of (S)-(-)-2-carboxamido-N-(allyl)pyrrolidine (2):
[0019] Mix (S)-(-)-2-carboxamidopyrrolidine (0.12g, 1.0mmol) with 4ml of tetrahydrofuran, add sodium carbonate (0.05g, 0.5mmol), allyl bromide (0.12g, 1.0mmol ), reacted in an oil bath at 80°C for 24h. After the reaction, tetrahydrofuran was removed, and a small amount of water was added to the residue to adjust the pH to 10. Extracted with dichloromethane, combined organic layers, dried over anhydrous magnesium sulfate, filtered, concentrated to give white needle-like solid (2) (0.13g, 85%), mp 76~77°C (document [3] : 40%, 79-81°C).
[0020] Preparation of (S)-(-)-2-methylamino-N-(allyl)pyrrolidine (3):
[0021] (2) (0.16g, 1.04mmol) was dissolved by adding 2ml of tetrahydrofuran, cooled to -78°C in a low-temperature bath, added dropwise diisobutylaluminum hydride (0.89g, 6.23mmol), stirred for 30min, raised to room temperature, and continued to stir 48h. Add 1mol / L hydrochloric acid to terminate the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com