Cage-shaped supramolecular catalyst for catalyzing thioether oxidation as well as preparation method and application of cage-shaped supramolecular catalyst
A technology for catalyzing sulfides and supramolecules, applied in the preparation of organic compounds, organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, etc., can solve the problems of poor stability and low activity of catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
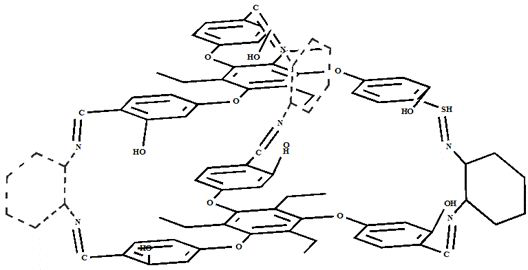
[0030] Example 1. Synthesis of covalent cage compound SNC containing Salen ligand
[0031] Weigh 122 mg of 2,4,6-triethyl-1,3,5-tris(4-oxomethyl-1-formyl)benzene c into a 50 mL round bottom flask, add 30 mL of trichloro After methane was completely dissolved, 45 μL of 1,2-cyclohexanediamine was added to the reaction system, and reacted at 60 °C for 12 h. After the reaction is complete, the reaction solution is distilled off under reduced pressure to remove the solvent, the filter cake is washed with acetonitrile, and the washed yellow solid powder is vacuum-dried to obtain the supramolecular cage compound SNC-2. 1 H NMR (400 MHz, CDCl3) δ 13.93(s, 6H), 8.34 – 8.10 (m, 6H), 7.12 (ddd, J = 20.9, 15.5, 8.8 Hz, 6H), 6.69 –6.35 (m, 12H), 3.94 – 3.19 (m, 12H), 2.92 – 2.58 (m, 12H), 1.35 – 1.10 (m, 18H).
Embodiment 2
[0032] Embodiment 2. The synthesis of caged supramolecular catalyst compound Ni(II)-SNC
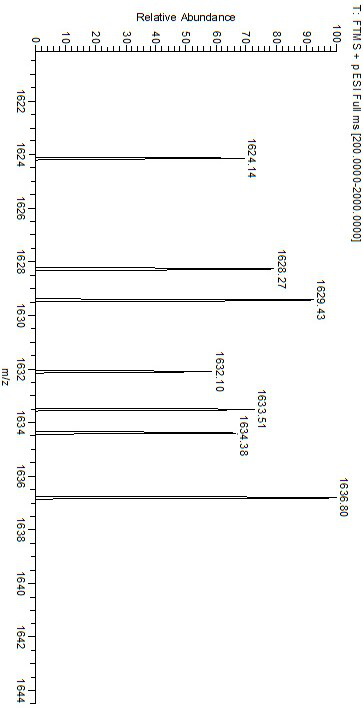
[0033] Into a 250 mL three-neck flask, 290 mg SNC and 150 mg nickel acetate tetrahydrate were added sequentially, and 120 mL chloroform / ethanol (2:1, v: v) mixed solution was added under nitrogen atmosphere, and the reaction was refluxed at 80 °C for 12 h. After the reaction was complete, it was cooled to room temperature, concentrated to remove the solvent, washed with water and dried in vacuum to obtain Ni(II)-SNC. Infrared (FT-IR) spectrum results show at 535 cm -1 The characteristic absorption peak of Ni-N bond appears at 469 cm -1 The stretching vibration peak of the Ni-O bond appears. Electrospray-high resolution mass spectrometry (HR-MS, ESI): [C 90 h 96 N 6 o 12 Ni 3 +H + ] m / z, calculated value: 1629.50 m / z; experimental value: 1629.43 m / z.
Embodiment 3
[0034] Example 3. Catalyzed oxidation of sulfide anisole by caged supramolecular compound Ni(II)-SNC
[0035] Add 80 mg Ni(II)-SNC catalyst, 600 mg sulfide anisole, 210 mg 4-methylpyridine oxide, 60 mL methanol and 2.0 g iodobenzenediacetic acid to a 100 mL round-bottomed flask and react at 40 °C After 6 h, it was allowed to stand at room temperature, and the reaction solution was concentrated by rotary evaporation. Then, 0.666 g of benzoin was obtained by separation on a silica gel column with ethyl acetate / n-pentane as the eluent. Yield: 99.1%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com