Method for preparing 2-bromofluorenone by catalyzing oxidizing of molecular oxygen in water phase
A technology for bromofluorenone in aqueous phase, applied in the field of 2-bromofluorenone synthesis, can solve problems such as toxicity, environmental pollution, danger, etc., and achieve the effects of less waste, high catalytic activity, and strong industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
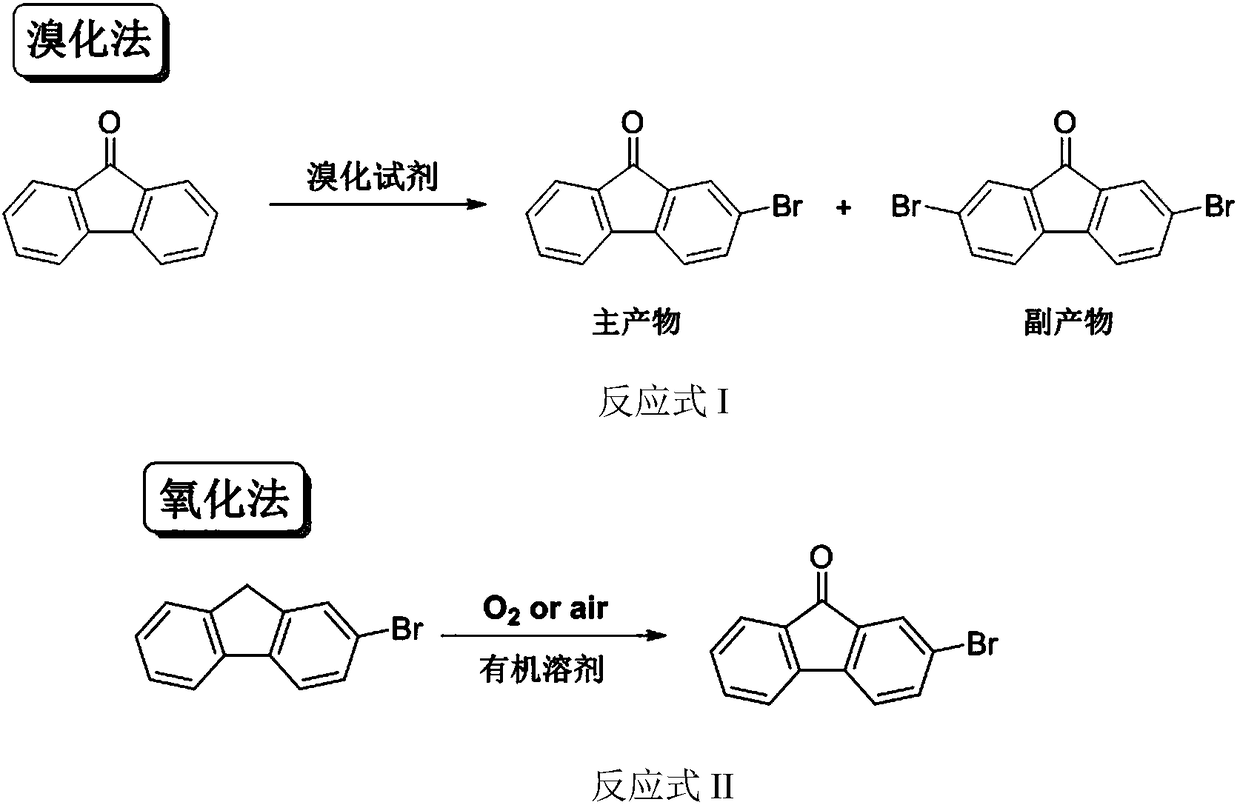
[0021] Example 1: Catalytic oxidation of 2-bromofluorene to synthesize 2-bromofluorenone.
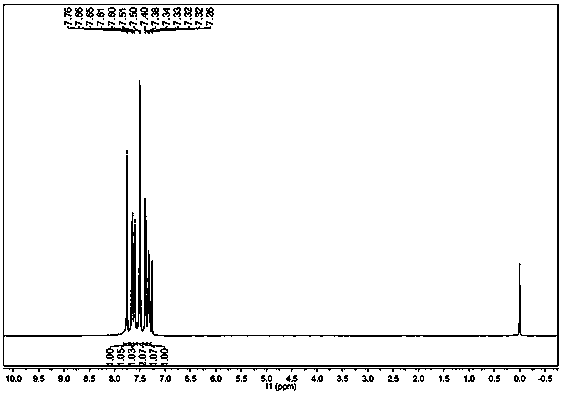
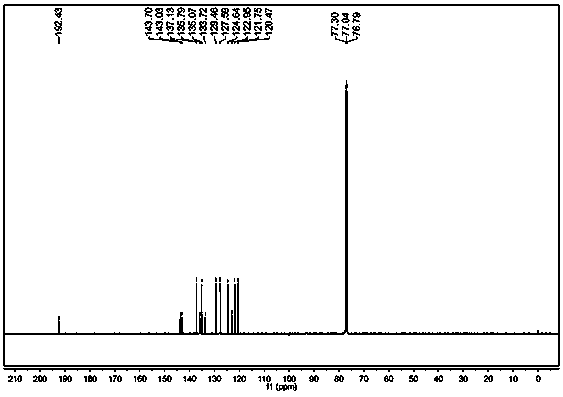
[0022] In a 50L glass reactor equipped with a reflux condenser and mechanical stirring, add 5Kg 2-bromofluorene, 0.5g Salen manganese catalyst, 0.5g Salen cobalt catalyst and 30L water, heat to 80°C while stirring, and bubble in air to react After 16 hours, the reaction was stopped, cooled, filtered, and the filter cake was dried to obtain 5.2Kg of a yellow solid. 1 H-NMR and 13 The structure was determined to be 2-bromofluorenone by C-NMR, and the yield was 99%. The purity of the product analyzed by liquid chromatography was 99.8%. The aqueous phase obtained by filtration was retained and continued to be used in the next batch.
Embodiment 2
[0023] Example 2: Synthesis of 2-bromofluorenone by catalytic oxidation of 2-bromofluorene in the aqueous phase recovered by filtration.
[0024] In a 50L glass reactor equipped with a reflux condenser and mechanical stirring, add 5Kg 2-bromofluorene, 0.5g Salen manganese catalyst, 0.5g Salen cobalt catalyst and the water phase recovered by filtration in Example 1, and heat to 80°C under stirring , feed air bubbles, stop the reaction after 16 hours of reaction, cool, filter, and dry the filter cake to obtain 5.2Kg of yellow solid. 1 H-NMR and 13 The structure was determined to be 2-bromofluorenone by C-NMR, and the yield was 99%. The purity of the product analyzed by liquid chromatography was 99.6%. The aqueous phase mother liquor obtained by filtration is retained and used in the next batch.
Embodiment 3
[0025] Example 3: Catalytic oxidation of 2-bromofluorene to synthesize 2-bromofluorenone.
[0026] In a 50L glass reactor equipped with a reflux condenser and mechanical stirring, add 5Kg 2-bromofluorene, 1g Salen cobalt catalyst and 30L water, heat to 100°C while stirring, and bubble in air, and stop the reaction after 24 hours of reaction , cooled, filtered and dried to obtain 5.0Kg of yellow solid, the product was 1 H-NMR and 13 C-NMR confirmed the structure as 2-bromofluorenone, and the yield was 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com