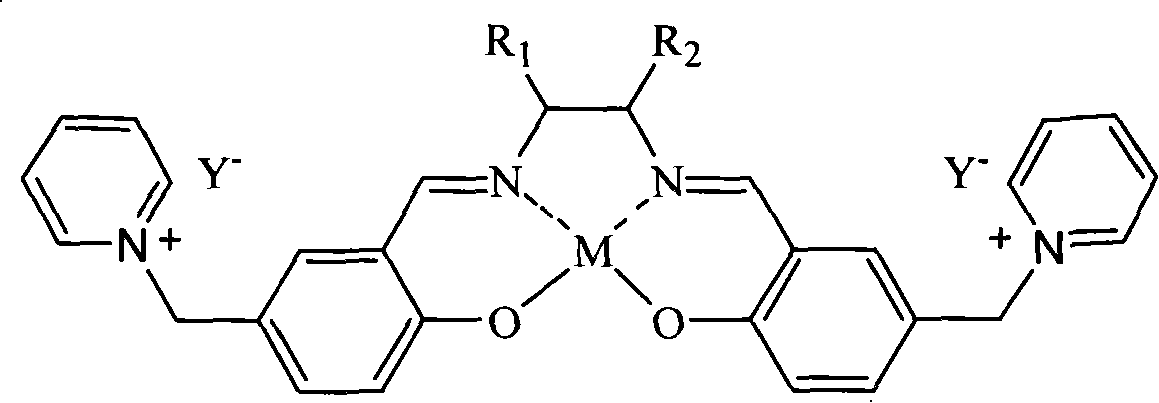
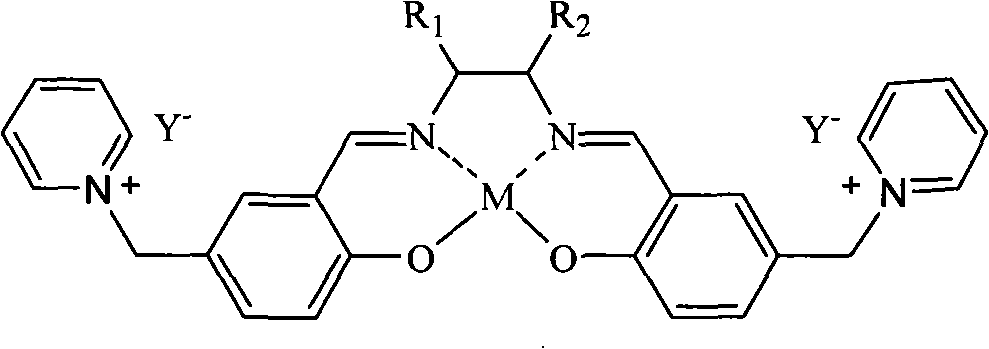
Salen metallic catalyst and preparation method
A metal catalyst, fe2 technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc., can solve water and air sensitivity, long reaction cycle, and low catalytic efficiency and other issues to achieve the effect of easy operation, water and air stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
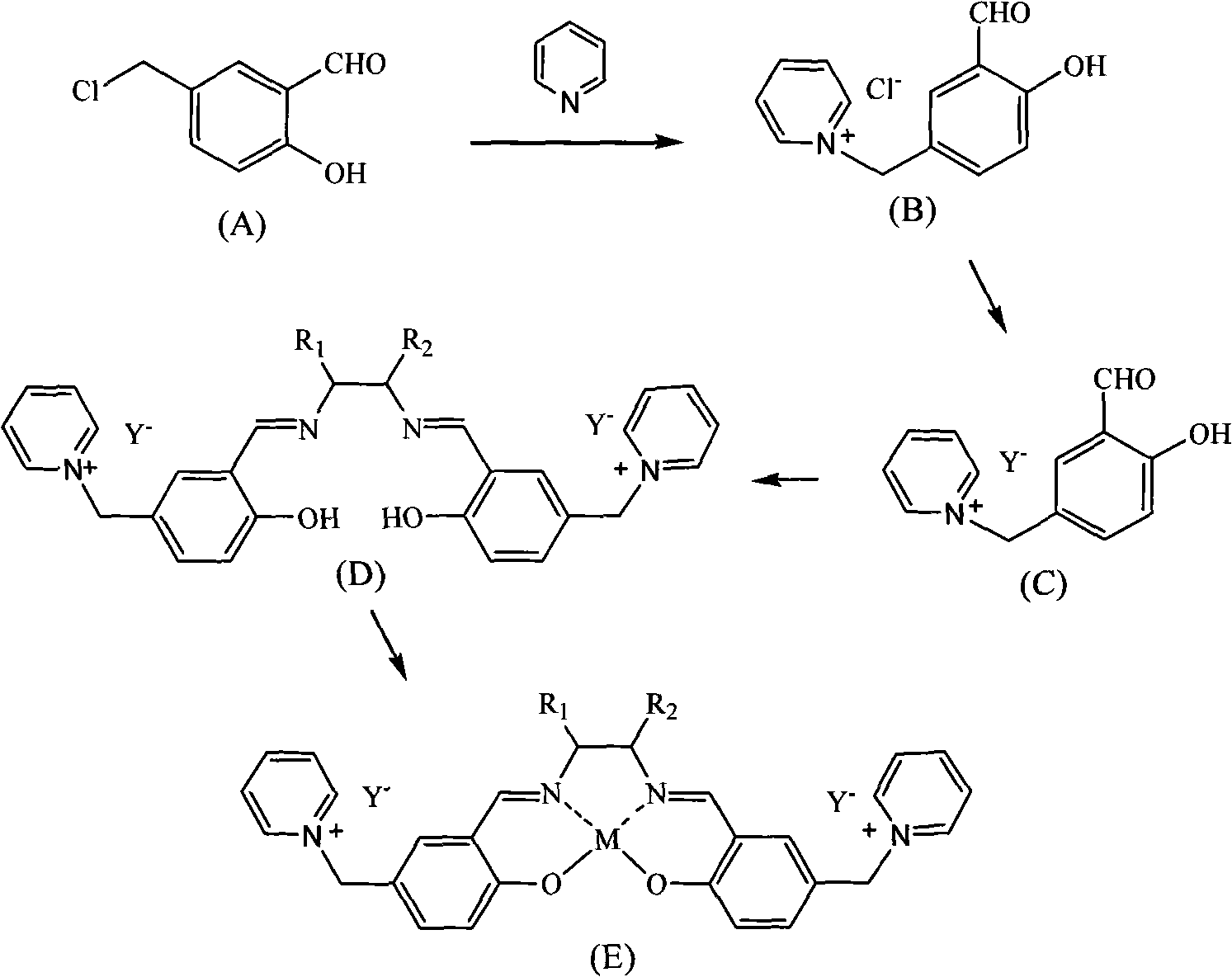
[0018] a. Synthesis of pyridine organic chloride salt (B)
[0019] Add 0.5 mol (85.5 g) of chlorinated salicylaldehyde and 200 ml of toluene to a 500 ml four-necked flask, protect it with nitrogen gas, add 1 mol (79 g) of pyridine dropwise at 100° C. Evaporate to remove solvent toluene and excess pyridine. After cooling, 150 ml of ethyl acetate was added to wash for 3 times, and vacuum-dried to obtain 100 g of pyridinium chloride salt (B) with a yield of 80%.
[0020] b. Synthesis of pyridine hexafluorophosphate (C)
[0021] Add 0.2mol (50g) of pyridinium chloride salt (B) into a 500ml three-necked flask, 200ml of deionized water, and add 0.2ml (36.8g) of KPF at room temperature 6 , reacted for 48h, washed 5 times with water, and removed the inorganic salt KCl. Rotary evaporation under reduced pressure at 80°C, removal of solvent water to obtain 70.4 g of pyridinium hexafluorophosphate, yield 97%.
[0022] c.SalenH 2 Synthesis of Ligand (D)
[0023] Weigh 0.05 mol (18 g)...
Embodiment 2
[0027] a. The synthesis of triethylamine organic chloride salt (B)
[0028] Add 0.5 mol (85 g) of chlorinated salicylaldehyde and 200 ml of benzene into a 500 ml three-neck flask, add 0.5 mol (50.5 g) of triethylamine dropwise at 100°C, and keep the reaction for 10 hours after dropping, then rotary steam under reduced pressure at 80°C, The solvent benzene was removed. After cooling, 150 ml of ethyl acetate was added to wash for 3 times, and vacuum-dried to obtain 115 g of triethylamine chloride salt (B), with a yield of 86%.
[0029] b. Synthesis of triethylamine nitrate (C)
[0030] In a 500ml three-necked flask, add 0.5mol (136g) of triethylamine chloride salt (B), 200ml of water, and add KNO at room temperature 3 , reacted for 48h, washed 5 times with water, removed KCl, and rotated under reduced pressure at 80°C to remove solvent water to obtain triethylamine nitrate with a yield of 98%.
[0031] c.SalenH 2 Synthesis of Ligand (D)
[0032]Dissolve 0.05 mol of triethyl...
Embodiment 3
[0036] a. Synthesis of organochlorine salt (B)
[0037] Add 0.5 mol (85 g) of chlorinated salicylaldehyde and 200 ml of chloroform into a 500 ml three-neck flask, add 0.5 mol (41 g) of methylimidazole dropwise at 70 ° C, and keep the reaction for 10 h after dropping, and rotate under reduced pressure at 60 ° C to remove Solvent chloroform. After cooling, 150 ml of ethyl acetate was added for washing three times, and vacuum-dried to obtain salicylaldehyde-substituted imidazolium chloride salt (B), with a yield of 86%.
[0038] b. Synthesis of salicylaldehyde-substituted imidazolium tetrafluoroborate (C)
[0039] In a 500ml three-necked flask, add 0.5mol (126.5g) of salicylaldehyde substituted imidazolium chloride salt (B), 200ml of water, and add NaBF at room temperature 4 , reacted for 48h, washed with water for 5 times, and rotated under reduced pressure at 80°C to remove the solvent water to obtain triethylamine tetrafluoroborate with a yield of 99%.
[0040] c.SalenH 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com