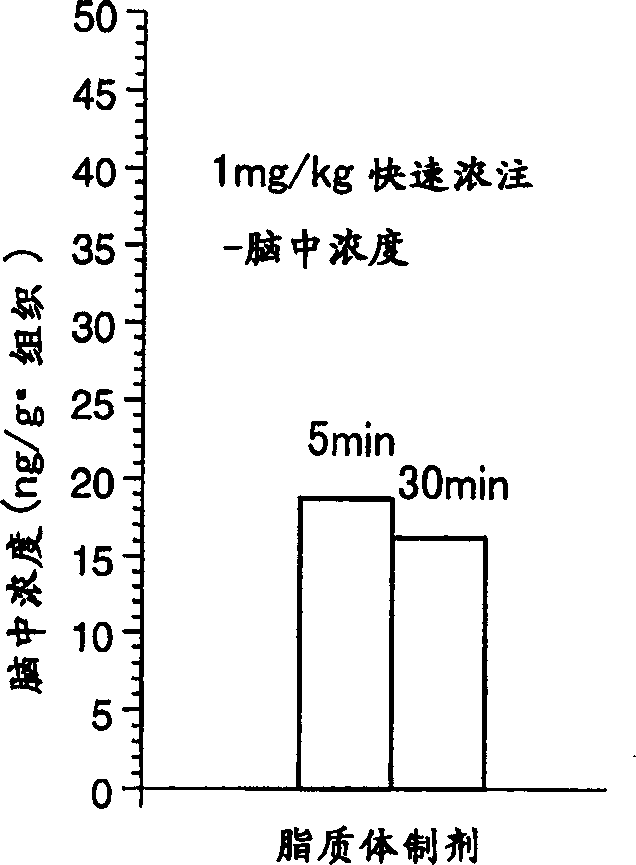
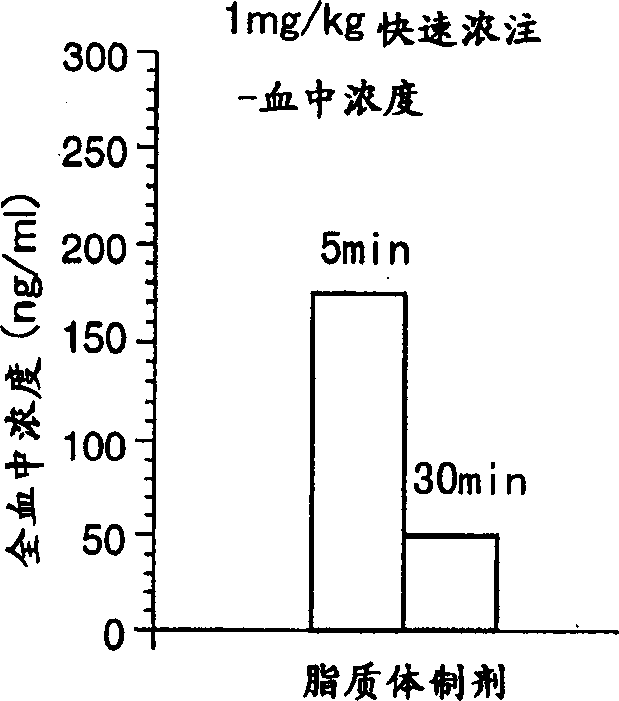
Liposome preparations
A liposome preparation and liposome technology, applied in the field of macrolide compounds, can solve the problem that liposome preparations cannot show fast enough action and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 3
[0101] (3) Preparation Example 3 (high pressure emulsion method): A solution obtained by dissolving purified egg yolk lecithin (20 g) and tacrolimus (1 g) in ethanol (10 mL) was vacuum-dried to form a thin film. This film was roughly dispersed in a 10% maltose aqueous solution (2000 mL) with a magnetic stirrer. The dispersion was treated with a high-pressure emulsifier manufactured by Nanomizer Co. The formed liposome emulsion was filled into a vial of 10 mL, and then freeze-dried. The freeze-dried liposome preparation formed was redispersed in 9 mL of water for injection to obtain liposomes containing 0.5 mg / mL tacrolimus and an average particle diameter of 80 nm (measured by dynamic light scattering method, C370 type manufactured by NICOMP Co.) Dispersions.
preparation Embodiment 4
[0103] In the same manner as Preparation Example 1, the following formula was used to obtain freeze-dried liposomes, and a proper amount of water for injection was used to make a liposome dispersion.
[0104] Tacrolimus 3mg
[0105] Purified egg yolk lecithin 100mg
[0106] Lactose monohydrate 1000mg
[0107] Composition 1103mg
preparation Embodiment 5
[0109] In the same manner as in Preparation Example 3, the following formula was used to obtain a freeze-dried liposome preparation, and an appropriate amount of water for injection was used to make liposome preparations 1) and 2)
[0110] 1) Tacrolimus 3mg
[0111] Purified egg yolk lecithin 100mg
[0112] Alpha-fertility 0.3mg
[0113] Maltose 1000mg
[0114] Composition 1103.3mg2) Tacrolimus 5mg
[0115] Purified egg yolk lecithin 100mg
[0116] Alpha-Tocopherol 0.3mg
[0117] Maltose 1000mg
[0118] Composition 1105.3mg
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com