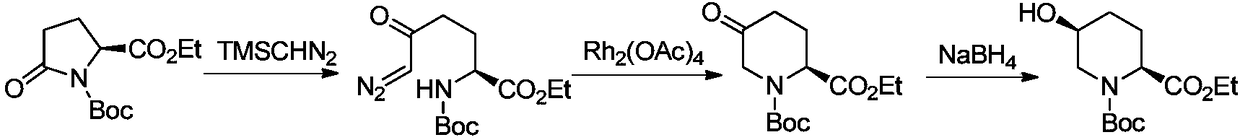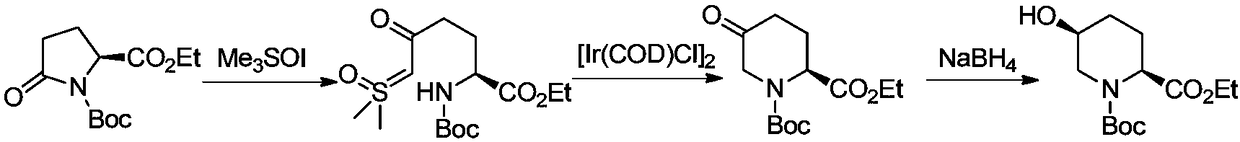Synthetic method of (2S,5S or 5R)-N-tert-butoxycarbonyl-5-hydroxy-2-piperidinecarboxylic acid ethyl ester
A technology of ethyl piperidinecarboxylate and tert-butoxycarbonyl, which is applied in the field of pharmaceutical intermediate synthesis, can solve problems such as difficulty in realizing large-scale production, long synthesis routes, expensive reagents, etc., and achieves advantages of large-scale production, easy operation, Inexpensive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0045] The present invention will be further described in conjunction with specific embodiments, but the content of the present invention is not limited by this embodiment.
[0046] 1.5-Hydroxy-2-pyridinecarboxylic acid ethyl ester hydrochloride (abbreviated as compound 1)
[0047]
[0048] Add 5-hydroxy-2-picolinic acid (20g, 143.8mmol) and 200ml ethanol to a 500ml single-neck flask, stir and heat to reflux. Thionyl chloride (52.2ml, 719mmol) was slowly added dropwise to the reaction solution, kept refluxed until the reaction was complete, and the solvent was removed by rotary evaporation under reduced pressure to obtain compound 1 (27.5g, 93.9%). 1 H NMR(500MHZ, DMSO-d 6 ): 8.30 (d, 1H), 8.02 (d, 1H), 7.47 (dd, 1H), 4.32 (q, 2H), 1.32 (t, 3H); MS (m / z): 168.1 [M+1] + .
[0049] Synthesis of ethyl 2.5-hydroxy-2-piperidinecarboxylate hydrochloride (abbreviated as compound 2)
[0050]
[0051] Compound 1 (27.5g, 135mmol), rhodium carbon (2.75g, 10% Rh / C) and 150ml of ethanol were added...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com