Hiv Prodrugs Cleavable by Cd26
a technology of prodrug and cd26, which is applied in the field of prodrug of hiv inhibitory compounds, can solve the problems of unsatisfactory physicochemical and biopharmaceutical properties of many existing drug molecules already on the market, unrealized medical advances, and unfavorable new chemical structures. , to achieve the effect of convenient preparation, and reducing the risk of infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
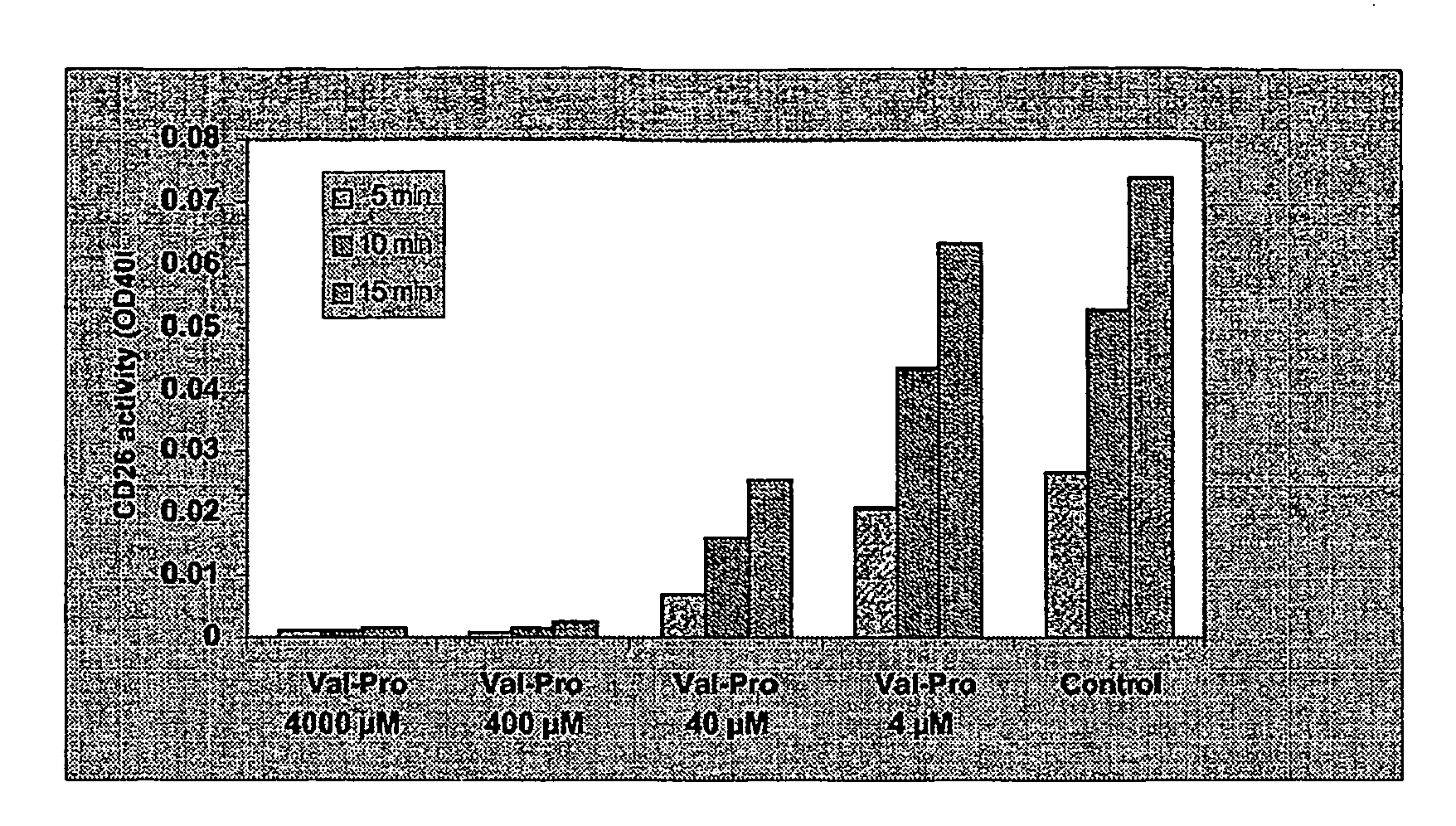
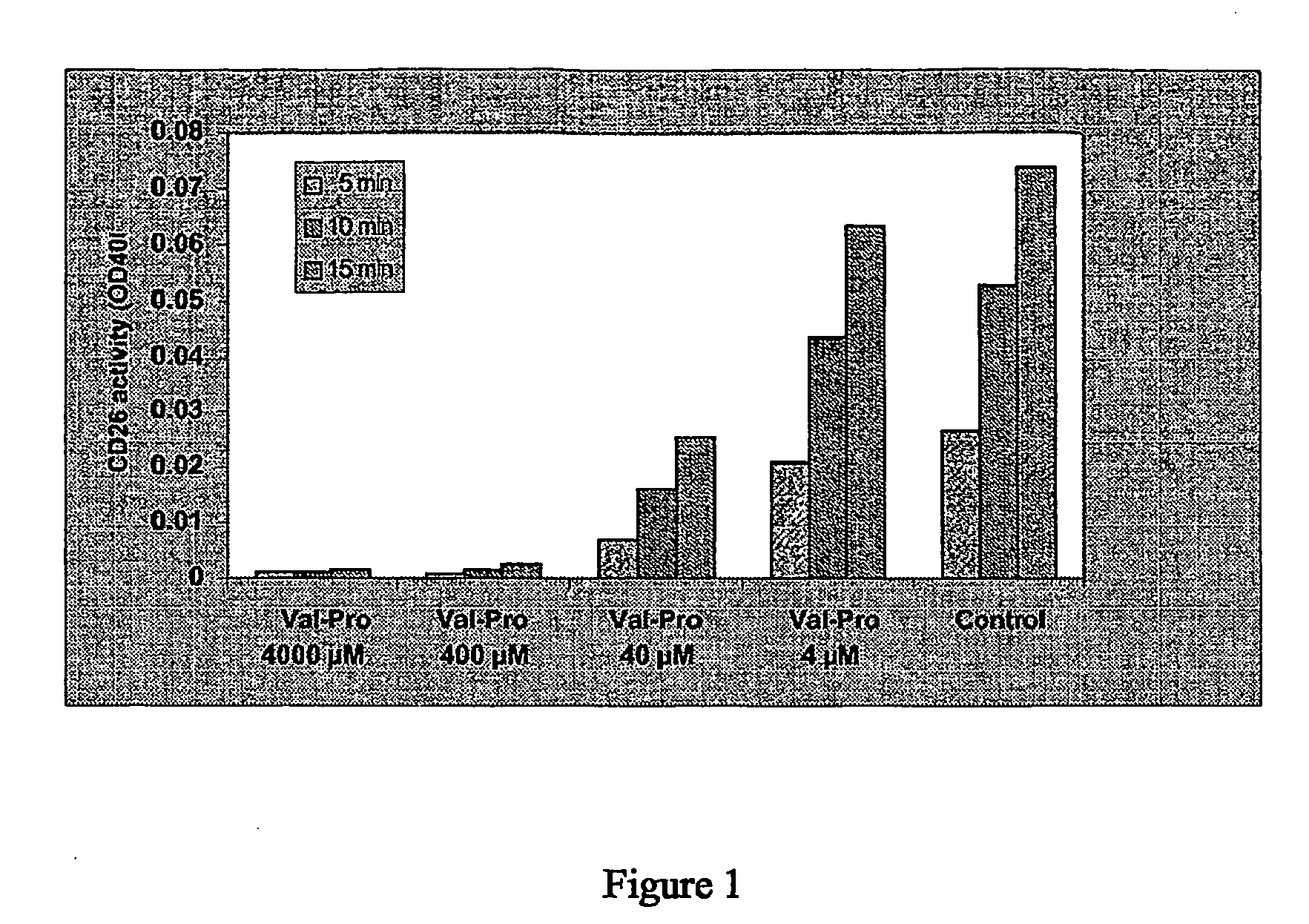
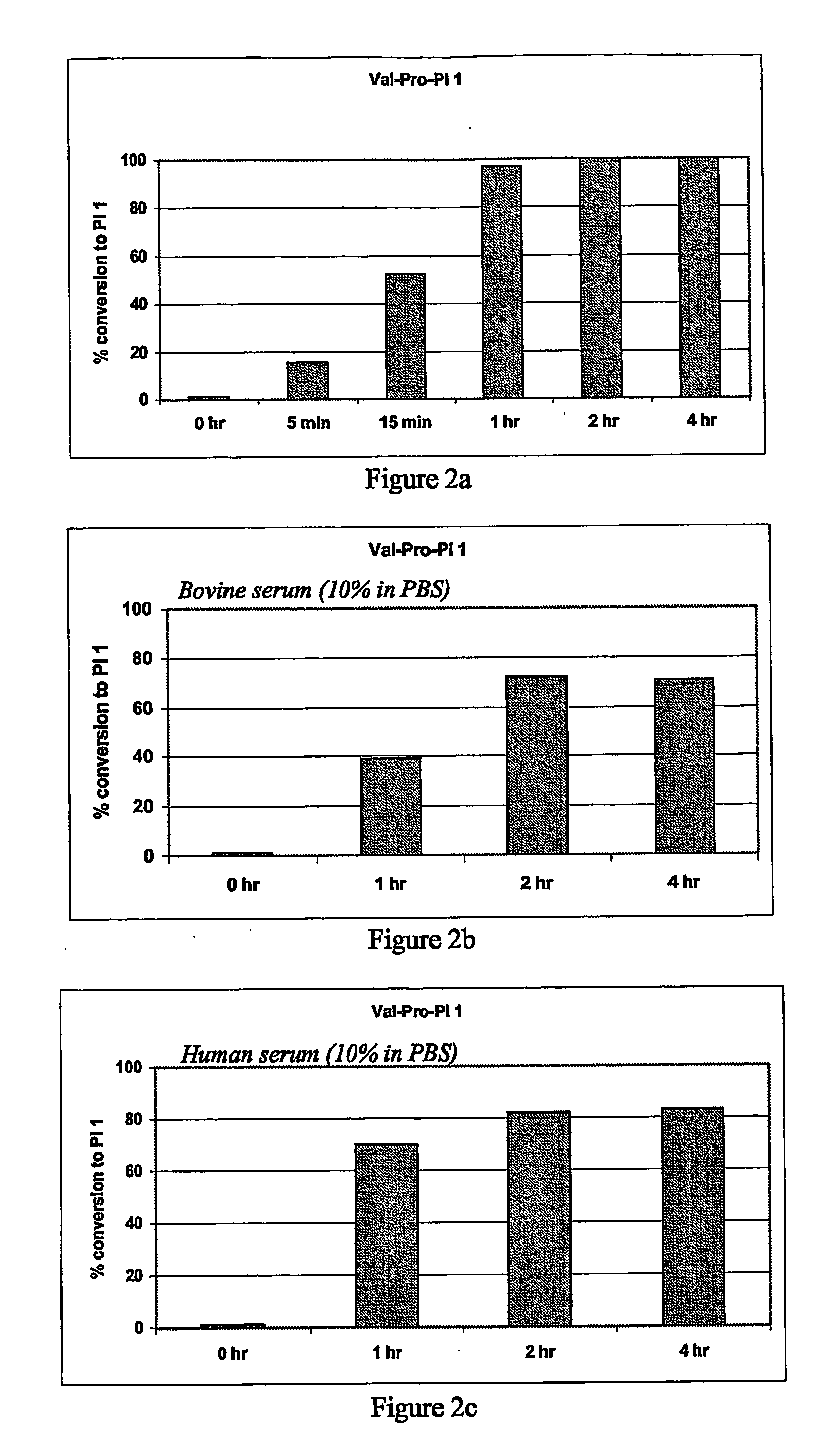
Val-Pro-PI 1
[0174]Step 1
[0175]Compound 1.1 (0.95 g; 1.69 mmol) and Boc-Val-Pro-OH (0.53 g; 1.7 mmol) were dissolved in 10 ml N,N-dimethylformamide. EDCI (0.36 g; 1.9 mmol) and HOAt (0.023 g; 0.17 mmol) were added and stirred at room temperature for 20 hours. The reaction mixture was poured in H2O and extracted twice with ethylacetate. The combined organic layer was washed with brine and then dried over Na2SO4. Solvent was removed and the obtained crude product purified by column chromatography (eluent:ethylacetate). Compound 1.2 was obtained as a white solid (yield 55%, purity 95% LC-MS).
[0176]Step 2
[0177]To a solution of compound 1.2 (0.77 g; 0.9 mmol) in 10 ml CH2Cl2 was added 10 ml trifluoroacetic acid. After stirring the reaction mixture at room temperature for 3 hours, the solvent was removed. The crude mixture was purified by column chromatography yielding 0.42 g of compound 1.3 (yield 61%, purity 95% LC-MS)
example 2
Asp-Pro-PI 1
[0178]Step 1
[0179]Compound 2.1 (3.16 g; 5.63 mmol) and Boc-Pro-OH (1.33 g; 6.18 mmol) were dissolved in 30 ml N,N-dimethylformamide. EDCI (1.18 g; 6.18 mmol) and HOAt (0.077 g; 0.5 mmol) were added and stirred for 36 hours. Ethylacetate and 0.1 N HCl were added and the resulting reaction mixture was extracted 3 times with ethylacetate. The combined organic layer was washed with 0.1 N HCl, H2O, saturated NaHCO3, water and brine. After drying over Na2SO4 and evaporation of the solvent a white foam (4.39 g, 103%) was obtained. After trituration in diisopropylether, 3.9 g of compound 2.2 was obtained (yield 93%, purity 97% LC-MS)
[0180]Step 2
[0181]A mixture of compound 2.3 (3.7 g, 4.8 mmol) and 15 ml trifluoroacetic acid in 40 ml CH2Cl2 was stirred at room temperature for 2 hours. After evaporation of solvent the crude sure was partitioned between ethylacetate and saturated NaHCO3. The organic layer was separated, washed with brine and dried over Na2SO4. Re-slurry of the crud...
example 3
Asp-Pro-Lys-Pro-PI 1
[0186]
[0187]Using analogous reaction procedures as described in examples 1 and 2, Boc-Lys(Fmoc)-OH was coupled to compound 3.1 (as prepared in example 2), yielding compound 3.2. After Boc-deprotection, compound 3.3 was obtained. Boc-Pro-OH was then coupled to compound 3.3, yielding compound 3.4 which was subsequently Boc-deprotected thus yielding compound 3.5. Compound 3.5 was coupled with Boc-Asp(OtBu)-OH yielding compound 3.6 which was first Boc-deprotected and then Fmoc-deprotected using dimethylamine in tetrahydrofuran, thus yielding compound 3.8 corresponding to Asp-Pro-Lys-Pro-PI 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| hydrophobic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com