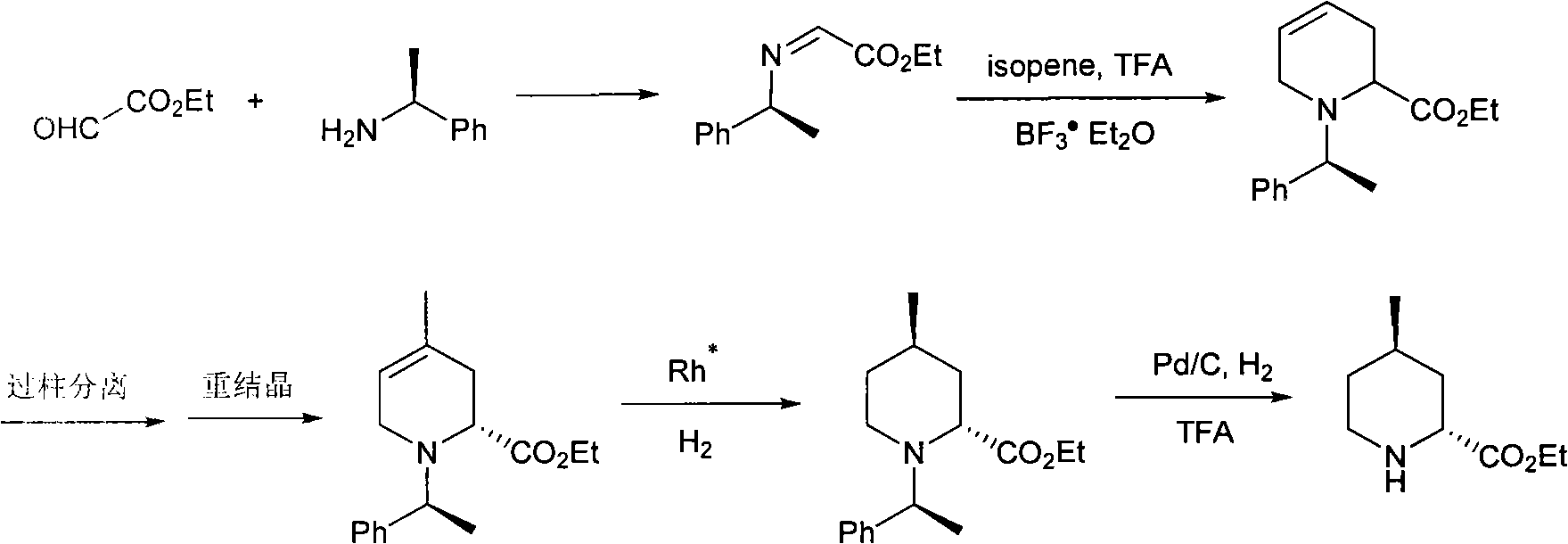
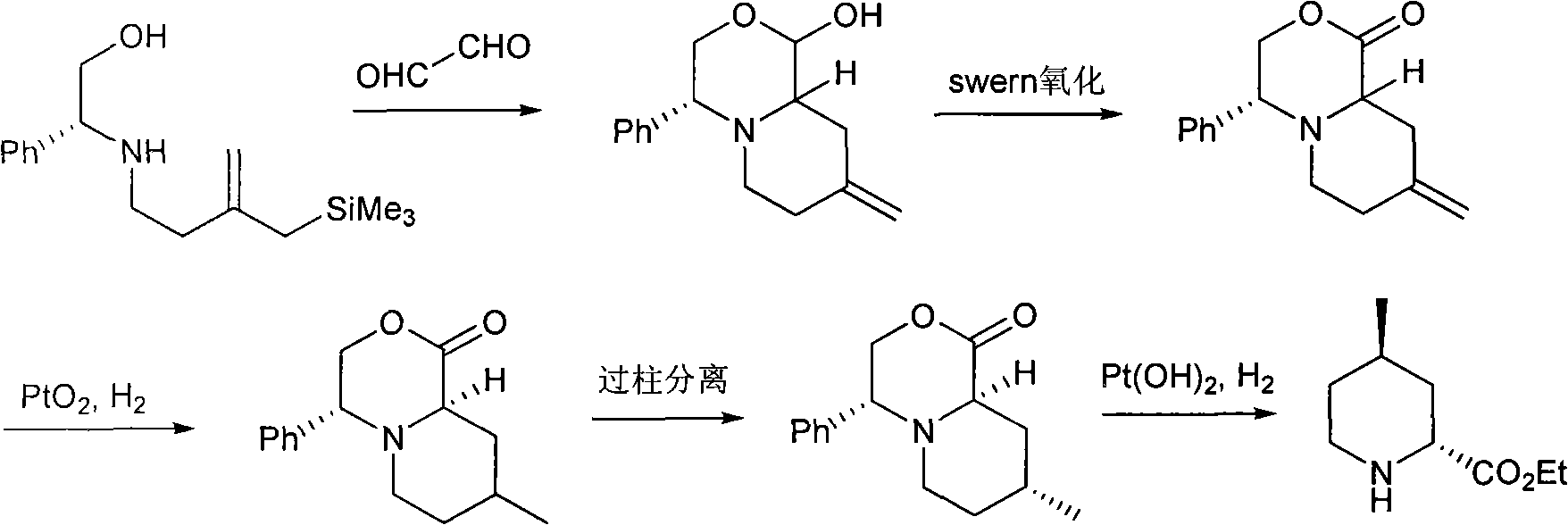
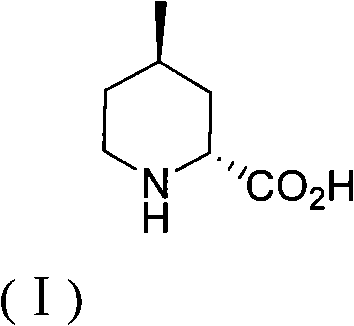Method for preparing (2R,4R)-4-methyl-2-pipecolic acid
A technology of -4-, pipecolic acid, which is applied in the field of preparation of the argatroban intermediate -4-methyl-2-pipericolic acid, can solve the problems of difficult industrialization, expensive starting materials, and high prices. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] The preparation of embodiment one L-aspartic acid-4-methyl ester hydrochloride (II)
[0062] Add 400g of L-aspartic acid into 1L of methanol, control the internal temperature at 0°C, and slowly add 350g of acetyl chloride dropwise to the system. After reacting for 20 hours, 5 L of diethyl ether was added to the system, and a solid was precipitated. After stirring for 2 hours, the solid was filtered and dried to obtain 460 g of a white solid, with a yield of 82%.
Embodiment 2
[0063] Preparation of Example Two N-(2-methoxycarbonyl) ethyl-N-benzyl-L-aspartic acid-4-methyl ester (III)
[0064] Add 860g of L-aspartic acid-4-methyl ester hydrochloride (II), 1200g of methyl acrylate, and 1500g of triethylamine into 3L of water, and react at room temperature for 12 hours. Subsequently, 3 L of diethyl ether was added to the reaction system, stirred for 1 hour, and the diethyl ether layer was separated and removed. Add 2200 g of benzyl bromide to the water layer, and stir at room temperature for 24 hours. Add 5 L of diethyl ether to the system, extract and separate to obtain the aqueous phase. 4N hydrochloric acid aqueous solution was added dropwise to the aqueous phase until the pH was 2, and then 5 L of diethyl ether was added to the aqueous phase for extraction and separation to obtain an organic phase. After the organic phase was dried over anhydrous sodium sulfate, the organic solvent was removed under reduced pressure to obtain 1170 g of oil, with a...
Embodiment 3
[0065] The preparation of embodiment three (R)-N-benzyl-4-oxopiperidine-2-carboxylic acid (IV)
[0066] Dissolve 600g of N-(2-methoxycarbonyl)ethyl-N-benzyl-L-aspartic acid-4-methyl ester (III) in 2.5L of tetrahydrofuran, and add 1.2 L dissolved 300 g of sodium methoxide in methanol, followed by reflux for 5 hours. After cooling off, 3L of water was added to the system, and the reaction was further refluxed for 12 hours. After the reaction solution was cooled, most of the organic solvent was removed under reduced pressure. Add 3L of diethyl ether, extract and separate the aqueous phase. 4N hydrochloric acid aqueous solution was added to the aqueous phase until the pH of the system was 2, and ethyl acetate was added for extraction and separation to obtain an organic phase. Under stirring, 200 mL of tert-butylamine was added to the organic phase, and a solid appeared. After stirring for 5 hours, the solid was obtained by filtration. After drying, the solid was recrystallized...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com