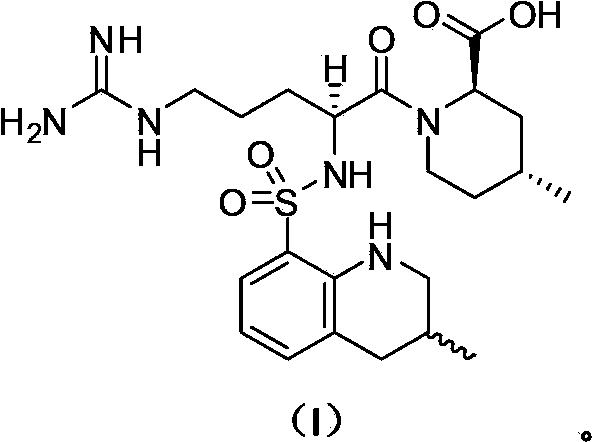
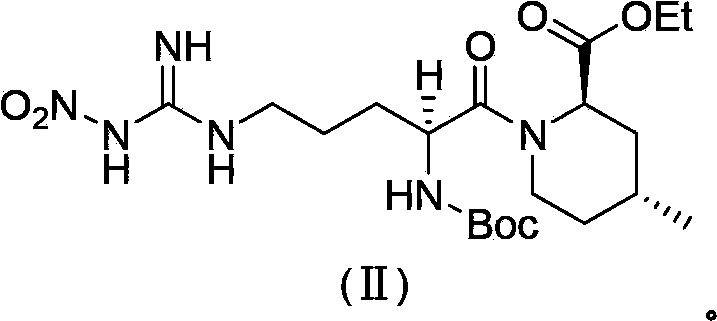
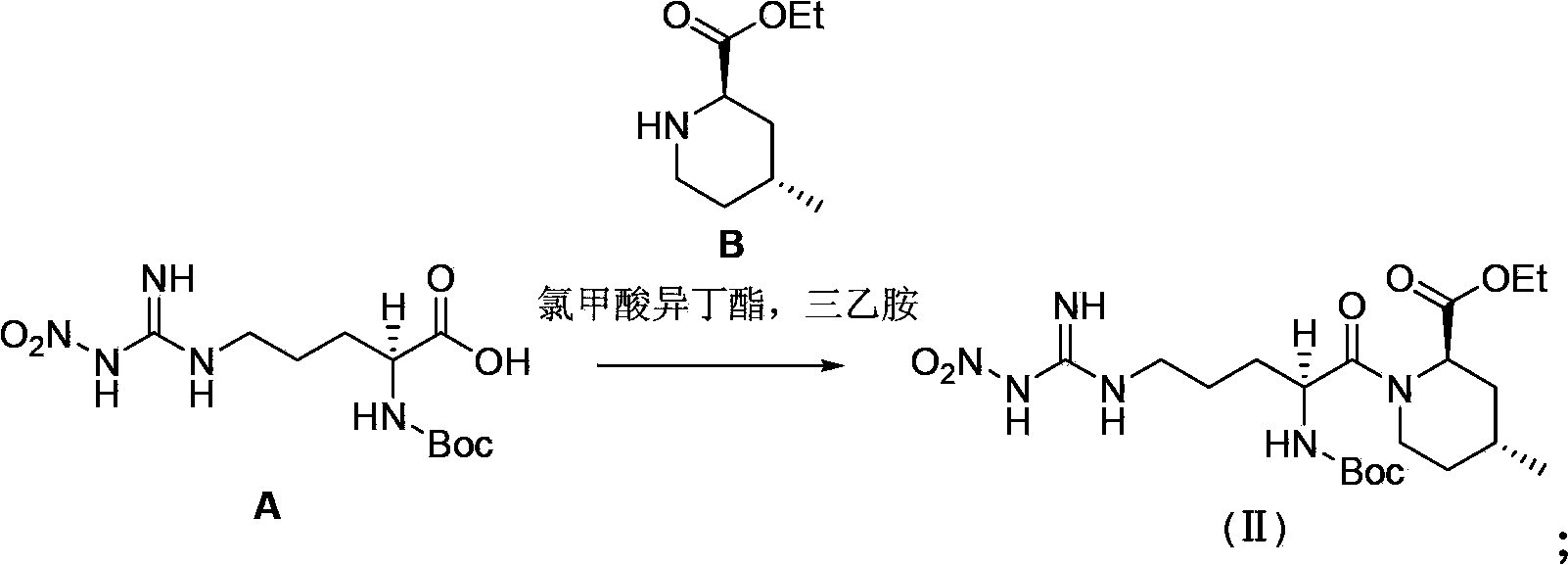
Preparation method of argatroban intermediate
A technology for argatroban and intermediates, which is applied in the field of preparation of argatroban intermediates, can solve the problems of being unsuitable for large-scale industrial production, difficult to purify products, unfriendly to the environment, etc., and achieves low production cost and good precision. , Environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Add 20.0g of compound A, 24.0g of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride, 200mL of tetrahydrofuran into a dried 500mL four-neck flask under nitrogen protection, and control the temperature at 20~ 30°C, add 11.0g of compound B in batches, and stir at 20~30°C for 2h. Concentrate the reaction solution to dryness, add 200mL of toluene to dissolve the material, wash once with 150mL of saturated brine, separate the liquid, concentrate the organic phase to 50mL, stir at 10~15°C for 8h, filter the material liquid, and wash the filter cake with 10mL of toluene. Dry the filter cake to obtain an off-white solid, which is the intermediate of argatroban. Yield: 80.0%, HPLC purity: 98.0%, content 98.6%, impurity formula III 1.20%, impurity formula IV not detected.
Embodiment 2
[0058] Add 20.0g compound A, 20.0g 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride, 20mL dichloromethane to the dried 500mL four-necked bottle under nitrogen protection, control Temperature -10~0°C, add 11.0g of compound B in batches, stir at -10~0°C for 8h. Wash once with 150 mL of saturated brine, separate the liquid, concentrate the organic phase to dryness, add 50 mL of methyl tert-butyl ether, stir at 10~15°C for 8 hours, filter the feed liquid, wash the filter cake with 10 mL of methyl tert-butyl ether, and dry The off-white solid obtained from the filter cake is the intermediate of argatroban. Yield: 82.0%, HPLC purity: 97.0%, content 98.1%, impurity formula III 1.26%, impurity formula IV not detected.
Embodiment 3
[0060] Add 20.0g of compound A, 60.0g of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride, and 1000mL of acetonitrile to a dried 3000mL four-neck flask under nitrogen protection, and control the temperature from 0 to 10°C, add 11.0g of compound B in batches, and stir at 0~10°C for 6h. Concentrate the reaction solution to dryness, add 200mL of toluene to dissolve the material, wash once with 150mL of saturated brine, separate the liquid, concentrate the organic phase to 50mL, stir at 10~15°C for 8h, filter the material liquid, and wash the filter cake with 10mL of toluene. Dry the filter cake to obtain an off-white solid, which is the intermediate of argatroban. Yield: 80.0%, HPLC purity: 98.2%, content 98.6%, impurity formula III 1.14%, impurity formula IV not detected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com