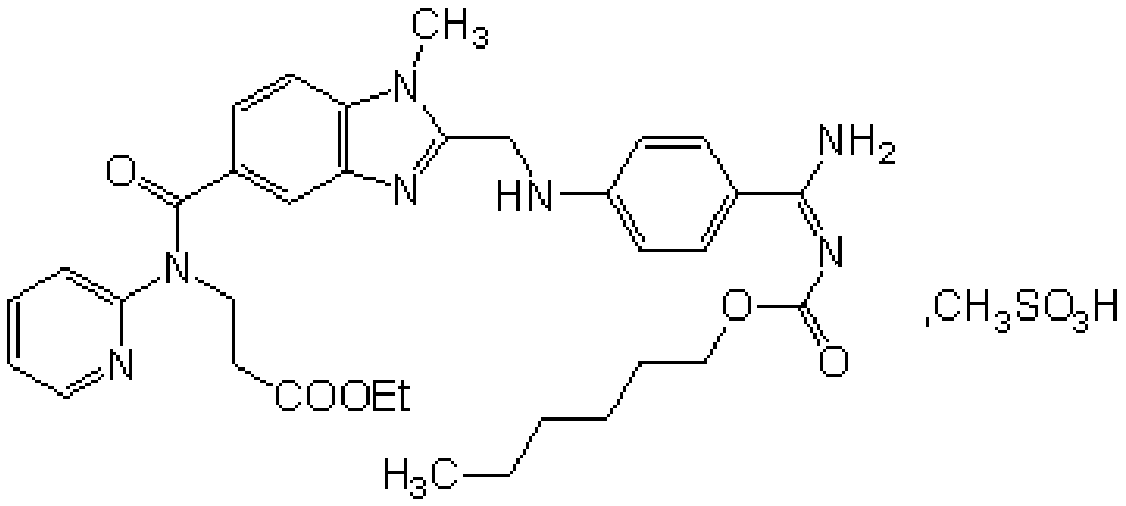
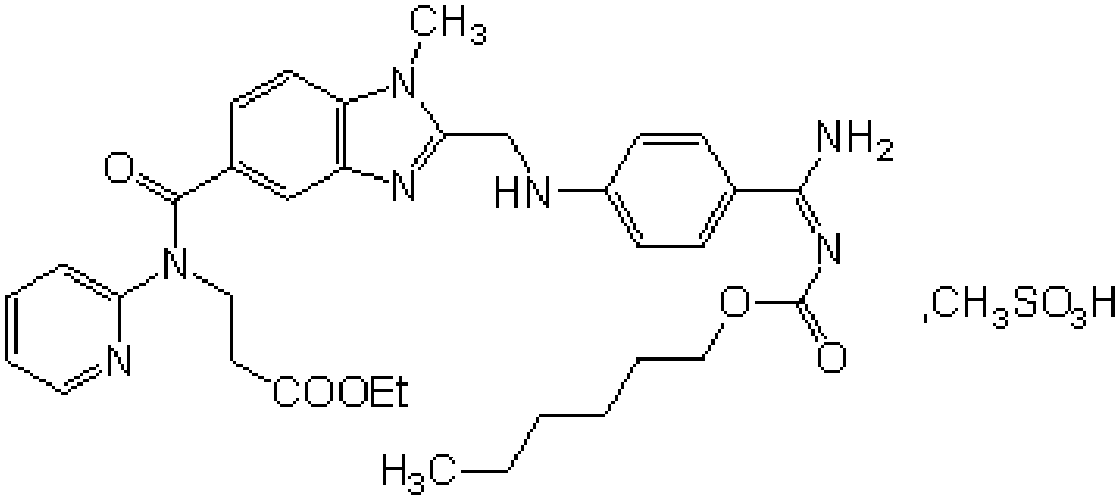
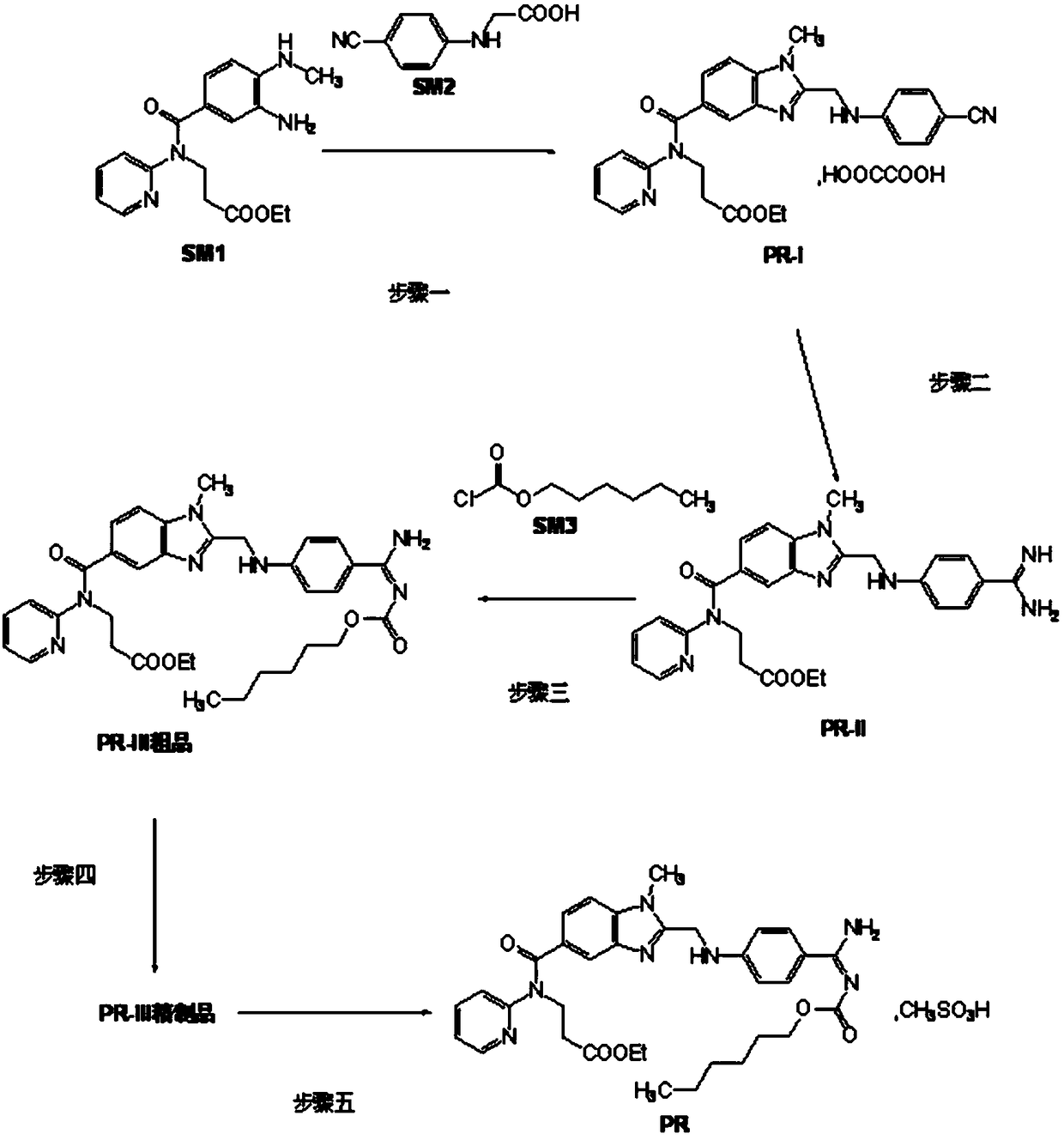
Production process of pradaxa mesylate
A technology for dabigatran etexilate mesylate and a production process, which is applied in the field of drug synthesis, can solve the problems of difficult separation of final products and intermediates, unfavorable industrialization of column chromatography separation, and difficulty in accurate quantification of solution concentration, and shortens the production cycle. , good color, low moisture content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] A kind of production technique of dabigatran etexilate mesylate, comprises the following steps:
[0072] Preparation of S1 intermediate PR-I
[0073] Add 1 kg of organic solvent a, 1 kg of SM2, and 1 kg of CDI to the reaction kettle in turn, stir and react at 10-50 ° C for 2.5 hours, then add 1 kg of SM1 and 1 kg of organic solvent b in sequence, continue to stir and react for 11 hours, evaporate the solvent under reduced pressure at 35 ° C, and transfer to the reaction kettle Put acetic acid into the kettle, heat to 100°C to react for 4.5 hours, evaporate the solvent under reduced pressure below 100°C, add dichloromethane and stir to dissolve, wash with water, recover the solvent under reduced pressure in the organic phase, add ethyl acetate to the obtained residue and stir Until dissolved, add a solution containing oxalic acid dihydrate, stir and crystallize at 20°C for 1.5 hours, centrifuge filter, wash the filter cake with ethyl acetate, and dry the filter cake unde...
Embodiment 2
[0099] A kind of production technique of dabigatran etexilate mesylate, comprises the following steps:
[0100] Preparation of S1 intermediate PR-I
[0101] Add 1 kg of organic solvent a, 1.5 kg of SM2, and 1.2 kg of CDI to the reaction kettle in sequence, stir and react at 10-50 ° C for 3 hours, then add 1 kg of SM1 and 1 kg of organic solvent b in sequence, continue to stir and react for 12 hours, evaporate the solvent under reduced pressure at 40 ° C, Put acetic acid into the reaction kettle, heat to 110°C and react for 5 hours, evaporate the solvent under reduced pressure below 100°C, then add dichloromethane and stir to dissolve, wash with water, recover the solvent under reduced pressure in the organic phase, add ethyl acetate to the obtained residue Stir the ester until dissolved, add a solution containing oxalic acid dihydrate, stir and crystallize at 22°C for 2 hours, centrifugally filter, wash the filter cake with ethyl acetate, dry the filter cake under reduced pres...
Embodiment 3
[0126] A kind of production technique of dabigatran etexilate mesylate, comprises the following steps:
[0127] Preparation of S1 intermediate PR-I
[0128] Add 1 kg of organic solvent a, 1.8 kg of SM2, and 1.3 kg of CDI to the reaction kettle in sequence, stir and react at 10-50 °C for 4 hours, then add 1 kg of SM1 and 1 kg of organic solvent b in sequence, continue to stir and react for 11.5 hours, evaporate the solvent under reduced pressure at 45 °C, Put acetic acid into the reaction kettle, heat to 105°C and react for 5 hours, distill off the solvent under reduced pressure below 100°C, add dichloromethane and stir to dissolve, wash with water, recover the solvent under reduced pressure in the organic phase, add ethyl acetate to the obtained residue Stir the ester until dissolved, add a solution containing oxalic acid dihydrate, stir and crystallize at 23°C for 2 hours, centrifugally filter, wash the filter cake with ethyl acetate, dry the filter cake under reduced pressur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com