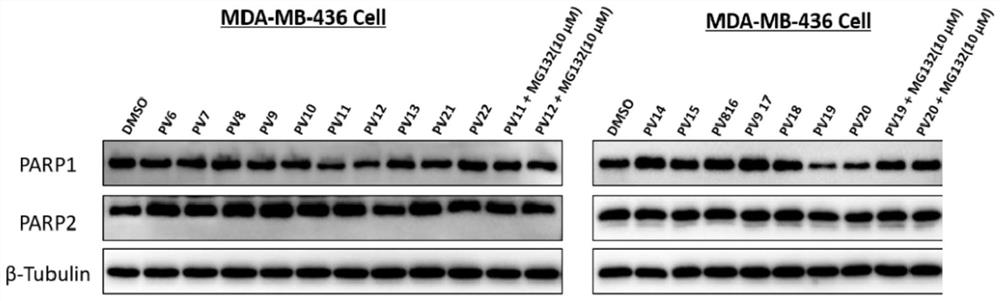
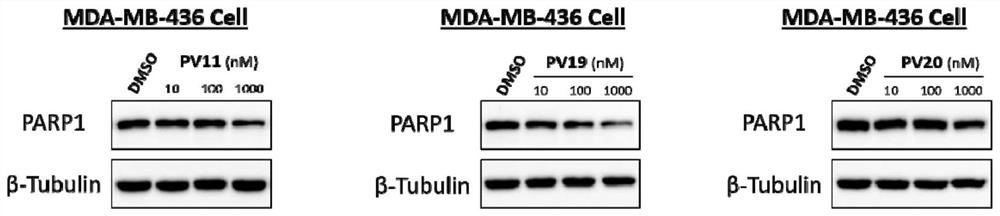
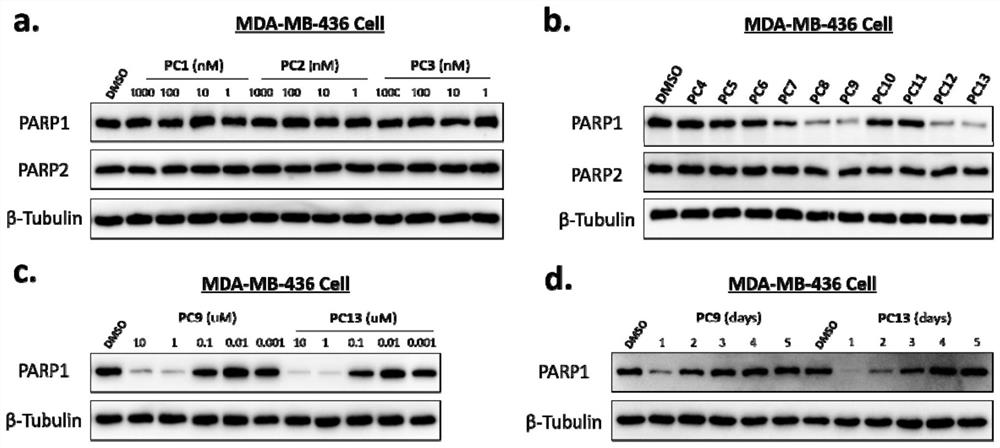
PARP1 protein degradation agent and application thereof in tumor resistance
A technology of anti-tumor drugs and compounds, applied in the direction of anti-tumor drugs, peptide/protein components, medical preparations containing active ingredients, etc., can solve problems such as recovery, drug action mechanism is not completely clear, and reduce efficacy Toxicity, induction of tumor cell apoptosis, and enhancement of chemotherapy efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] 1. Preparation of PARP1 ligand intermediate
[0056] Add L (4.45g, 15mmol), N-Boc-piperazine (3.35g, 18mmol) into a round bottom flask, add 100mL of DMF and cool the reaction solution to 0°C. TEA (4.2mL, 30mmol) and HATU (6.80g, 18mmol) were added under stirring, and the reaction was incubated for 5h. After the complete reaction of the raw materials was monitored by TLC, 500 mL of water was added to the reaction liquid, and stirred at 0°C for 1 h. A large amount of white solid was obtained by suction filtration, and the filter cake was washed with a small amount of ice water and ice EA to obtain a crude intermediate (6.43 g, 92% yield).
[0057] Add the above reaction crude product (6.43 g, 13.8 mmol) into a round bottom flask, add 50 mL of absolute ethanol, add 6N HCl (6.9 mL) dropwise under rapid stirring, and react at room temperature for 3 h after the dropwise addition. Concentrate the solvent after TLC monitors that the reaction is complete, add 50mL of water, an...
Embodiment 2
[0114] Example 2 Synthesis of PARP1 PROTAC molecules
[0115] General procedure E: Add V1 / V2 / V3 (0.11mmol) to a 25mL reaction flask, add 10mL DCM / MeOH (5:1) to dissolve, add dropwise 1mL 4M HCl-dioxane solution, react at room temperature for 2h, reduce After concentrating the solvent to obtain a white solid, add L2-L14 (0.1 mmol) to the reaction flask, then add 10 mL of anhydrous DMF to dissolve, put it in an ice-water bath, add DIPEA (0.2 mmol), and add HATU (380 mg, 0.1mmol), the reaction was placed in an ice-water bath for 1-2h. After the completion of the reaction monitored by TLC, 50 mL of semi-saturated brine was added to the reaction solution for dilution, and then extracted with ethyl acetate (3 × 60 mL), the combined organic layers were washed once with saturated aqueous sodium chloride solution, and anhydrous Na 2 SO 4 Dry, filter and distill off the solvent under reduced pressure to obtain an oily crude product. Finally, a white solid was obtained by column chrom...
Embodiment 3
[0117] The synthesis of PV6 refers to the general procedure E, and the product PV6 can be obtained as a white solid with a yield of 74% by using L7 and V1 as the reaction raw materials. 1 H NMR (400MHz,DMSO)δ12.59(s,1H),8.98(s,1H),8.63–8.51(m,1H),8.27(d,J=7.7Hz,1H),7.97(d,J= 7.9Hz, 1H), 7.90(t, J=9.9Hz, 2H), 7.83(t, J=7.4Hz, 1H), 7.49–7.32(m, 6H), 7.23(t, J=9.0Hz, 1H) ,5.13(s, 1H),4.53(d,J=9.2Hz,1H),4.48–4.38(m,2H),4.39–4.29(m,3H),4.28–4.17(m,1H),3.71–3.46 (m,6H), 3.42–3.36(m,2H),3.34(s,6H),3.17(d,J=25.0Hz,2H),2.61–2.53(m,2H),2.47–2.34(m,4H ),2.09–1.98 (m,1H),1.94–1.86(m,1H),0.94(s,9H).HRMS(DART-TOF)calculated for C 46 h 51 FN 8 NaO 7 S + [M+Na] + m / z 901.3483, found 901.3491. Its chemical structural formula is:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com