A treatment method for the solidification process of nano-suspension
A technology of nano-suspension and processing method, which is applied in the direction of pharmaceutical formula, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the unfavorable follow-up process of nano-crystalline powder and the low drug content of nano-crystalline powder , Reduce patient compliance and other issues, achieve the effects of reducing industrial production costs, easy to redisperse, and simple methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
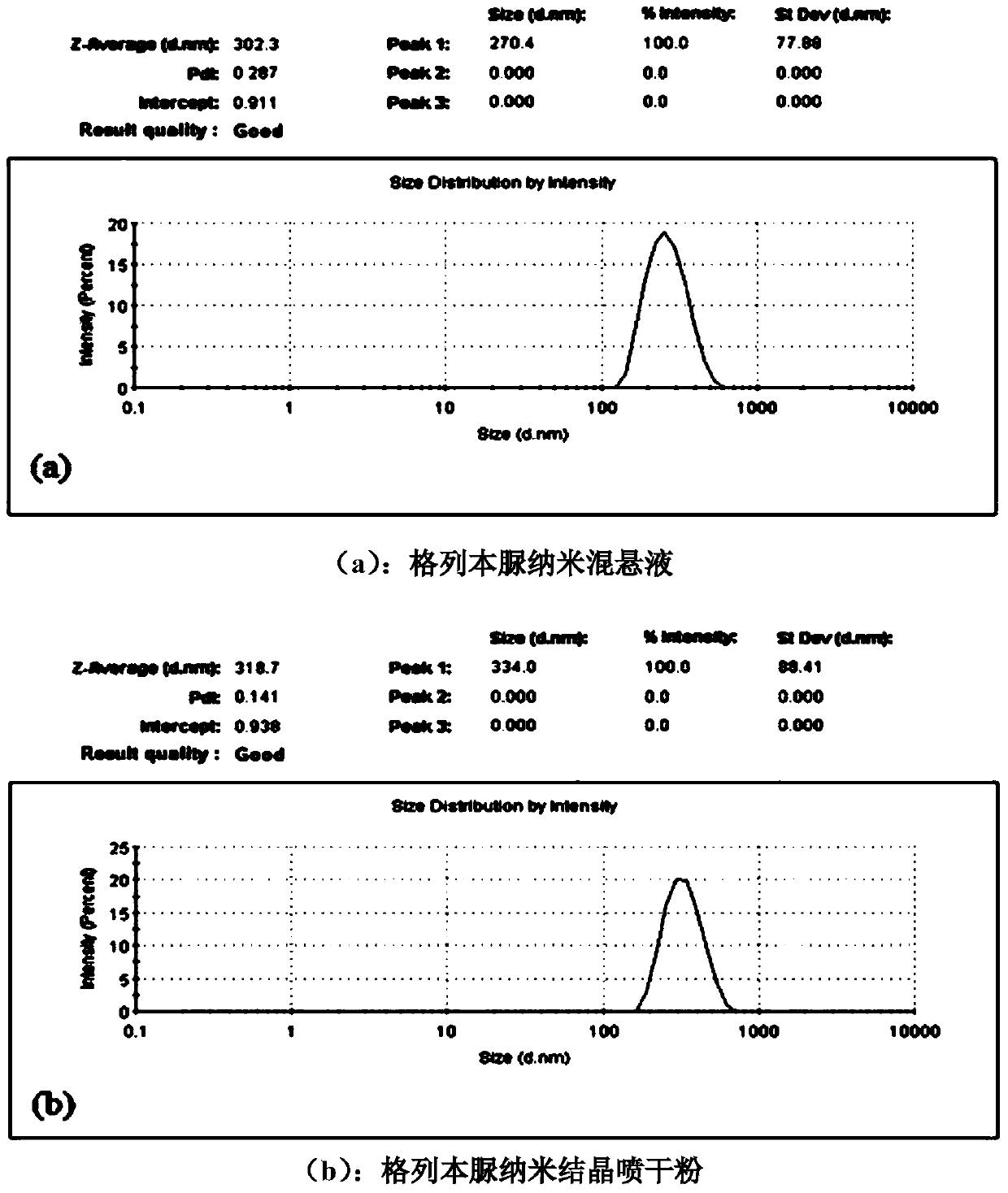
[0026] Embodiment 1: Preparation and post-treatment of a kind of glibenclamide nanosuspension
[0027] First, prepare the water phase containing the HPMC E5 of 0.3% (w / v), after stirring, the glibenclamide of 7% (w / v) is suspended in the above water phase, after that, the suspension is transferred to In the ball mill jar, the ball mill was used to grind for 80 minutes, and the average particle size of the obtained nanosuspension was measured to be 302.3 nm. Before spraying dry, add the distilled water of suspension total volume 50% (v / v), the mannitol of 1.0% (w / v), the Tween80 of 0.5% (w / v) in gained nanosuspension, after spraying Dry machine peristaltic pump feeding liquid speed is 5mL / min, air flow rate is 50mL / min, and the inlet temperature is 160 ℃ under the conditions of spray drying to obtain solidified glibenclamide nanocrystalline powder. After reconstitution, the average particle diameter was measured to be 318.7nm. The results show that the nano-suspension treatme...
Embodiment 2
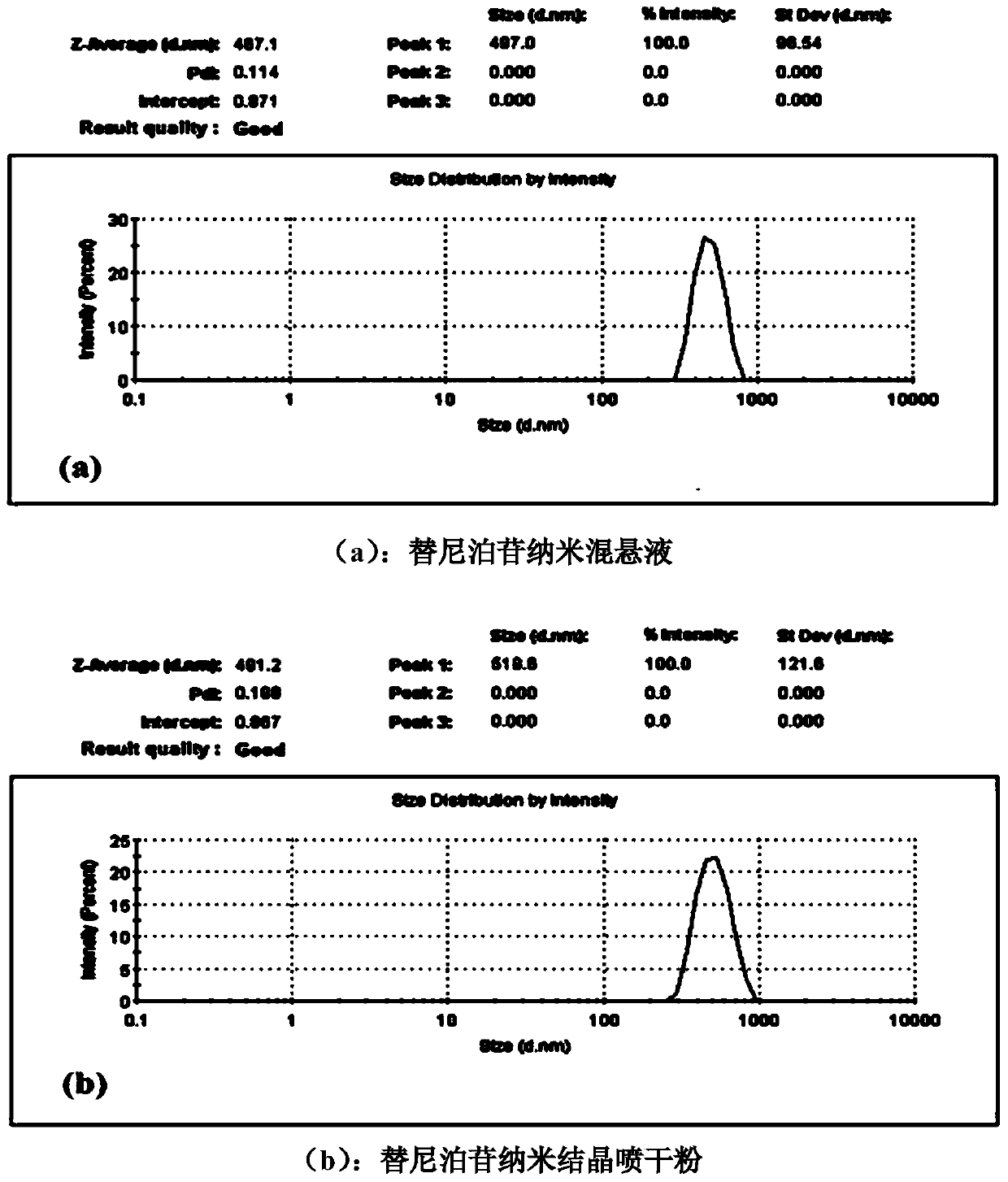
[0028] Embodiment 2: Preparation and post-processing of a teniposide nanosuspension
[0029] First, prepare an aqueous phase containing 0.5% (w / v) of PVP K30, and after stirring, suspend 6% (w / v) teniposide in the above aqueous phase, and then transfer the suspension to In a ball mill jar, use a ball mill to grind for 45 minutes. The average particle diameter of the obtained nanosuspension was measured to be 467.1 nm. Before spraying dry, add the distilled water of suspension total volume 100% (v / v), the Tween 80 of 3.5% (w / v) in gained nanosuspension, feed liquid speed in spray drying machine peristaltic pump is 3mL / min, an air flow rate of 60 mL / min, and an inlet temperature of 140° C. to obtain solidified teniposide nanocrystalline powder. After reconstitution, the average particle diameter was measured to be 491.2nm. The results show that the nano-suspension processing method of the present invention can effectively maintain the particle size of teniposide nanoparticle...
Embodiment 3
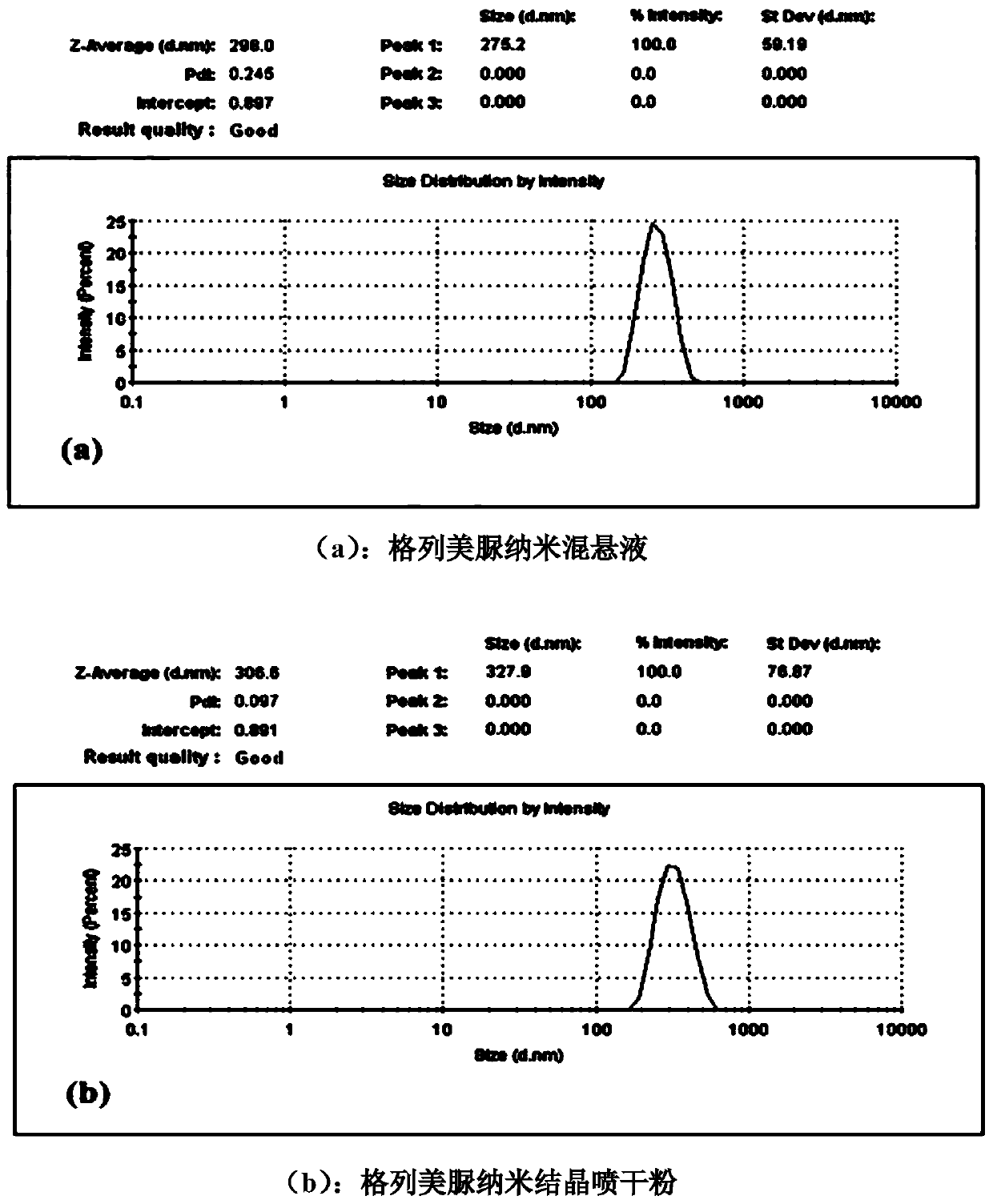
[0030] Embodiment 3: the preparation of a kind of glimepiride nanosuspension and its aftertreatment
[0031] First, prepare the water phase containing the HPMC E50 of 0.1% (w / v), after stirring, the glimepiride of 5% (w / v) is suspended in the above water phase, after that, the suspension is transferred to In a ball mill jar, the mixture was ground for 45 minutes with a planetary ball mill, and the average particle diameter of the obtained nanosuspension was measured to be 298.0 nm. Before spraying dry, add the glucose of 4.0% (w / v), the SDS of 0.02% (w / v) in gained nanosuspension, in the peristaltic pump feeding speed of spray drying machine, be 2.5mL / min, air velocity is Spray-dry under the conditions of 60mL / min and an inlet temperature of 130°C to obtain solidified glimepiride nanocrystalline powder. After reconstitution, the average particle diameter was measured to be 306.6nm. The results show that the nano-suspension treatment method of the present invention can effect...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com