Oil-soluble medicine degradable polymer microcapsule injecta and preparing method
A technology for degrading polymers and injection preparations, which is applied in the field of degradable polymer microcapsule injection preparations of oil-soluble drugs and their preparation, can solve the problem that the research reports and applications of drug release carriers are few, it is difficult to obtain long-acting sustained release, and it is difficult to Microcapsules and other problems, to achieve the effects of good physiological compatibility and biodegradability, good biocompatibility and biodegradability, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
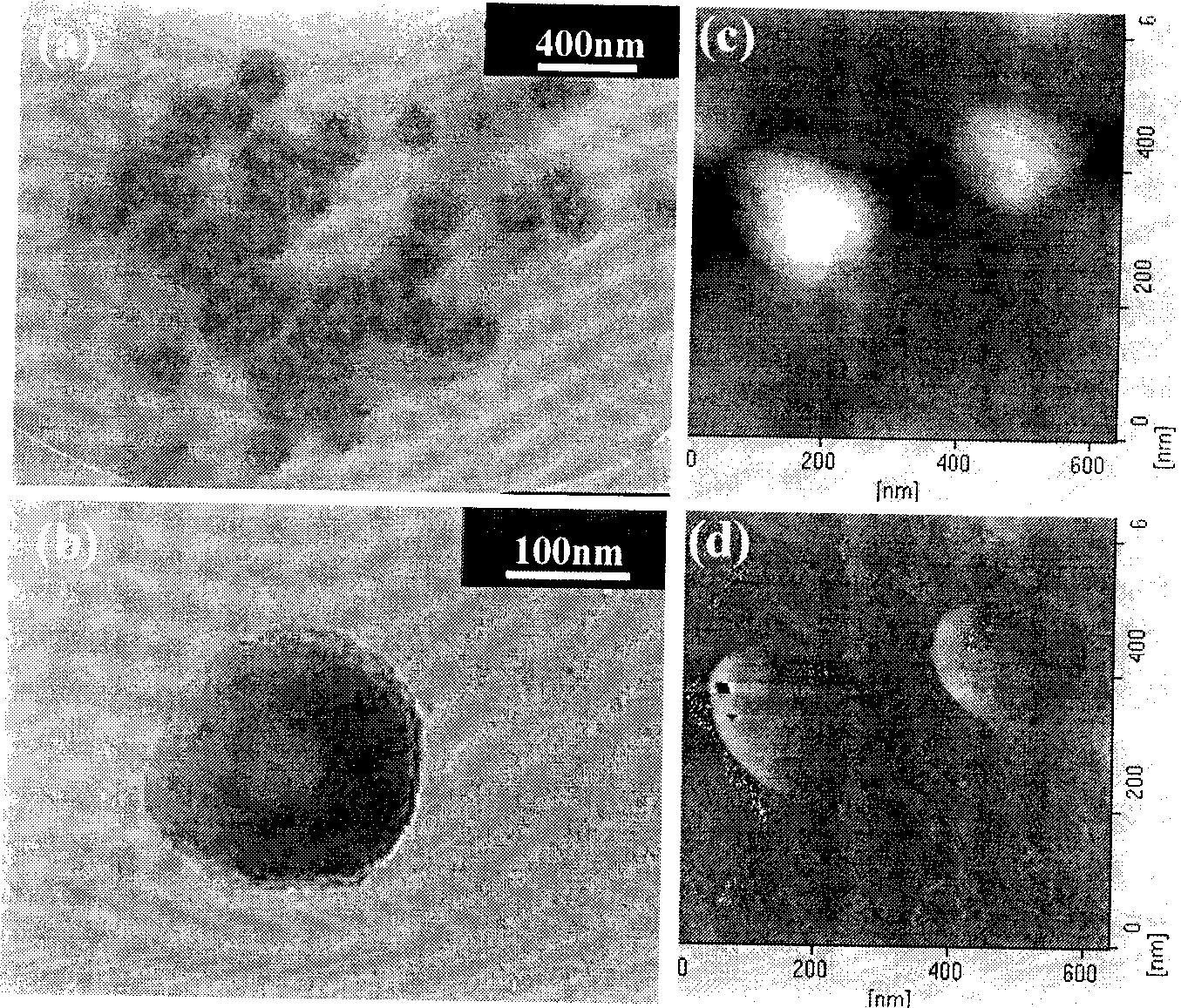
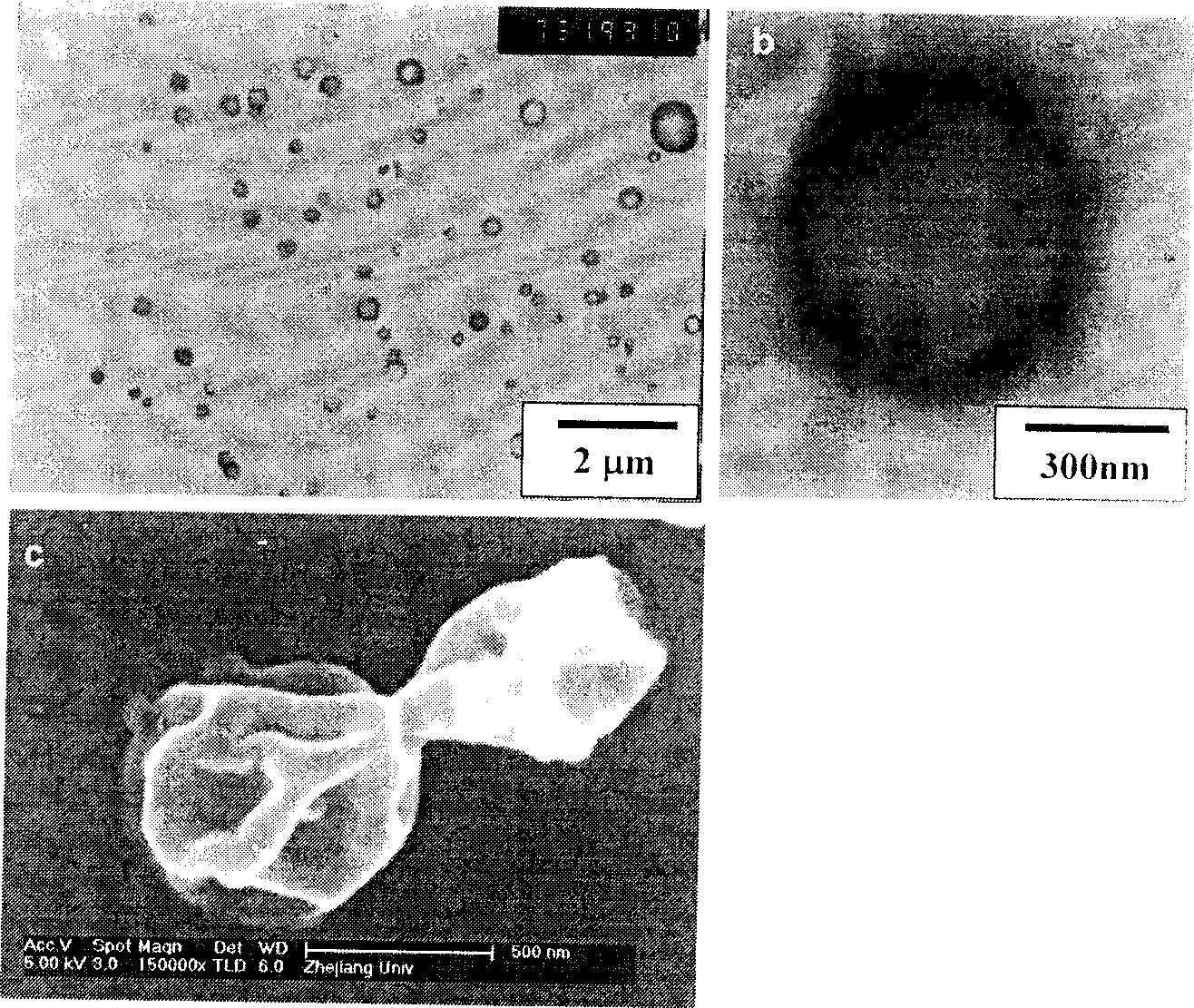
Image
Examples
example 1
[0030] (1) Grind lactide and stannous chloride crystals into powder, add a certain amount of allyloxyethylene glycol, place it in a reaction container, remove oxygen and nitrogen, and close the container. The amount of stannous chloride It is 0.8‰ of lactide weight, and the molar ratio of lactide / allyloxyethylene glycol is 55;
[0031] (2) The above reaction device is placed in an oil bath at 140°C, and the temperature is lowered after 8 hours at a constant temperature. Using acetone as a solvent and deionized water as a precipitating agent, it is purified by dissolution-precipitation to remove impurities, and vacuum-dried at 40°C to constant weight or freeze-drying to obtain double bond-containing polylactic acid;
[0032] (3) At 25° C. and a pH value of 7.0, methacrylic acid with a concentration of 1 g / ml is dissolved in an aqueous solution containing water-soluble carbodiimide, and the weight ratio of methacrylic acid and carbodiimide is 1:2, After stirring for 2 hours, ad...
example 2
[0038] (1) Grind lactide and stannous chloride crystals into powder, add a certain amount of allyloxyethylene glycol, place it in a reaction container, remove oxygen and nitrogen, and close the container. The amount of stannous chloride It is 2‰ of the weight of lactide, and the molar ratio of lactide / allyloxyethylene glycol is 200;
[0039] (2) The above-mentioned reaction device was placed in an oil bath at 200°C, and the temperature was lowered after 24 hours at a constant temperature. Using acetone as a solvent and deionized water as a precipitant, it was purified by dissolution-precipitation to remove impurities, and vacuum-dried at 40°C to constant weight. Obtain double bond-containing polylactic acid;
[0040] (3) At room temperature, after dissolving ciprofloxacin, medical grade polylactic acid (molecular weight 10,000), polylactic acid containing double bonds and polyethylene glycol diacrylate in chloroform, add 0.2% polyvinyl alcohol ( PVA) aqueous solution, an O / W ...
example 3
[0044] (1) step (1) and (2) in the synthetic repetition example 1 containing double bond polylactic acid, difference is that the consumption of stannous chloride is 10‰ of lactide weight;
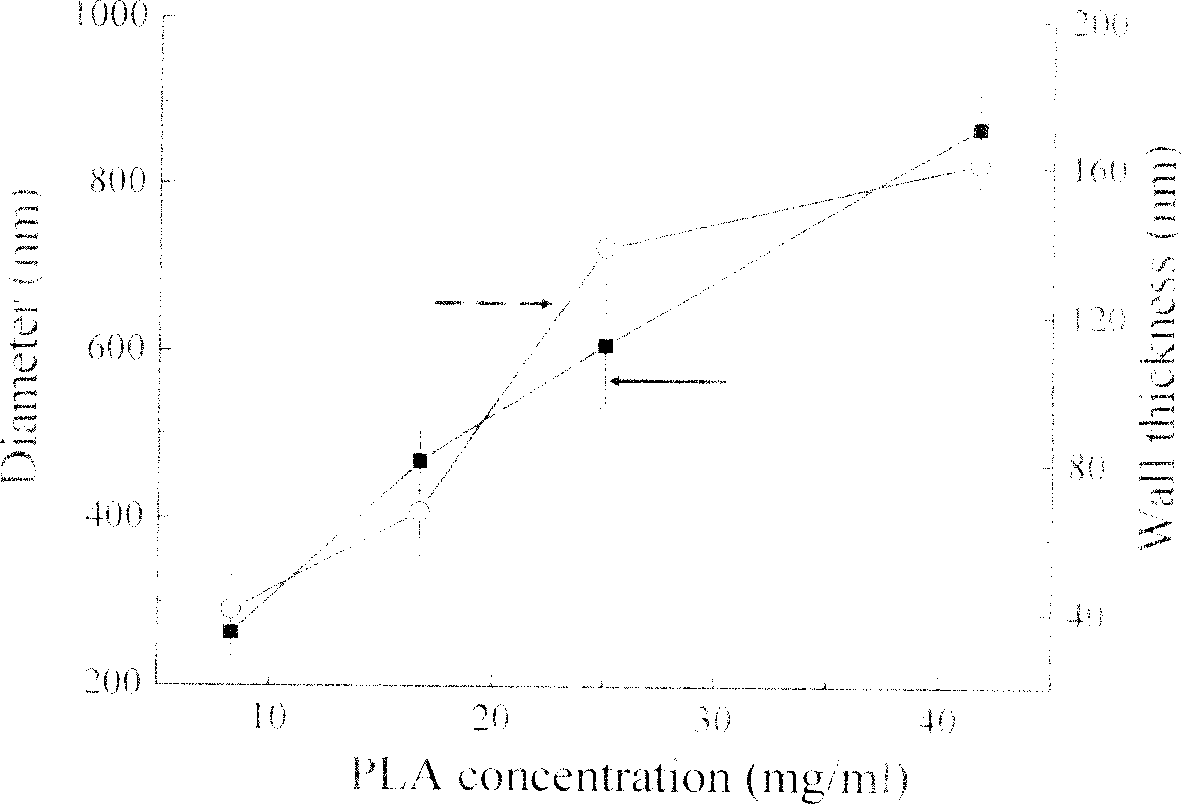
[0045] (2) At room temperature, after dissolving ciprofloxacin, medical grade polylactic acid (molecular weight 8,000), polylactic acid containing double bonds and divinylbenzene (DVB) in chloroform, add 0.2% polyvinyl alcohol (PVA ) in an aqueous solution to obtain an O / W emulsion. The concentration of polylactic acid in chloroform is respectively 8.5, 17, 25.5 and 42.5mg / ml, and the DVB weight accounts for 1% of the polylactic acid weight containing double bonds, and the consumption of ciprofloxacin is 0.1% of the polylactic acid gross weight, O / W volume ratio is 1:5;
[0046] (3) Pass nitrogen and maintain the nitrogen atmosphere in the above container, after mechanical stirring for 0.5 hours (600rmp), keep the temperature at 40°C, K 2 S 2 o 8 with NaHSO 3 (1:1 weight ratio, concen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com