Method of producing hepatic targeting drug microcapsule
A technology of liver targeting and microcapsules, which is applied in the field of preparation of drug microcapsules, can solve the problems of inability to precisely control drug sustained release, lack of tissue targeting, and normal tissue toxicity and side effects, and achieve a simple, efficient and easy-to-use copolymerization method Operation, high efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Synthesis of Galactose Vinyl Ester Monomer
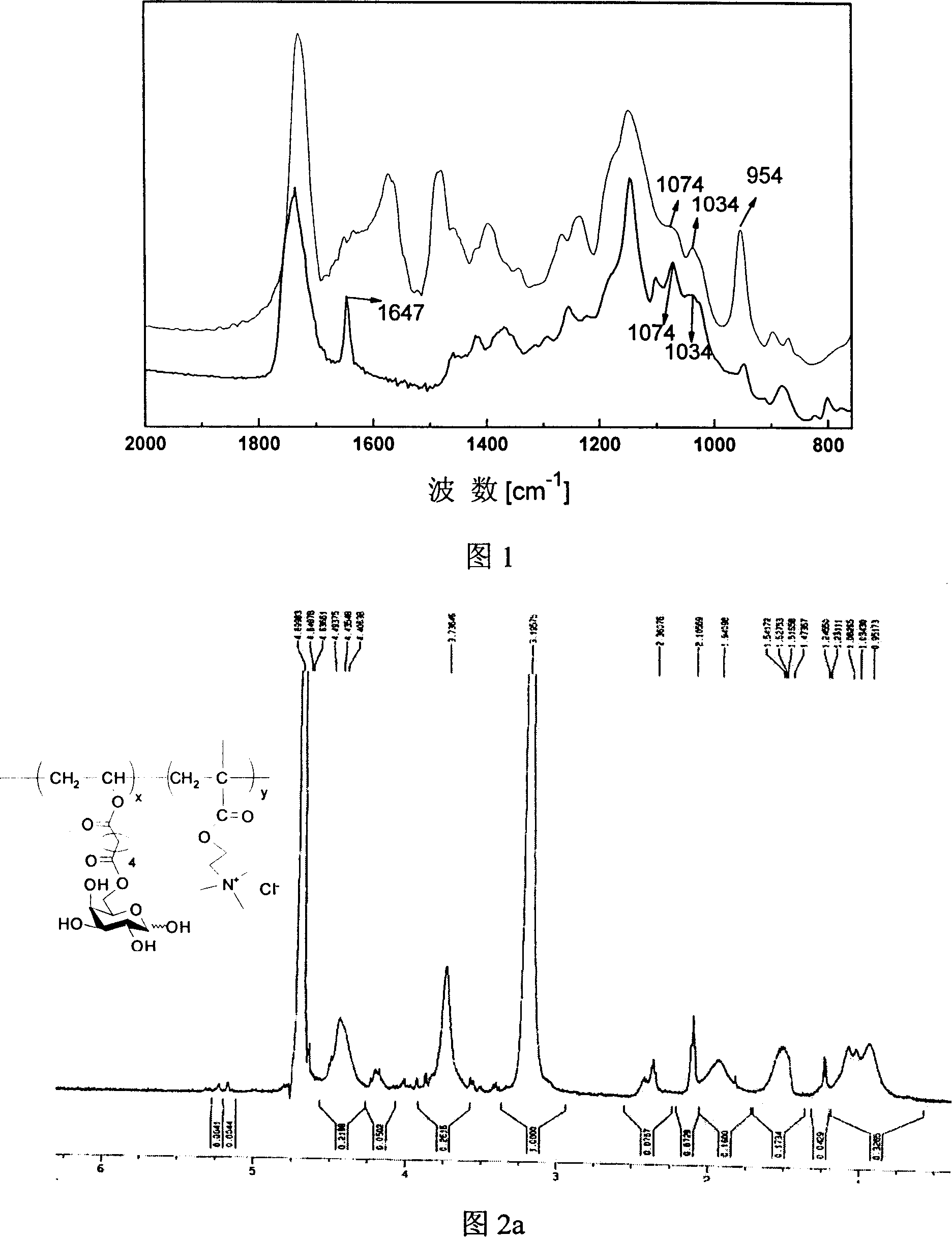
[0045] Put 16g of divinyl adipate, 4g of galactose, and 1g of subtilisin into a 250mL Erlenmeyer flask, add 40mL of pyridine, ultrasonically mix, and react on a shaker at 50°C at 200rpm for 3 days. The solid was removed by filtration, the crude product was isolated, and distilled under reduced pressure to obtain a galactose vinyl ester monomer containing a double bond. The infrared spectrogram of galactose vinyl ester monomer is shown in Figure 1 (upper curve), and the characteristic peaks of vinyl ester C=C double bond and sugar C-O characteristic peaks are obvious, indicating that galactose vinyl ester monomer was successfully prepared.
Embodiment 2
[0046] Example 2 Synthesis of sugar-containing polycations (1)
[0047] After vacuum-drying the galactose vinyl ester monomer, place it in a desiccator and dry slowly for 20 days, take 0.4g galactose vinyl ester and 0.7g methacryloyloxyethyltrimethylammonium chloride (DMC) in a 25mL small flask In, add 1.4ml of distilled water to dissolve, at the same time add 1ml of potassium persulfate solution with a concentration of 10mg / ml. Vacuumize the nitrogen repeatedly for 3 times, the polymerization solution is stirred and reacted at 70°C for 24 hours under the protection of nitrogen, pour into acetone to precipitate the polymer, dissolve it with 10mL water, put it in a dialysis bag with a molecular weight cut-off of 3500kD, and dialyze against water for one day. The solution in the bag was freeze-dried, and the infrared spectrum of the obtained sugar-containing polycation was shown in Figure 1 (lower curve). The characteristic peak of the C=C double bond disappeared, the characteri...
Embodiment 3
[0048] Example 3 Synthesis of sugar-containing polycations (2)
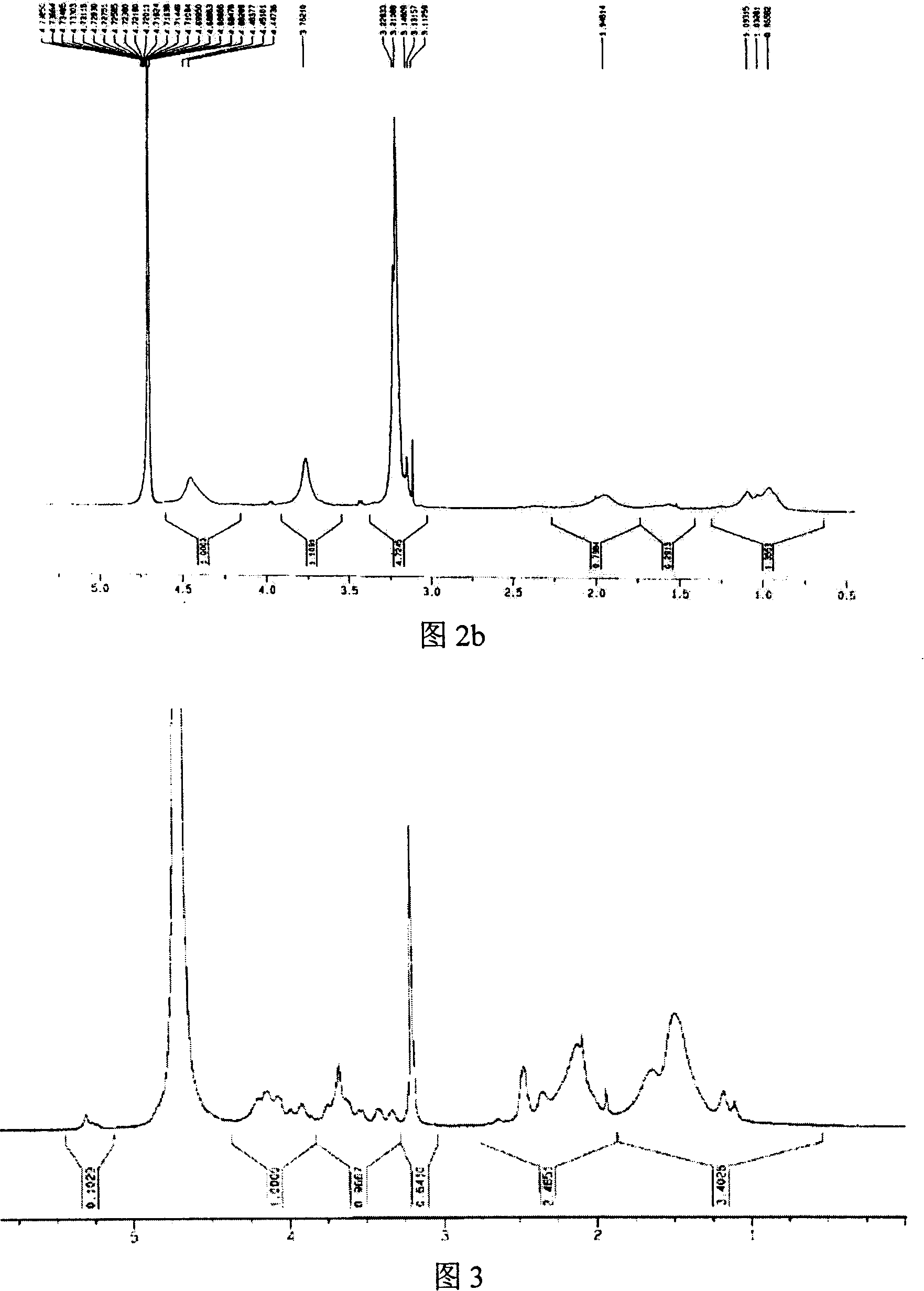
[0049] Using the method of Example 2, 0.1 g of galactose vinyl ester monomer was copolymerized with 0.7 g of DMC to finally obtain a sugar-containing polycation with a grafted sugar molar ratio of 5%. The H NMR spectrum characterization is shown in Figure 2b.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com