Doxorubicin hydrochloride self-assembled polymer nanoparticle and preparation method thereof
A doxorubicin hydrochloride and polymer technology, which is applied in the field of doxorubicin hydrochloride self-assembled polymer nanoparticles and their preparation, can solve the problems of unstable molecular structure, short plasma half-life, serious toxic and side effects, and reduce adverse reactions As well as toxic side effects, no physiological toxicity, and the effect of reducing toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
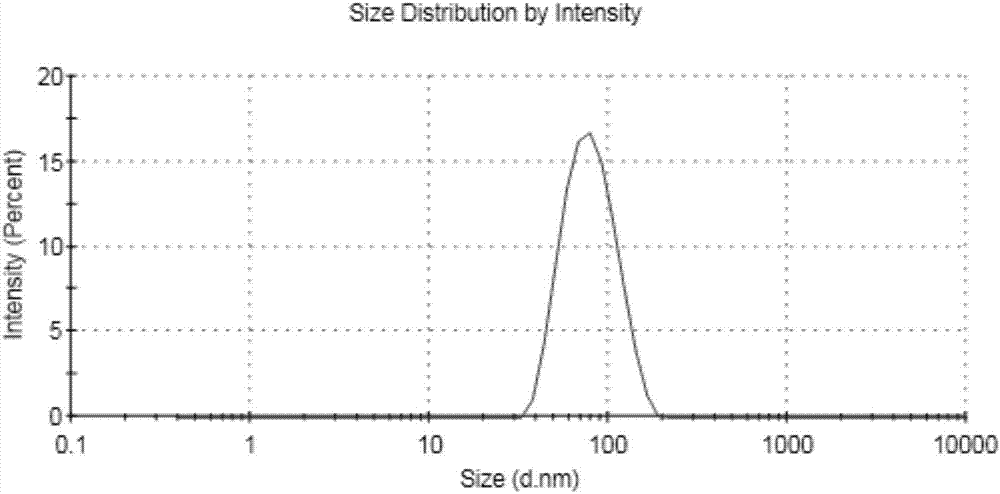
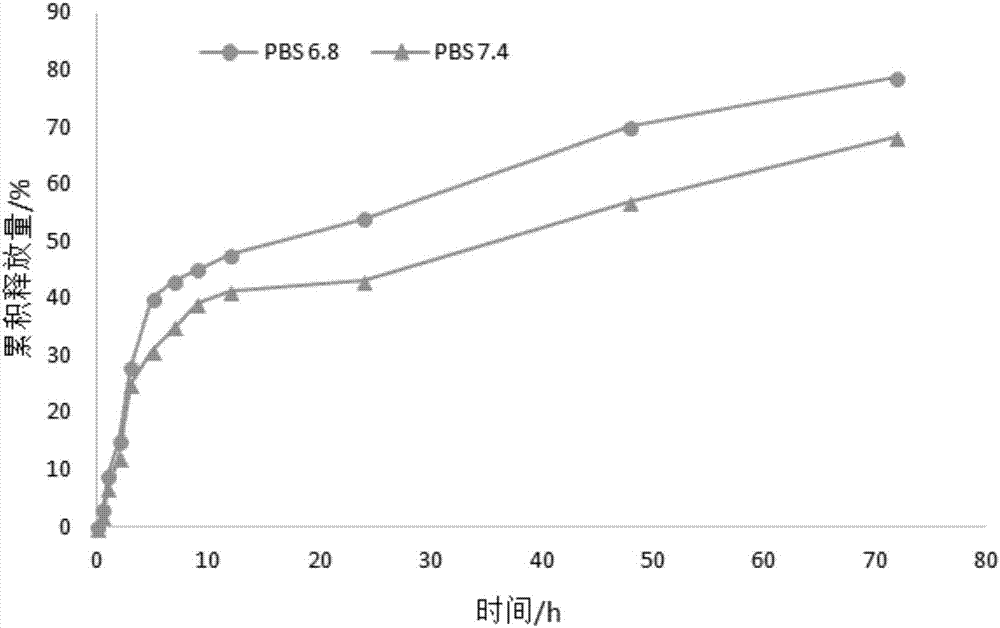
Image
Examples
Embodiment 1
[0024] Embodiment 1 Preparation of doxorubicin hydrochloride self-assembled polymer nanoparticles
[0025] Prescription composition: doxorubicin hydrochloride as the main drug, PLGA as the polymer carrier.
[0026] The specific preparation method is as follows:
[0027] 1) Accurately weigh 0.1 mg of doxorubicin hydrochloride and dissolve it in 1 ml of dimethylsulfoxide (DMSO) to obtain a DMSO solution with a concentration of doxorubicin hydrochloride of 0.1 mg / ml.
[0028] 2) Accurately weigh 1 mg of PLGA and dissolve it in 1 ml of acetonitrile to prepare a PLGA solution with a concentration of 1 mg / ml.
[0029] 3) Precisely pipette 25 μl of doxorubicin hydrochloride solution and add it dropwise to the PLGA solution, and mix well to obtain a co-solution. Take 3ml of deionized water and put it in a beaker. Under the condition of magnetic stirring, slowly add the co-solution dropwise to the deionized water, and keep stirring at room temperature with different stirring speeds o...
Embodiment 2
[0034] Embodiment 2 Preparation of doxorubicin hydrochloride self-assembled polymer nanoparticles
[0035] Prescription composition: doxorubicin hydrochloride as the main drug, PLGA as the polymer carrier.
[0036] The specific preparation method is the same as in Example 1, keeping other conditions constant, the stirring speed is selected at 30r / min, and only changing the solvent type of the PLGA solution in step 2), that is, accurately weighing 1mg PLGA dissolved in 1ml acetonitrile, 1ml acetone, 1ml dichloro A PLGA solution with a concentration of 1 mg / ml was prepared in methane, and the particle size and PDI of the doxorubicin hydrochloride-PLGA self-assembled polymer nanoparticles were measured, and the results are shown in Table 2.
[0037] Table 2
[0038] Organic solvents Particle size / nm PDI acetone 137.68 0.189 Acetonitrile 75.45 0.157 Dichloromethane 168.92 0.367
[0039] As can be seen from Table 2, when the organic solvent ...
Embodiment 3
[0040] Embodiment 3 Preparation of doxorubicin hydrochloride self-assembled polymer nanoparticles
[0041] Prescription composition: doxorubicin hydrochloride as the main drug, PLGA as the polymer carrier.
[0042] Concrete preparation method is the same as embodiment 1, keeps other conditions constant, and stirring speed selects 30r / min, just changes the input amount of doxorubicin hydrochloride in step 1), promptly is 1 by the mass ratio of doxorubicin hydrochloride and PLGA respectively: 25, 1:20, 1:10, and 1:5 ratios were used to feed materials, and the particle size, PDI, and encapsulation efficiency of doxorubicin hydrochloride-PLGA self-assembled polymer nanoparticles were measured, and the results are shown in Table 3.
[0043] table 3
[0044] Doxorubicin hydrochloride: PLGA Particle size / nm PDI Encapsulation Efficiency (EE%) 1:25 98.31 0.189 68.1 1:20 85.72 0.201 73.9 1:10 75.45 0.157 77.6 1:5 80.25 0.142 71.8
[0045...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com