Novel pharmaceutical application of apatinib or pharmaceutically acceptable salt thereof
A technology of apatinib and medicinal salt, applied in the field of drug application, can solve the problem that there is no research report on apatinib MPM, and achieve the effects of clear drug action mechanism, good safety and outstanding curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
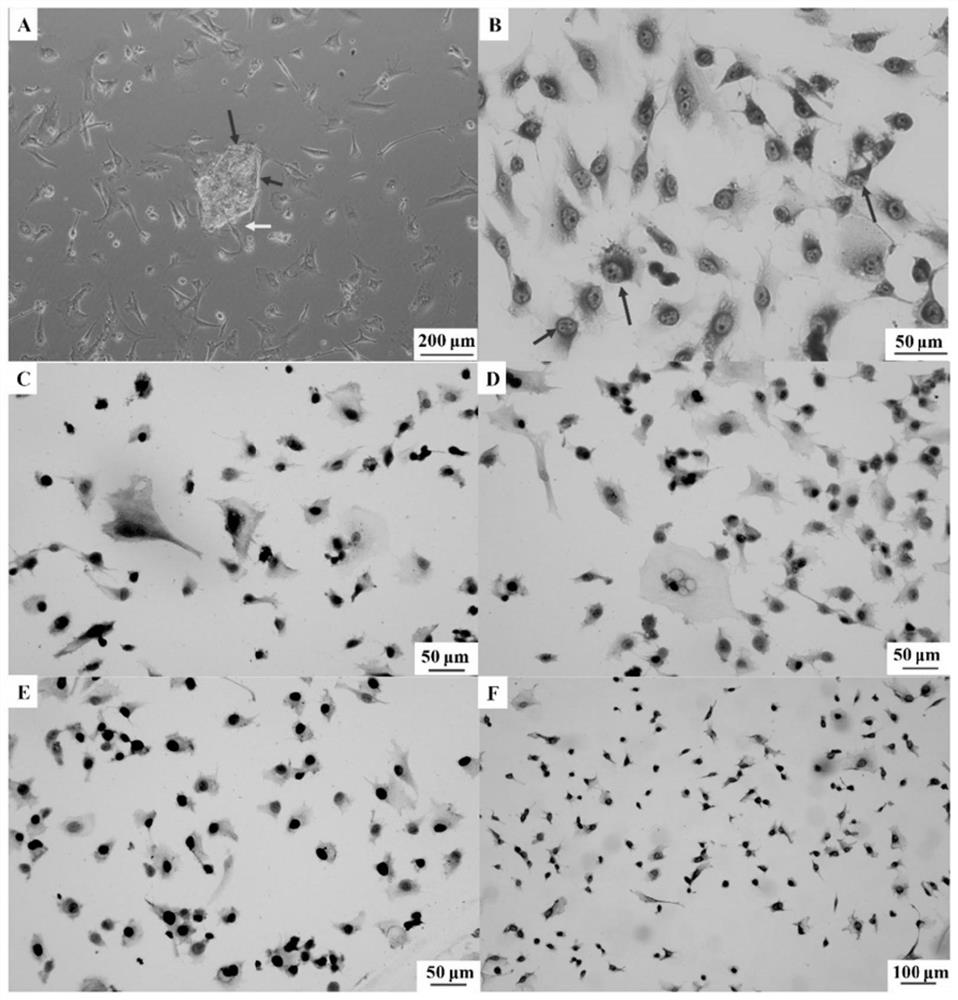
[0036] Example 1: Apatinib mesylate inhibits viability and proliferation of malignant peritoneal mesothelioma cells
[0037] Experimental method: The effect of apatinib mesylate on the viability and proliferation of MPM cells was verified by the CCK8 assay; MPM cells in the logarithmic growth phase were taken, and the cell concentration was adjusted to 1×10 5 / mL, add 50 μL of cell suspension or medium to the 96-well plate, and after the cells adhere to the wall, set up the blank control group and the apatinib mesylate group with different concentrations (0, 12.5, 25, 50, 100 μM respectively) ), incubate for 24, 48, and 72 hours, and use the CCK8 kit to detect the proliferation of MPM cells in each group (the specific steps are operated according to the instructions of the kit).
[0038] The result is as figure 2 Shown: (A) Different concentrations of apatinib mesylate can inhibit the proliferation of MPM cells; (B) apatinib mesylate can inhibit the viability of MPM cells; (...
Embodiment 2
[0041] Example 2: Apatinib mesylate affects MPM cell cycle progression
[0042] Experimental method: The effect of apatinib mesylate on the cell cycle of MPM was analyzed by flow cytometry; the cell concentration was adjusted to 3×10 6 / mL, inoculated in a 6-well plate with 1 mL, set up blank control group and apatinib mesylate group (concentration: 50 μM), cultured for 24 and 48 hours respectively, and used PI kit to detect apatinib mesylate according to the instructions. Effects of tinib on the cell cycle of MPM.
[0043] The result is as image 3 Shown: (A) MPM cells cultured for 24 hours, the percentage of cells in each phase of the cycle; (B) 50 μM apatinib mesylate acted on MPM cells for 24 hours, the percentage of cells in each phase of the cycle; compared with the control group , cells in G1 phase increased, cells in G2 phase decreased; (C) MPM cells were cultured for 48 hours, the percentage of cells in each phase of the cycle; (D) 50 μM apatinib mesylate was applie...
Embodiment 3
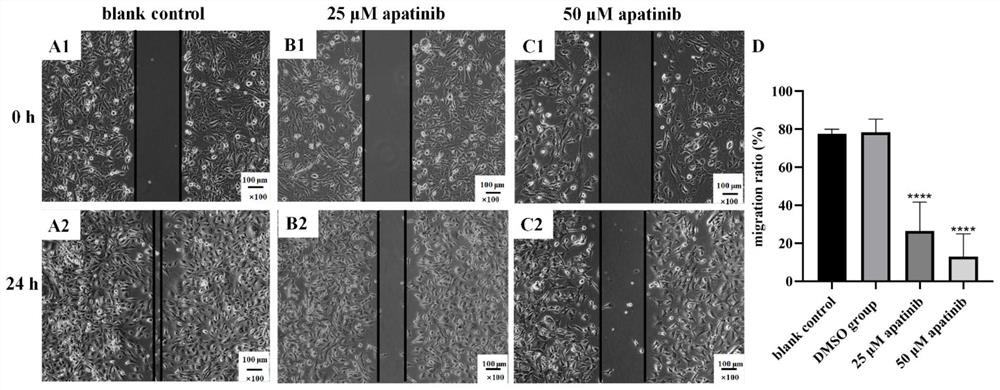
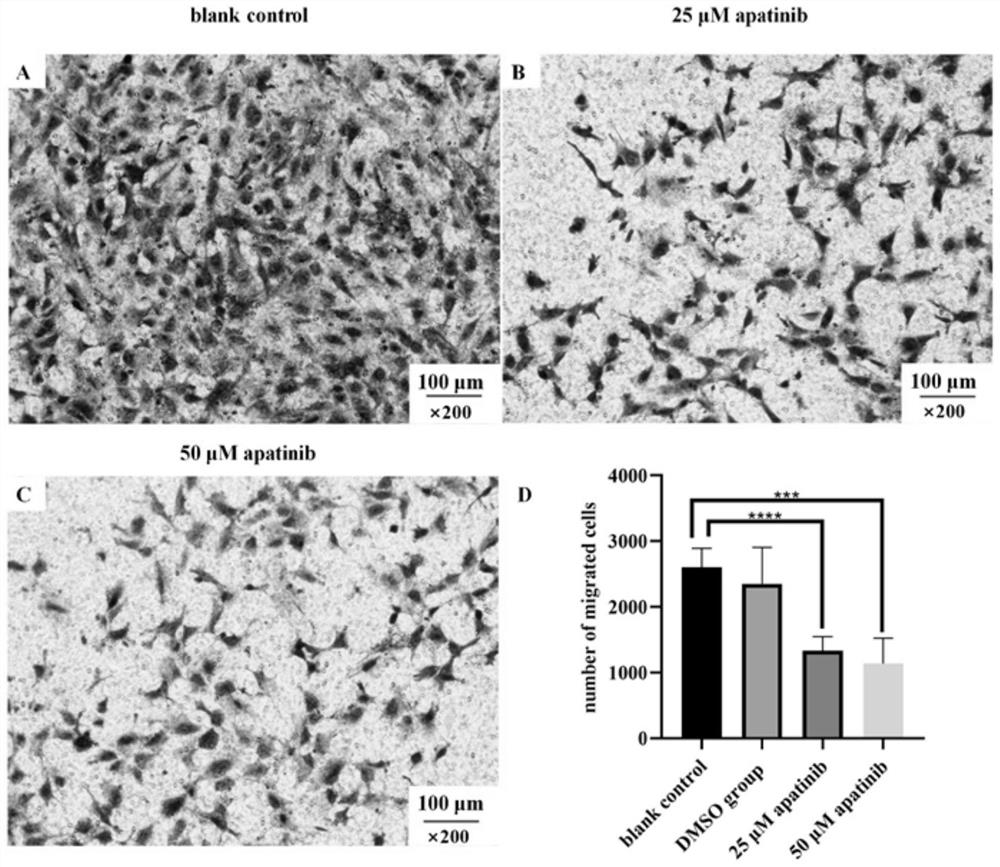
[0044] Example 3: Scratch test to verify the effect of apatinib mesylate on the movement and migration of MPM cells
[0045] Experimental method: adjust the cell concentration to 1×10 6 / mL, add 80 μL to the 6-well plate, after the cells adhere to the wall, scratch the cell surface with the tip of the 200 μL sampler perpendicular to the cell surface, after light washing with PBS, replace the serum-free DMEM medium, set blank control, solvent control and methanesulfonate After culturing for 24 hours in the apatinib group (concentrations of 25 and 50 μM, respectively), the cell migration rate of each group was calculated.
[0046] The result is as Figure 4 Shown: (A1) and (A2) are the migration status of MPM cells in the blank control group at 0h and 24h respectively; (B1) and (B2) are the migration status of 25μM apatinib mesylate at 0h and 24h respectively; (C1) , (C2) 50μM apatinib mesylate 0h and 24h cell migration respectively; (D) cell migration rate of four groups. De...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com