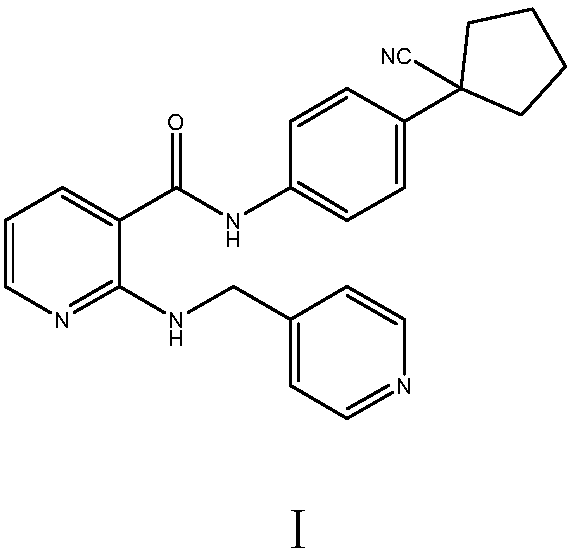
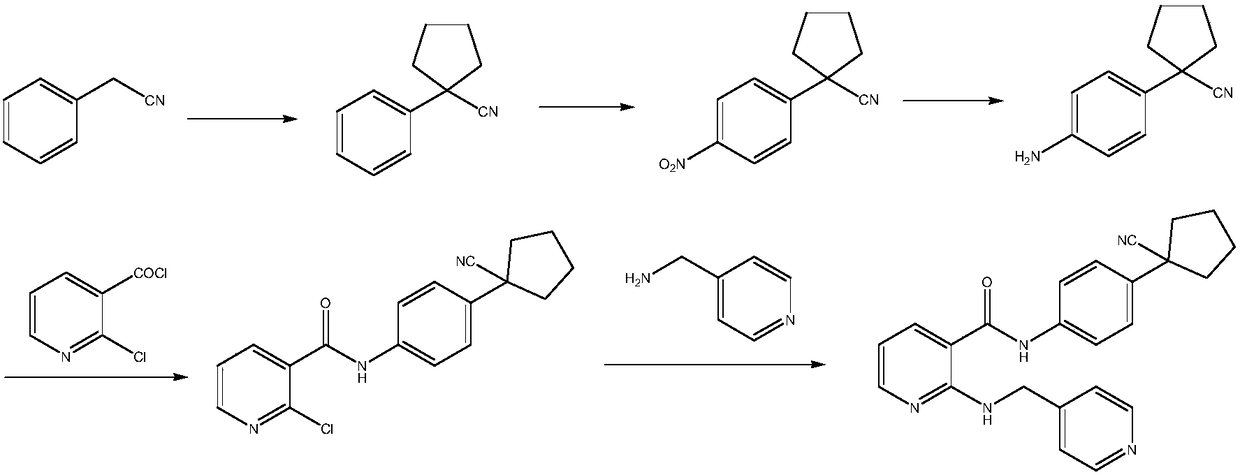
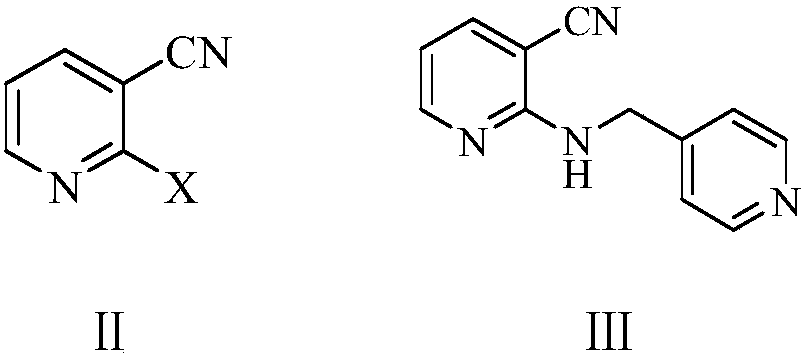
Preparation method of apatinib
A technology of apatinib and its compounds, which is applied in the field of preparation of apatinib, can solve problems such as unfavorable product purity assurance and impurity control, multiple side reactions in reaction conditions and product degradation, insufficient activation of chlorine atom substitution reactions, etc., to achieve Low cost, stable reaction operation, and less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1: Preparation of N-(pyridin-4-ylmethyl)-2-amino-3-cyanopyridine (Ⅲ)
[0058] To a 500 ml four-neck flask connected with a stirring, thermometer, and vacuum distillation device, add 180 g of N,N-dimethylformamide, 27.7 g (0.2 moles) of 2-chloro-3-cyanopyridine (II1) , 34.5 grams (0.25 moles) of potassium carbonate, 22.0 grams (0.2 moles) of 4-aminomethylpyridine, 95 to 100 ° C stirring reaction for 4 hours, cooled to 20-25 ° C, filtered, filter cake with 30 grams of N, N- Dimethylformamide was washed, the combined filtrates were distilled under reduced pressure to recover the solvent, and the residue was recrystallized with methyl tert-butyl ether to obtain 38.9 grams of N-(pyridin-4-ylmethyl)-2-amino-3-cyano Pyridine (Ⅲ), yield 92.6%, liquid phase purity 99.72%.
Embodiment 2
[0059] Example 2: Preparation of N-(pyridin-4-ylmethyl)-2-amino-3-cyanopyridine (Ⅲ)
[0060] To a 500 ml four-neck flask connected with a stirring, thermometer, and vacuum distillation device, add 200 g of N,N-dimethylformamide, 36.6 g (0.2 moles) of 2-bromo-3-cyanopyridine (II2) , 34.5 grams (0.25 moles) of potassium carbonate, 22.0 grams (0.2 moles) of 4-aminomethylpyridine, stirred and reacted at 80 to 85 ° C for 5 hours, cooled to 20-25 ° C, filtered, and the filter cake was washed with 30 grams of N, N- Wash with dimethylformamide, combine the filtrates, and distill under reduced pressure to recover the solvent, and the residue is recrystallized with methyl tert-butyl ether to obtain 39.3 grams of N-(pyridin-4-ylmethyl)-2-amino-3-cyano Pyridine (Ⅲ), yield 93.6%, liquid phase purity 99.65%.
Embodiment 3
[0061] Example 3: Preparation of N-(pyridin-4-ylmethyl)-2-amino-3-pyridinecarboxylic acid methyl ester (Ⅳ1)
[0062] In a 500 ml four-neck flask connected with stirring, a thermometer, and a reflux condenser, add 160 grams of methanol, 21.0 grams (0.1 mole) of N-(pyridin-4-ylmethyl)-2-amino- 3-cyanopyridine (Ⅲ), 22.0 grams of 35wt% concentrated hydrochloric acid, stirred and reacted at 60 to 65°C for 5 hours, cooled to 20-25°C, added 100 grams of water, 150 grams of toluene, and adjusted the pH of the system with 5wt% sodium carbonate aqueous solution The value is 7.0-8.0, separate layers, the water layer is extracted twice with toluene, 50 grams of toluene each time, the toluene organic layer is combined, washed once with 30 grams of saturated sodium chloride aqueous solution, methanol and toluene are recovered by atmospheric distillation, and the residue is washed with toluene tert-butyl ether was recrystallized to obtain 21.6 g of methyl N-(pyridin-4-ylmethyl)-2-amino-3-pyr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com