Novel application of Apatinib for preparing medicine for treating acute myelogenous leukemia
An apatinib, acute myeloid technology, applied in the field of drug application, can solve problems such as no relevant reports, and achieve the effects of good safety, good biocompatibility, and no toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Evaluation of the Inhibitory Effect of Apatinib on the Proliferation of AML Cell Lines
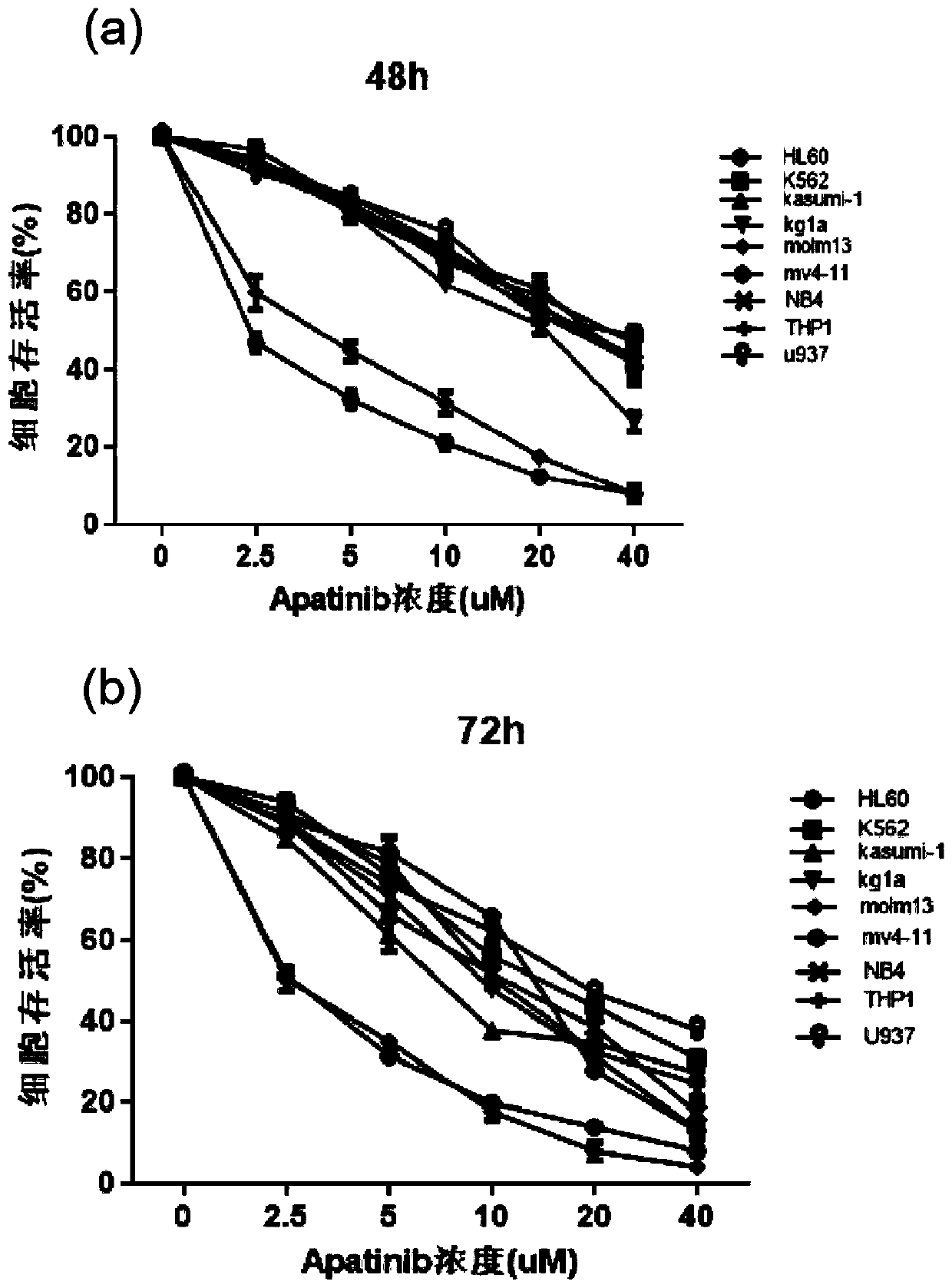
[0050] The operation method is: take the quantity as 2×10 4 AML cell lines in the logarithmic growth phase (including HL60, K562, Kasumi-1, Kg1a, Molm13, MV411, NB4, Thp1 and U937 cell lines) were inoculated in 96-well plates, and the control group and different concentrations of Apatinib groups (0, 2.5, 5, 10, 20, 40 μM), after each group was treated for 48h and 72h, respectively, the proliferation of AML cells in different groups was detected with the CCK8 kit (the specific operation steps were carried out according to the kit instructions).
[0051] The result is as figure 1 As shown, wherein (a) is the result of Apatinib acting for 48h, and (b) is the result of Apatinib acting for 72h. Depend on figure 1 It can be seen that: Apatinib can significantly inhibit the proliferation of various AML cell lines, especially Molm13 cells and MV411 cells; moreover, the inhibitory effect ...
Embodiment 2
[0056] Evaluation of Apatinib Inducing Apoptosis in AML Cell Lines
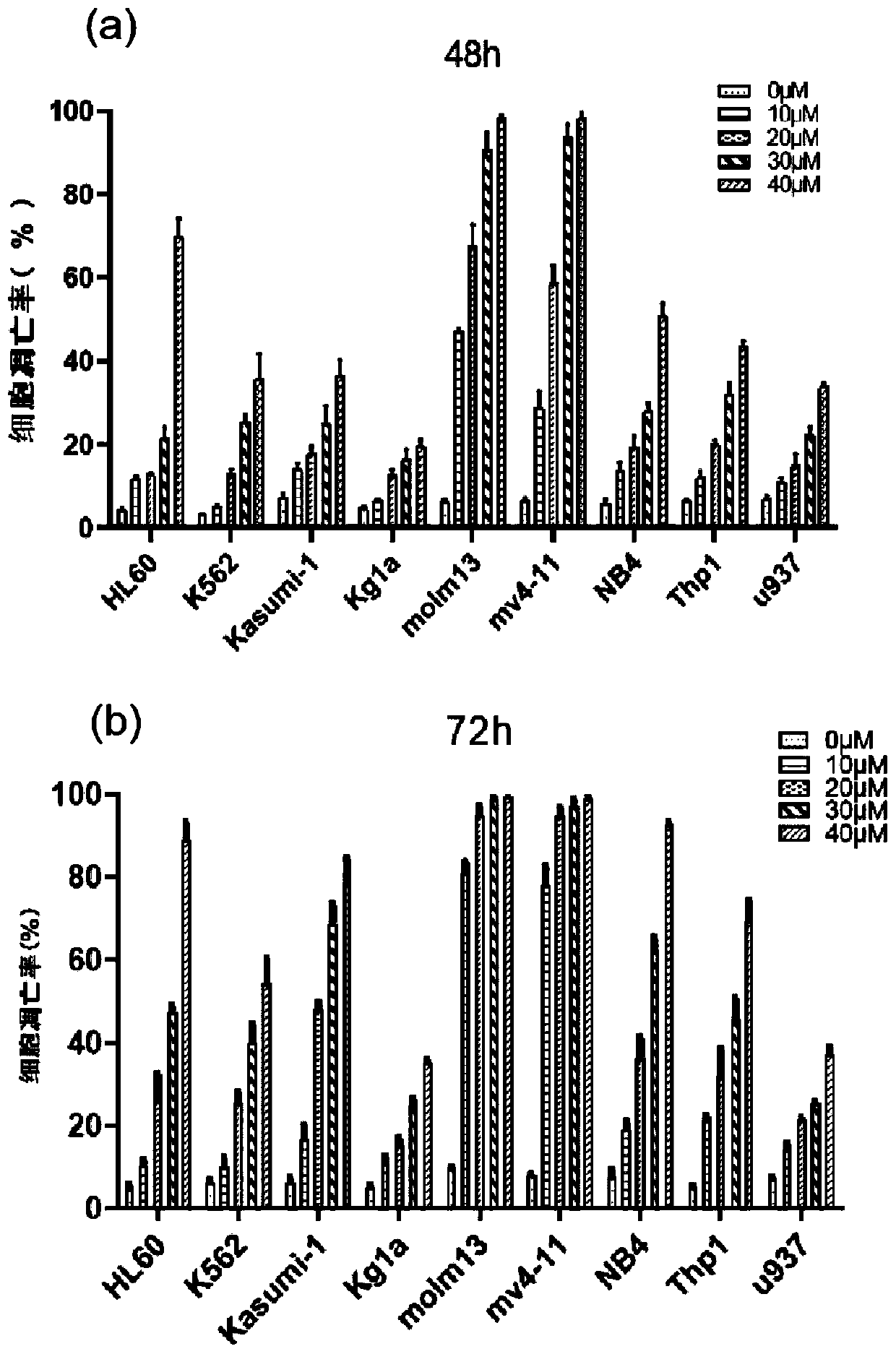
[0057] The operation method is: take the quantity as 2×10 5 AML cell lines in the logarithmic growth phase (including HL60, K562, Kasumi-1, Kg1a, Molm13, MV411, NB4, Thp1 and U937 cell lines) were inoculated in 24-well plates, and the control group and different concentrations of Apatinib groups (0, 10, 20, 30, 40 μM), each group was treated for 48h and 72h respectively, and Annexin V / PI kit was used to detect the apoptosis of AML cells in different groups (the specific operation steps were carried out according to the kit instructions).
[0058] The result is as figure 2 Shown, wherein (a) is the result of Apatinib acting for 48h, (b) is the result of Apatinib acting for 72h. Depend on figure 2 It can be seen that: Apatinib can significantly induce apoptosis of various AML cell lines, especially for Molm13 cells and MV411 cells; moreover, Apatinib has a significant concentration-dependent effect on indu...
Embodiment 3
[0060] Evaluation of the effect of apatinib on AML cells and normal bone marrow mononuclear cells from primary cell level
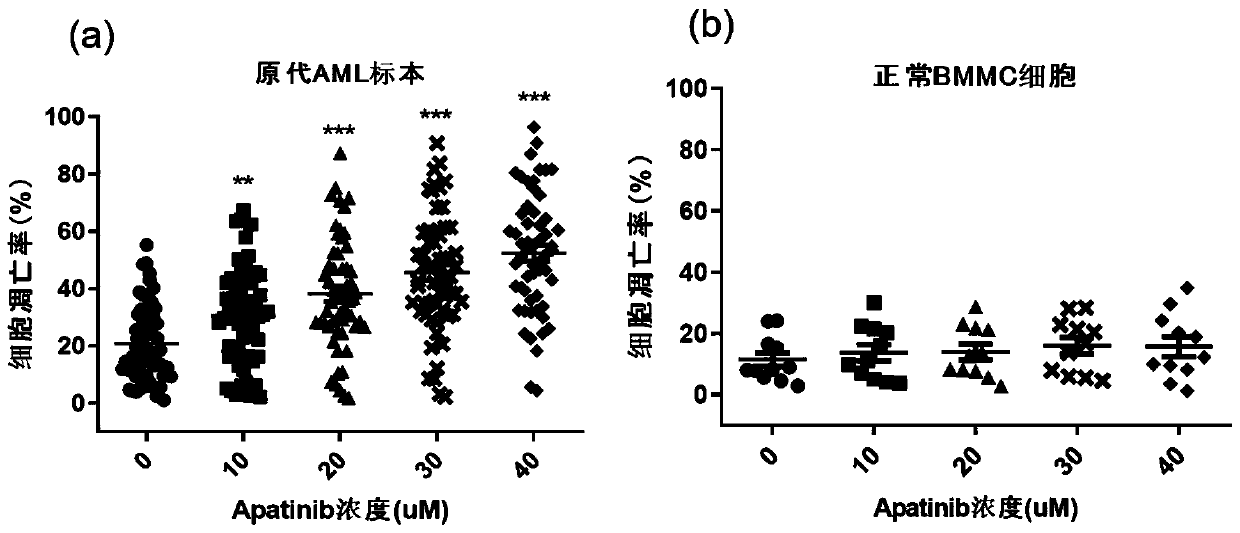
[0061] The operation method is as follows: collect 57 cases of bone marrow samples from patients with newly diagnosed or refractory recurrent AML and 11 cases of bone marrow samples (BMMCs) from hematopoietic stem cell transplantation donors, and extract mononuclear cells with lymphocyte separation medium. After treating primary AML cells and normal BMMCs cells with different concentrations of Apatinib (0, 10, 20, 30, 40 μM) for 48 hours, the apoptosis ratio of primary AML cells and normal BMMCs was detected by Annexin V / PI double staining method.
[0062] The result is as image 3 Shown, wherein (a) is the apoptosis result of primary AML cells, (b) is the apoptosis result of normal BMMCs cells. Depend on image 3 It can be seen that the addition of Apatinib can significantly increase the apoptosis rate of primary AML cells, and the apoptosis rate gradu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com