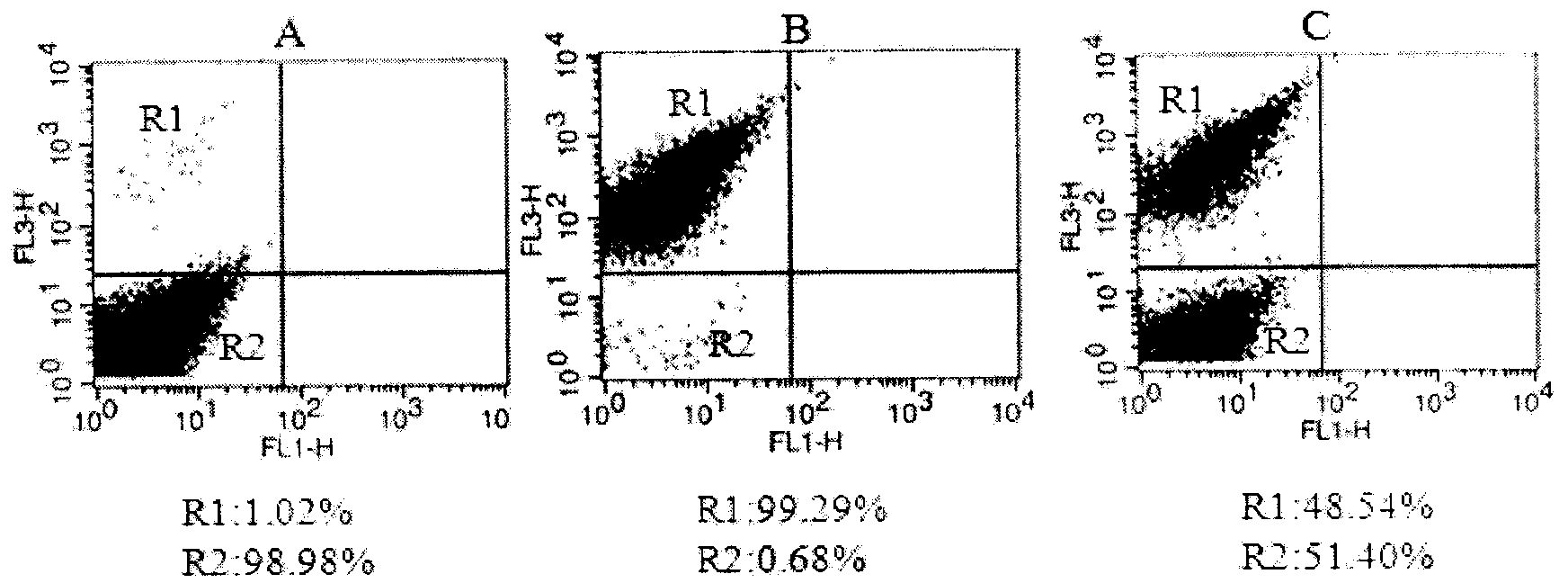
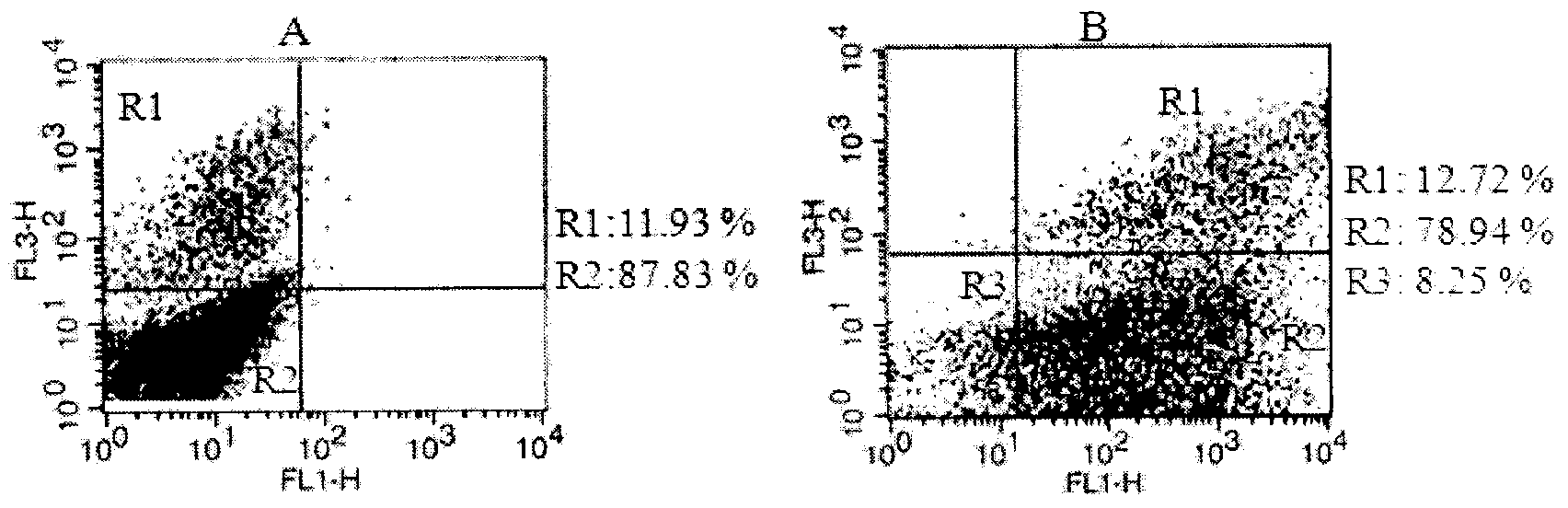
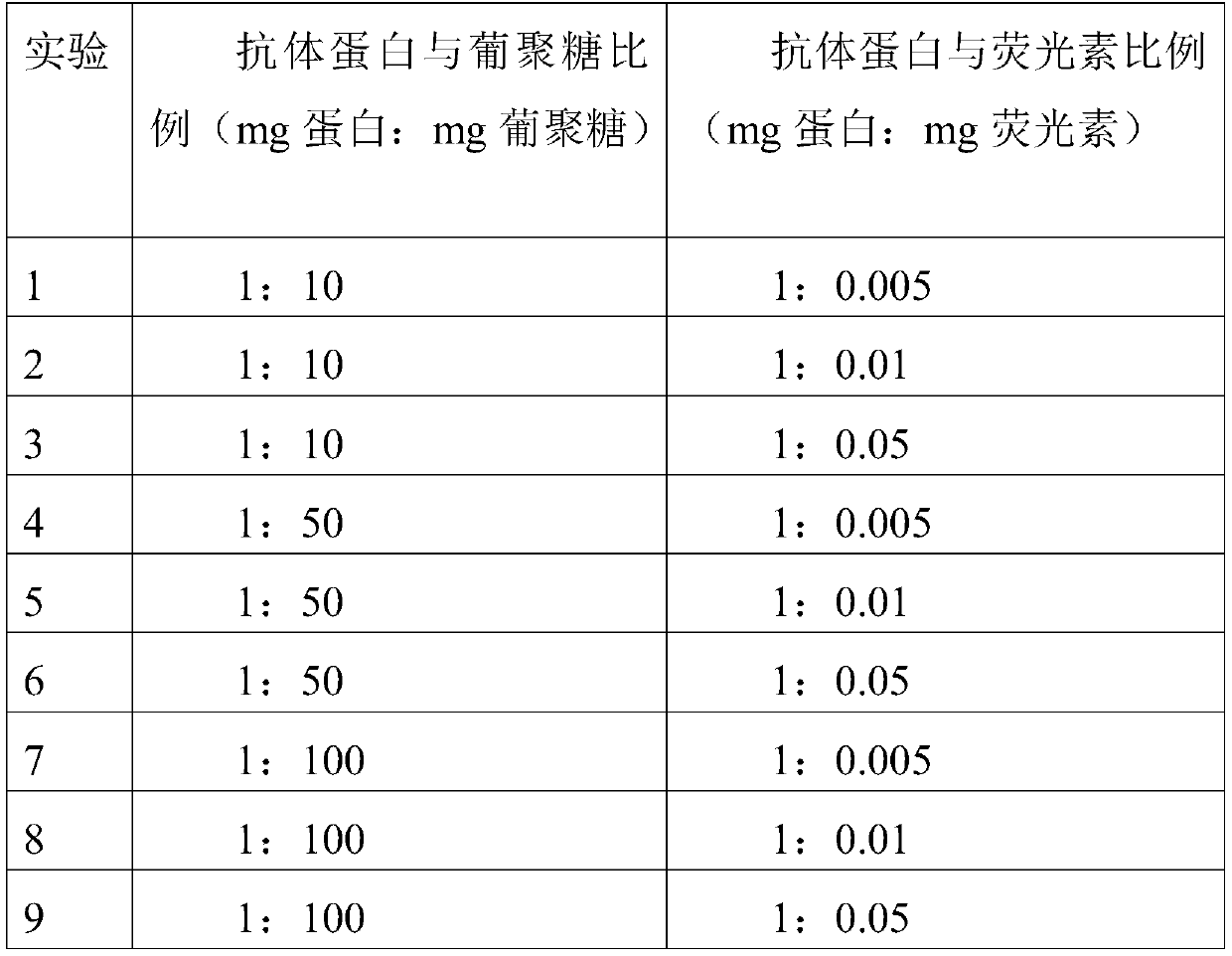
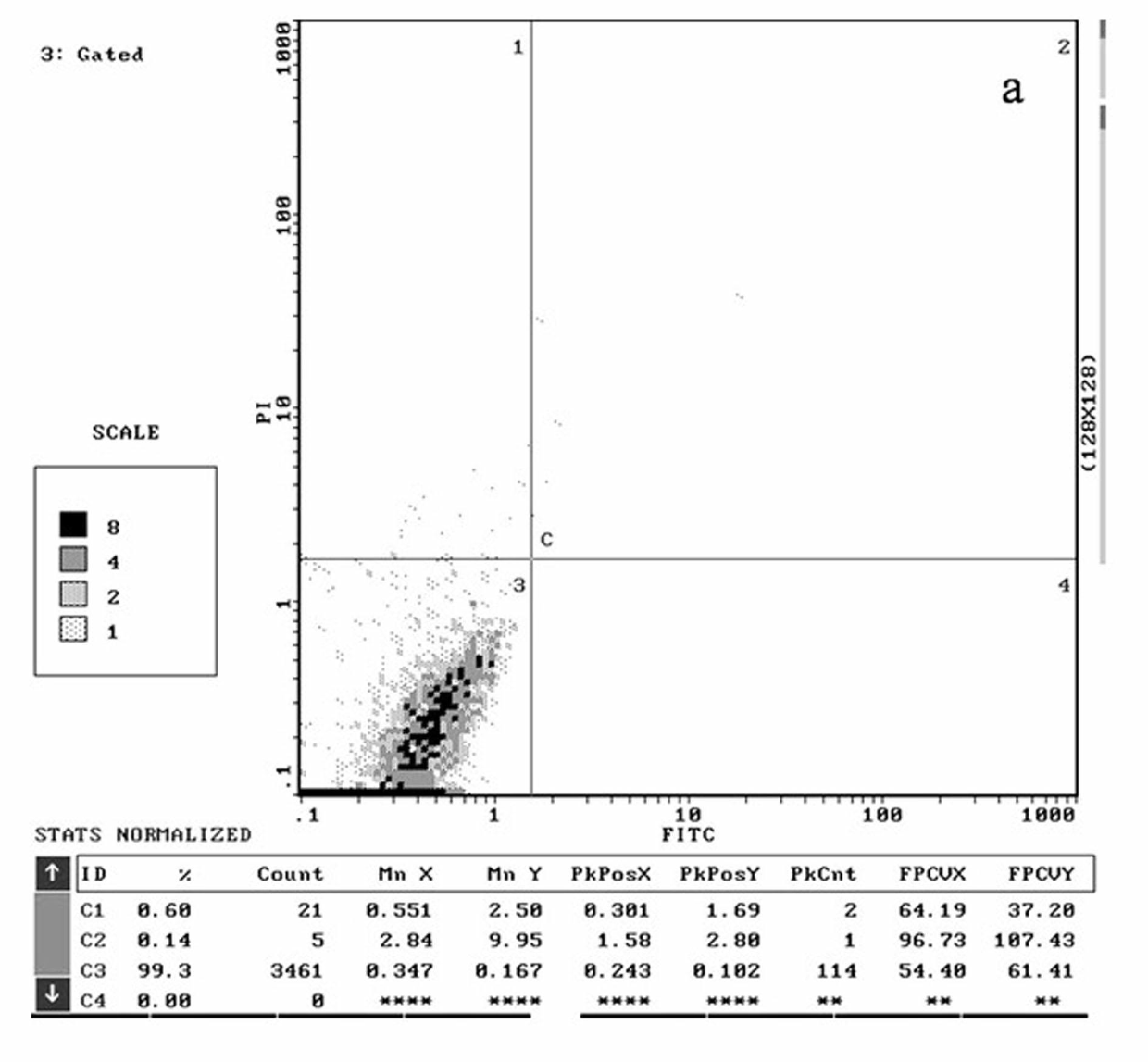
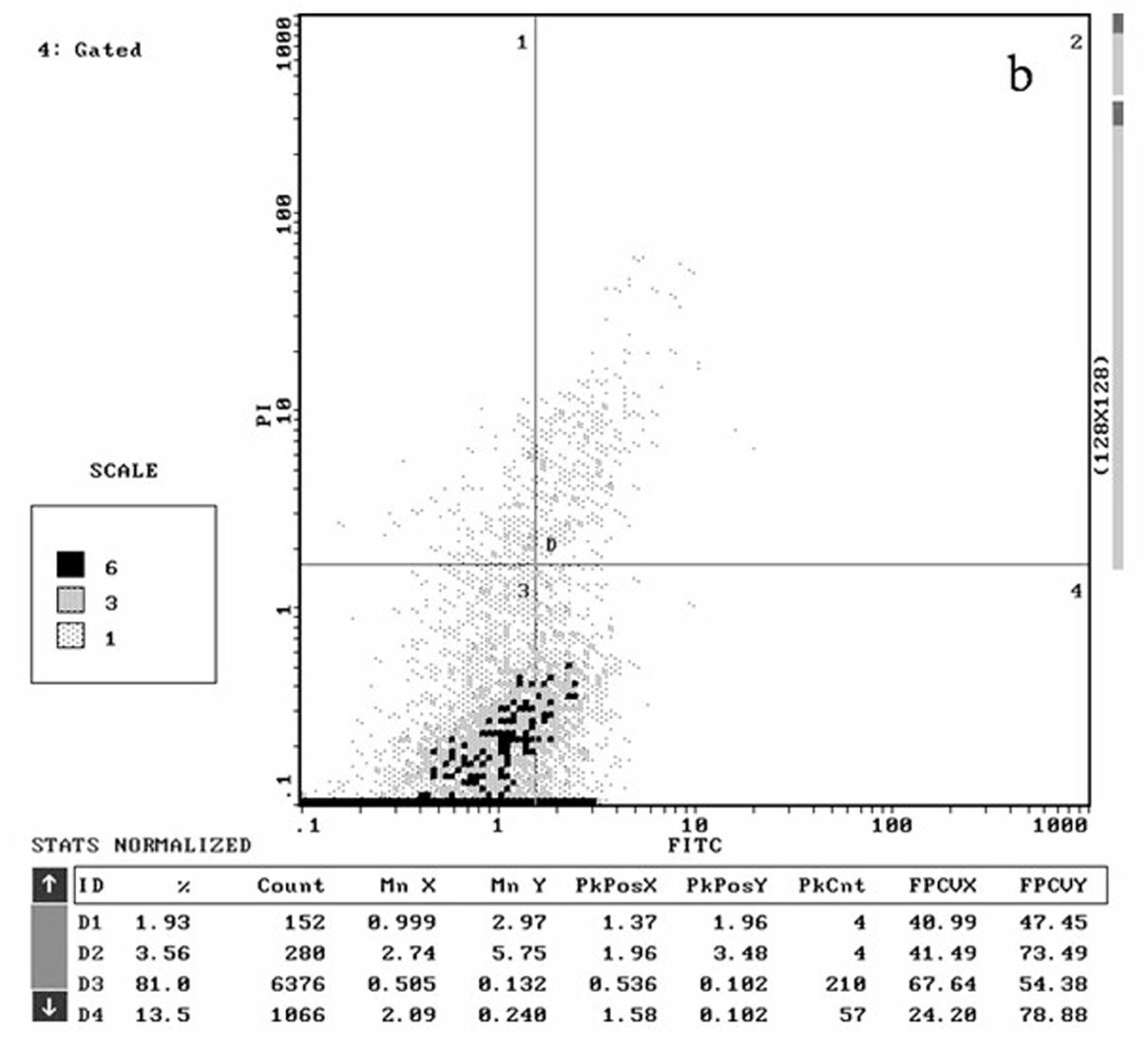
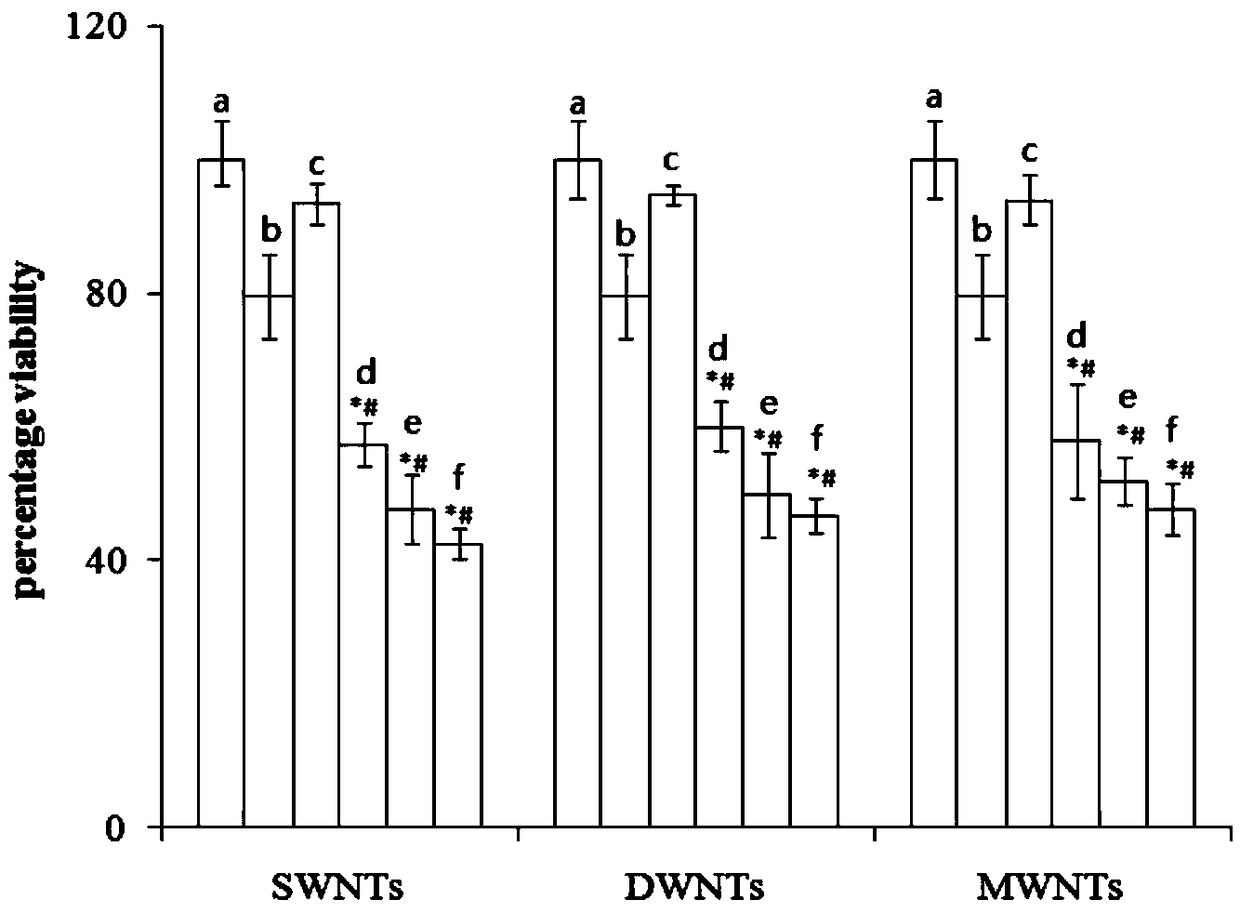
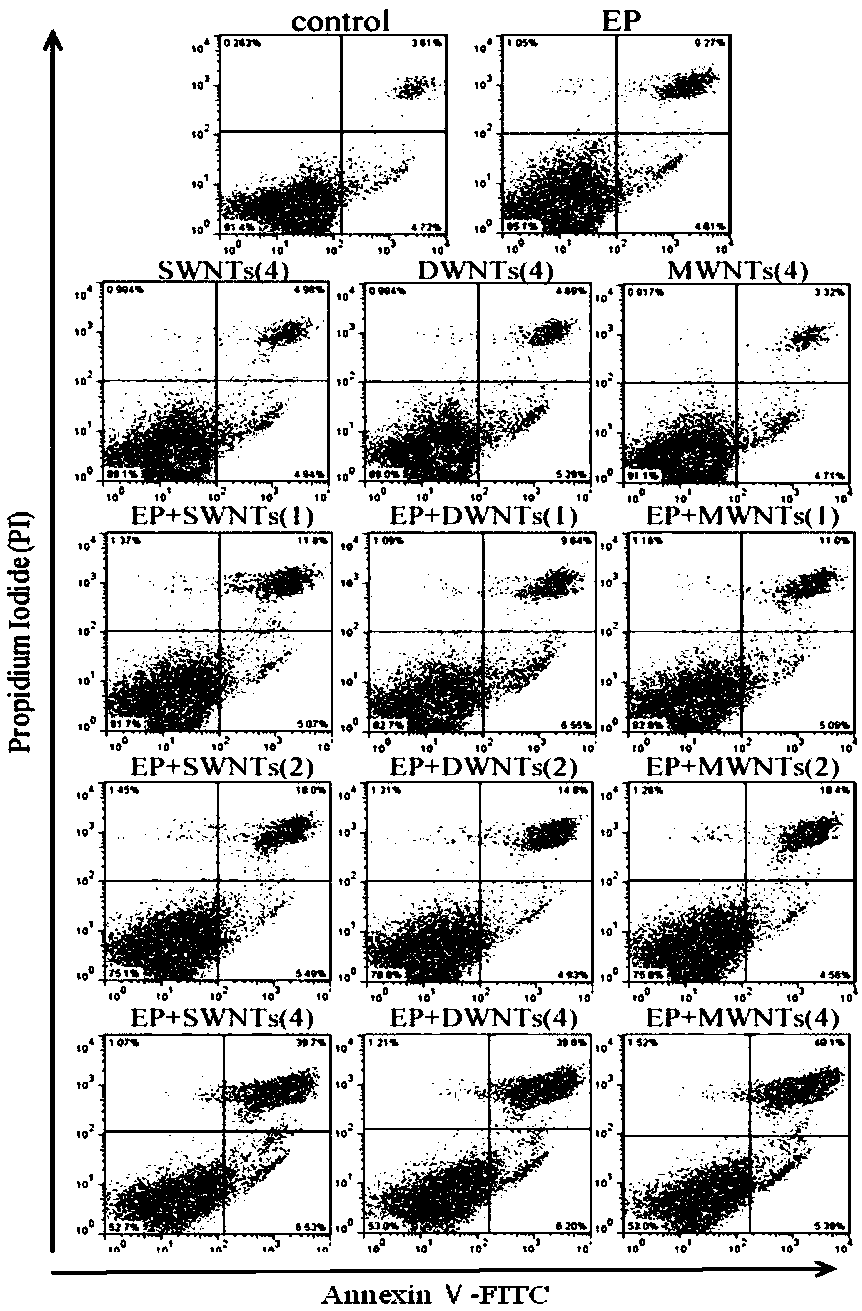
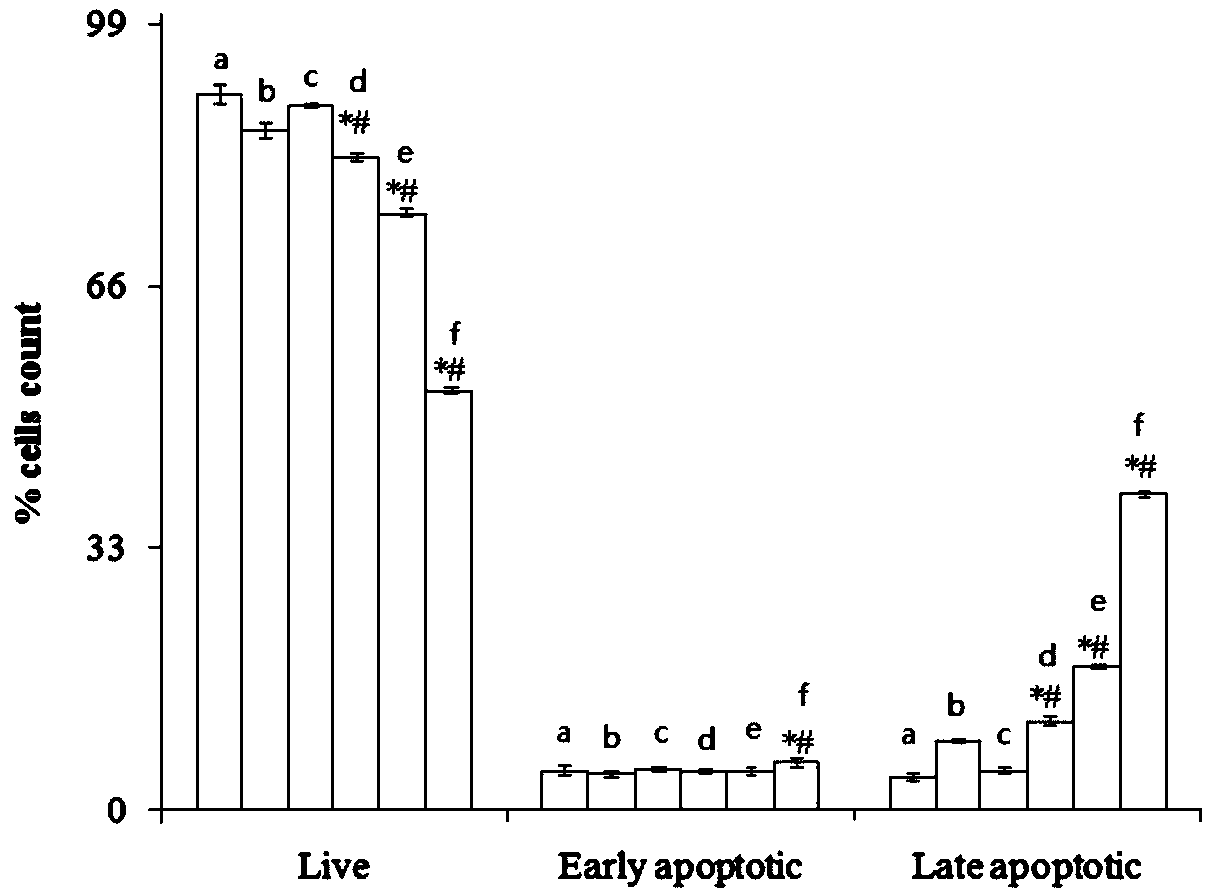
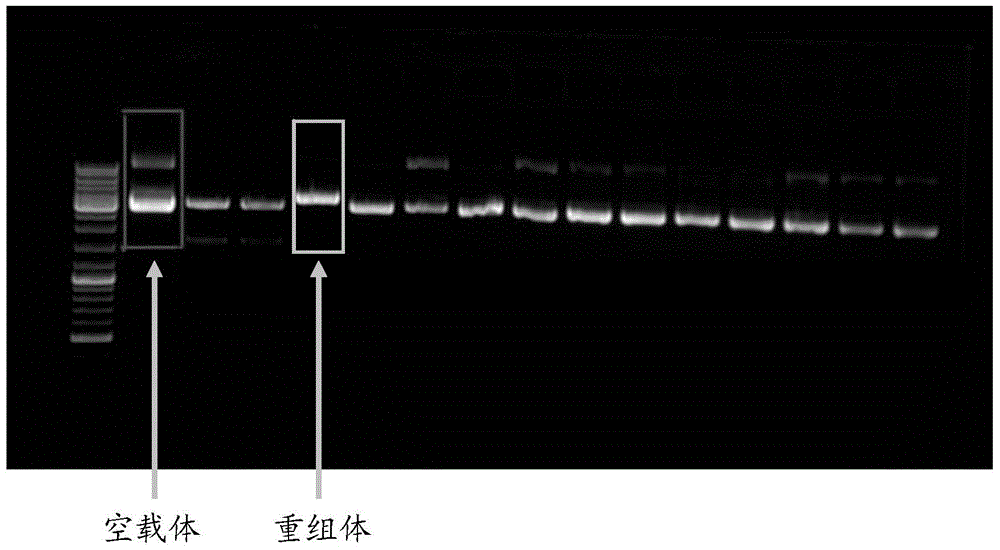
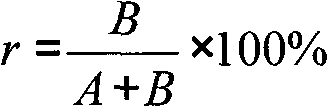Patents
Literature
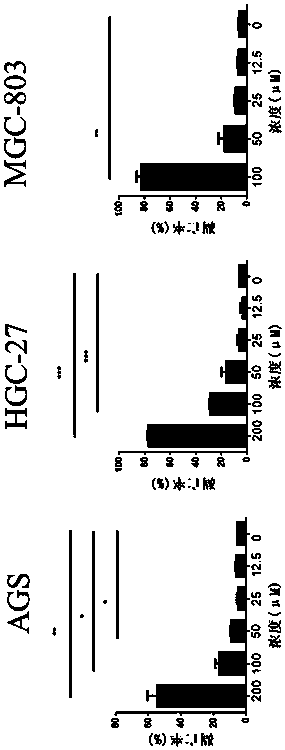
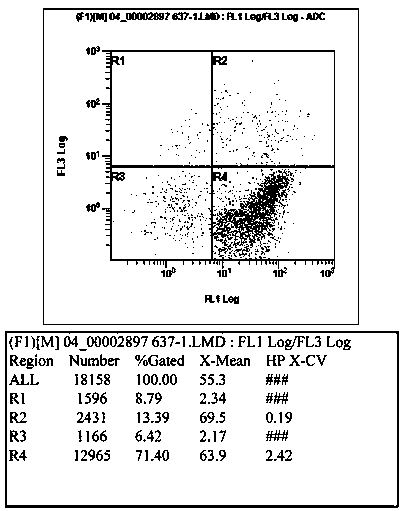
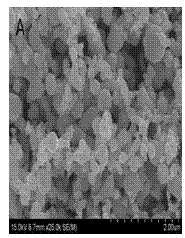
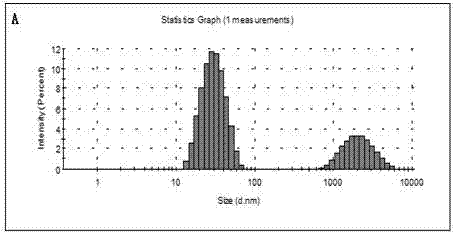
51 results about "Double staining" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
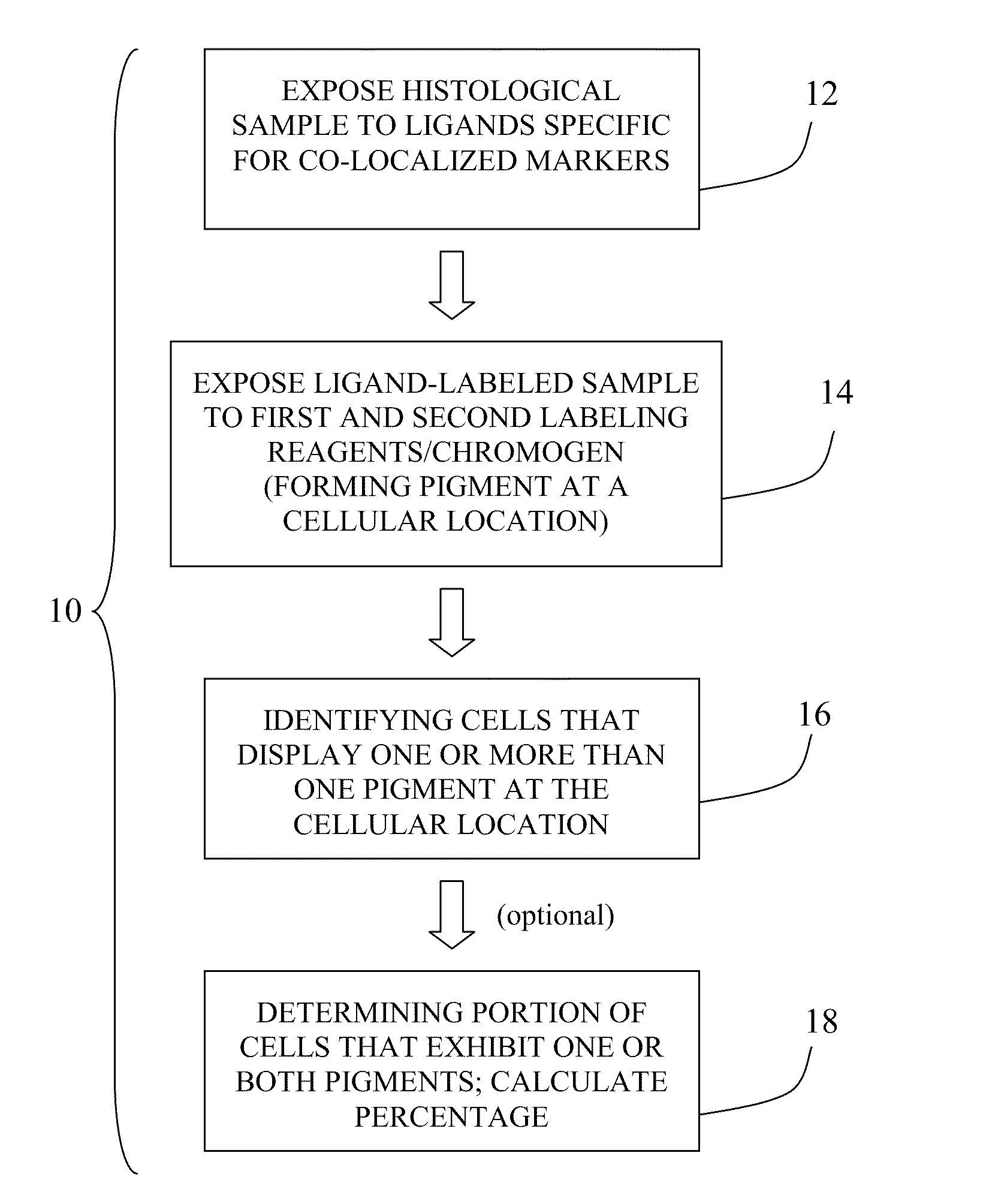
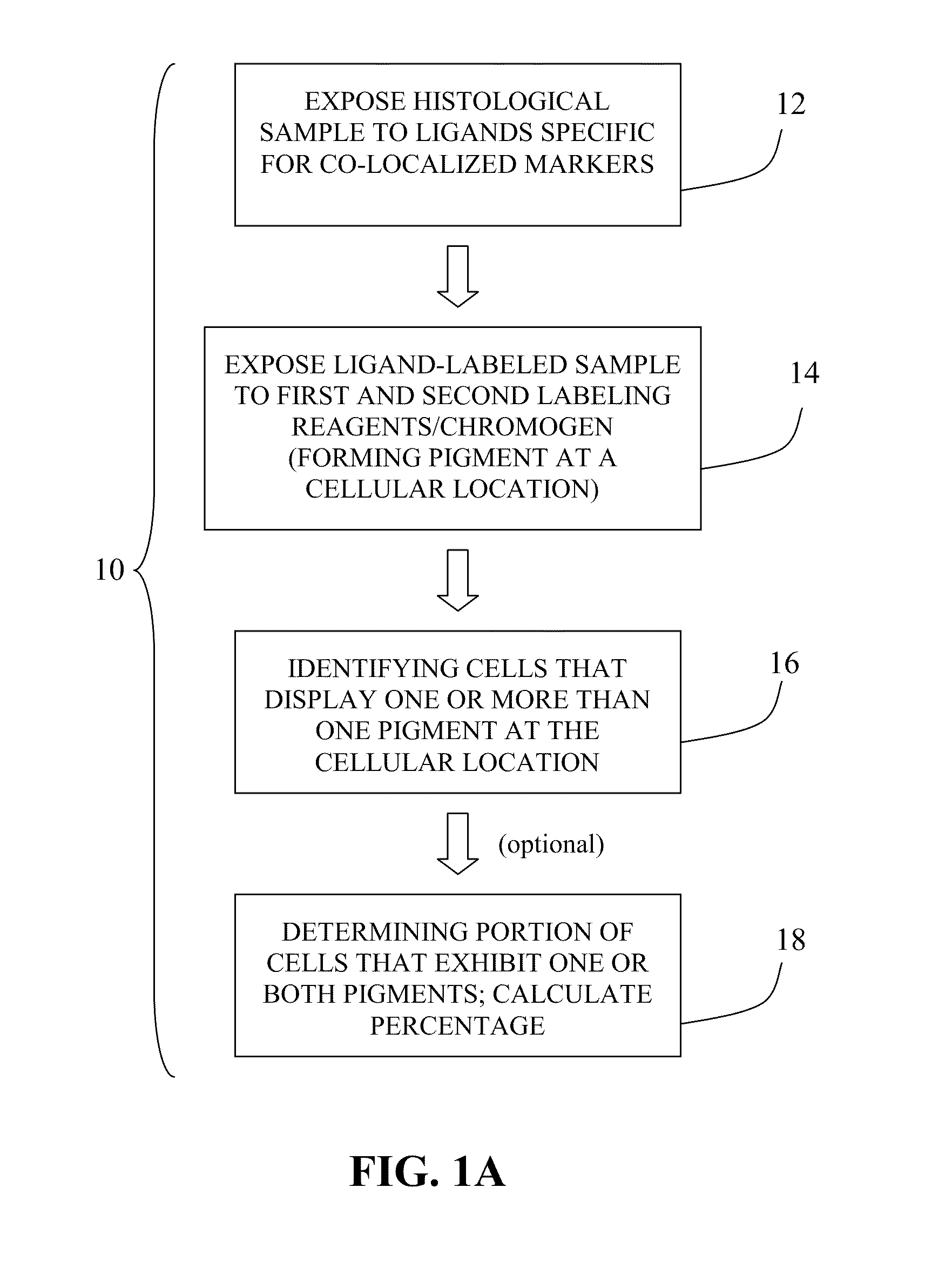
Patent Status
Application Year
Inventor
Dou·ble stain. A mixture of two dyes, each of which stains different portions of a tissue or cell. double stain. A mixture of two contrasting dyes, usually an acid and a basic stain.
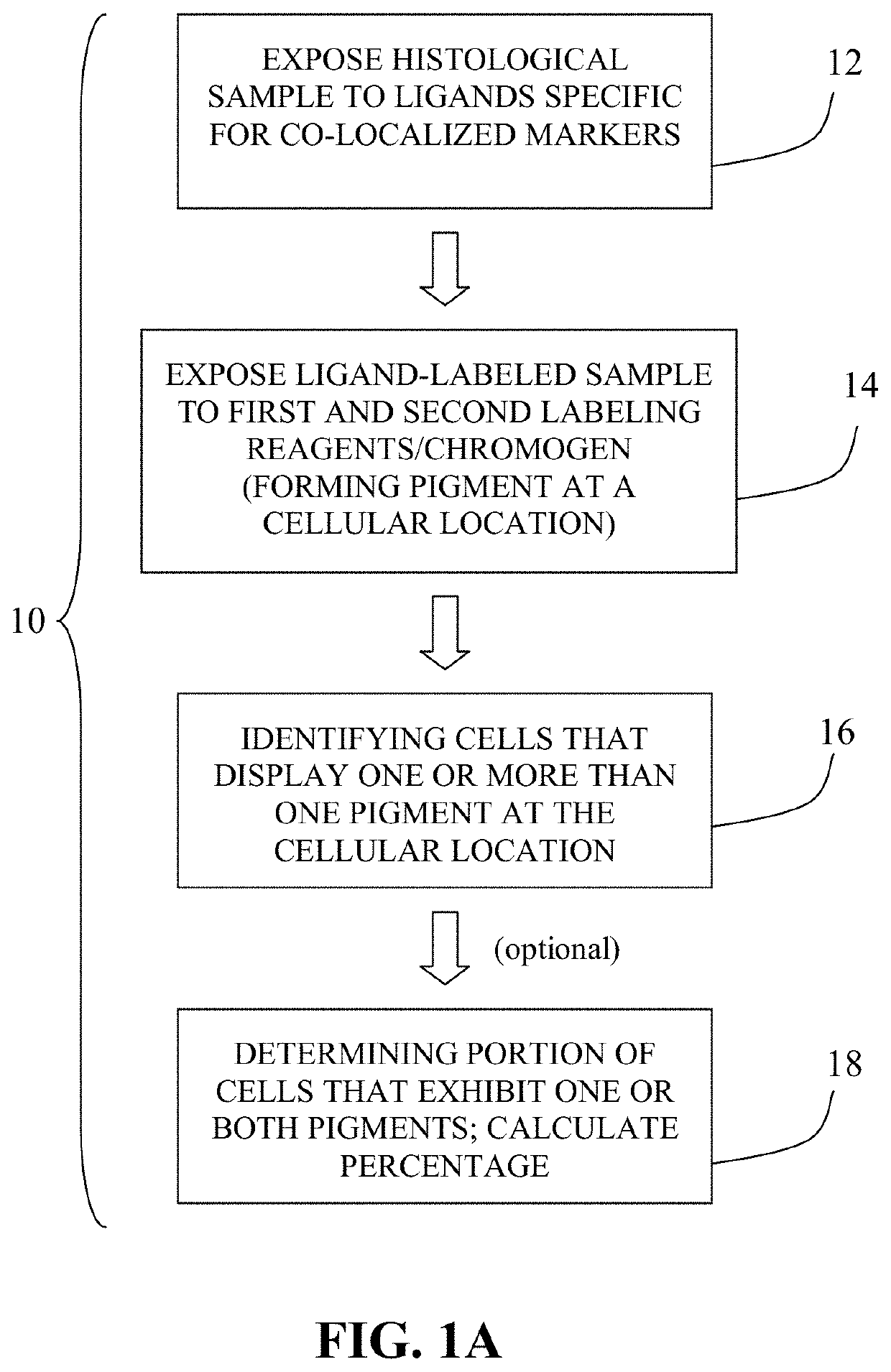
Methods of detecting cancer cells in biological samples
InactiveUS20040197839A1Preparing sample for investigationBiological testingBladder cancerBacteriuria
The invention provides methods of detecting cancerous cells in biological samples using a double staining / dual imaging approach, which can be used to diagnose cancer. More specifically, the present invention provides methods of diagnosing bladder cancer by a simultaneous scanning of cell morphology and FISH signals of cells derived from a urine sample.
Owner:BIOVIEW
Information processing apparatus, information processing method, and program
ActiveUS20130323825A1Improve accuracyEffectively suppresses the components of other light emission elements leakingBioreactor/fermenter combinationsBiological substance pretreatmentsInformation processingFluorescence
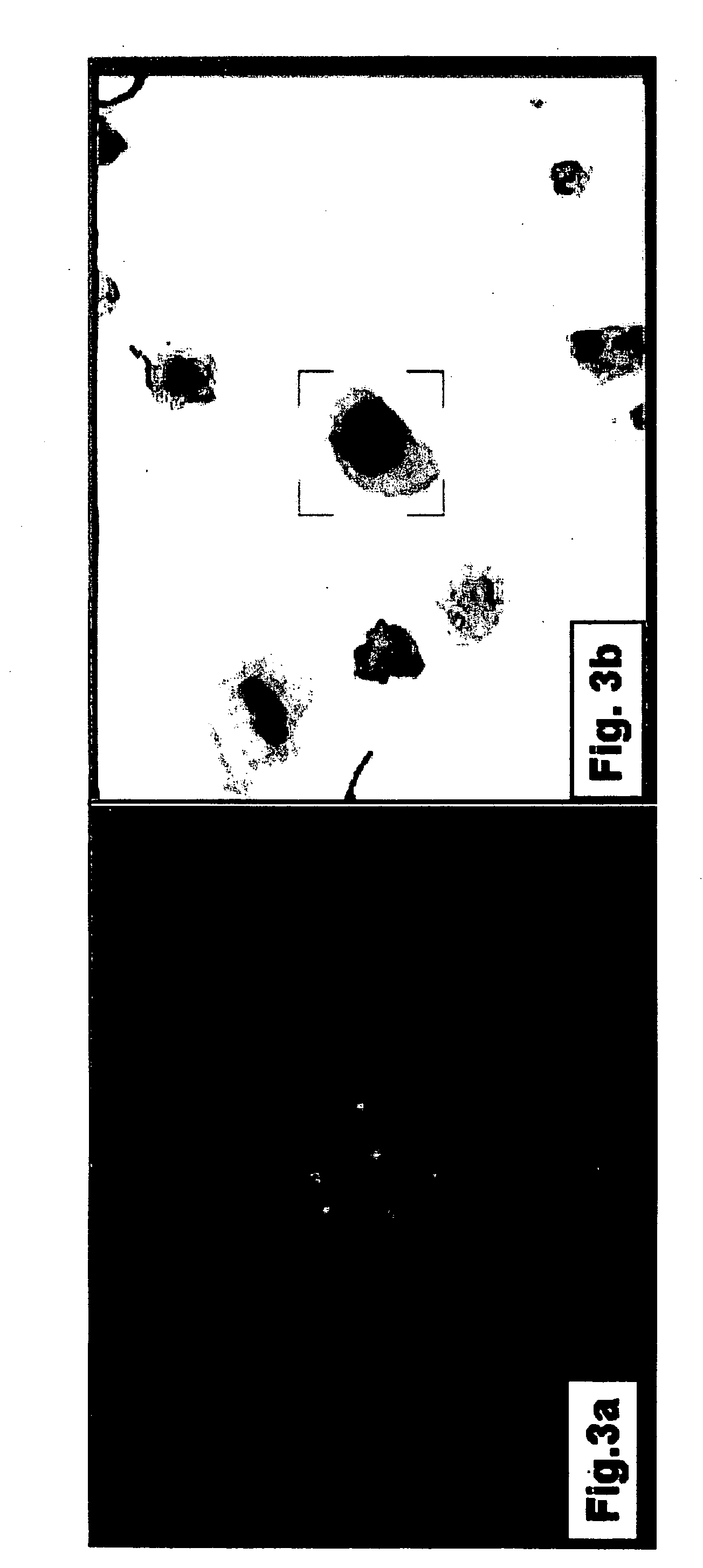
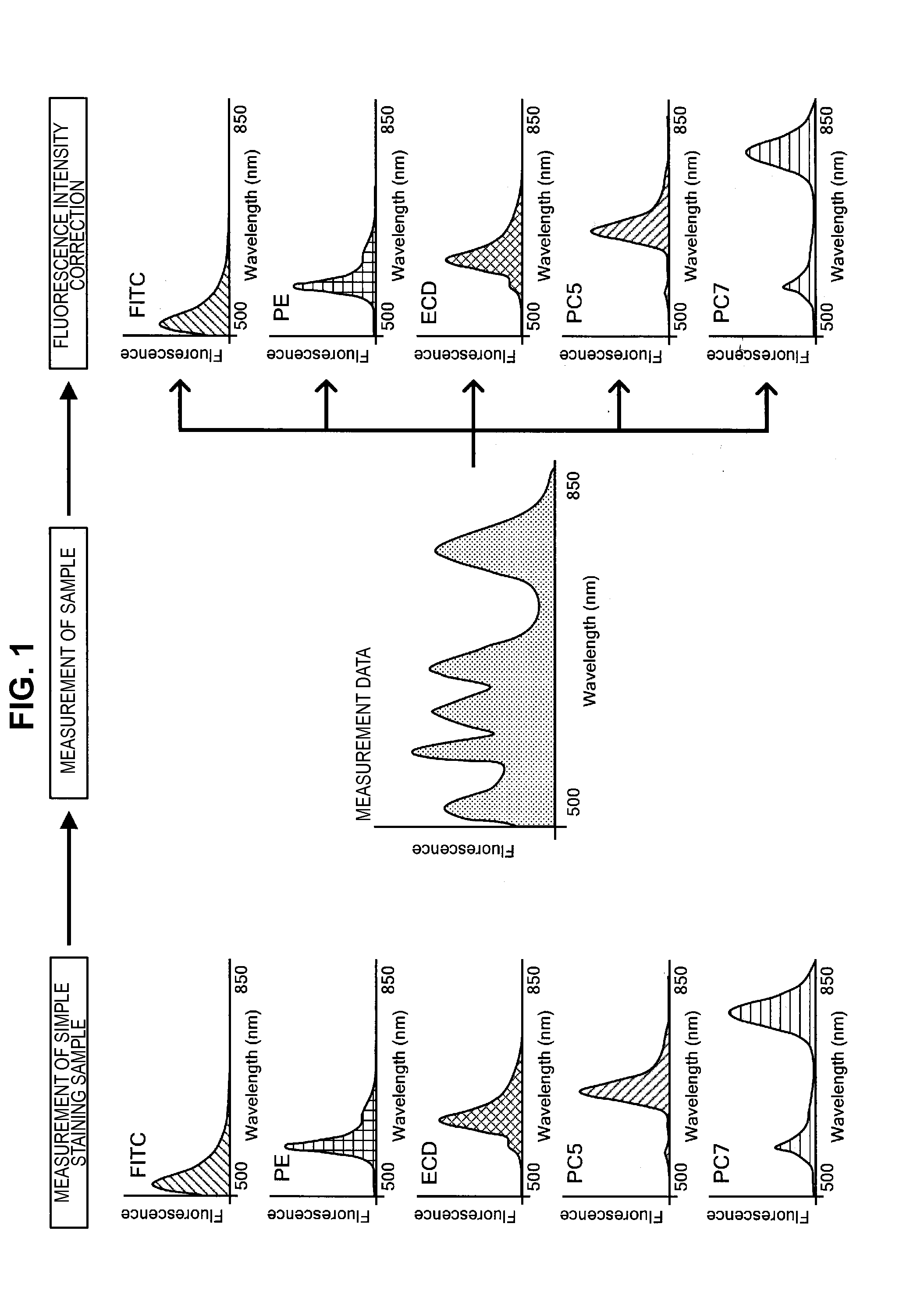
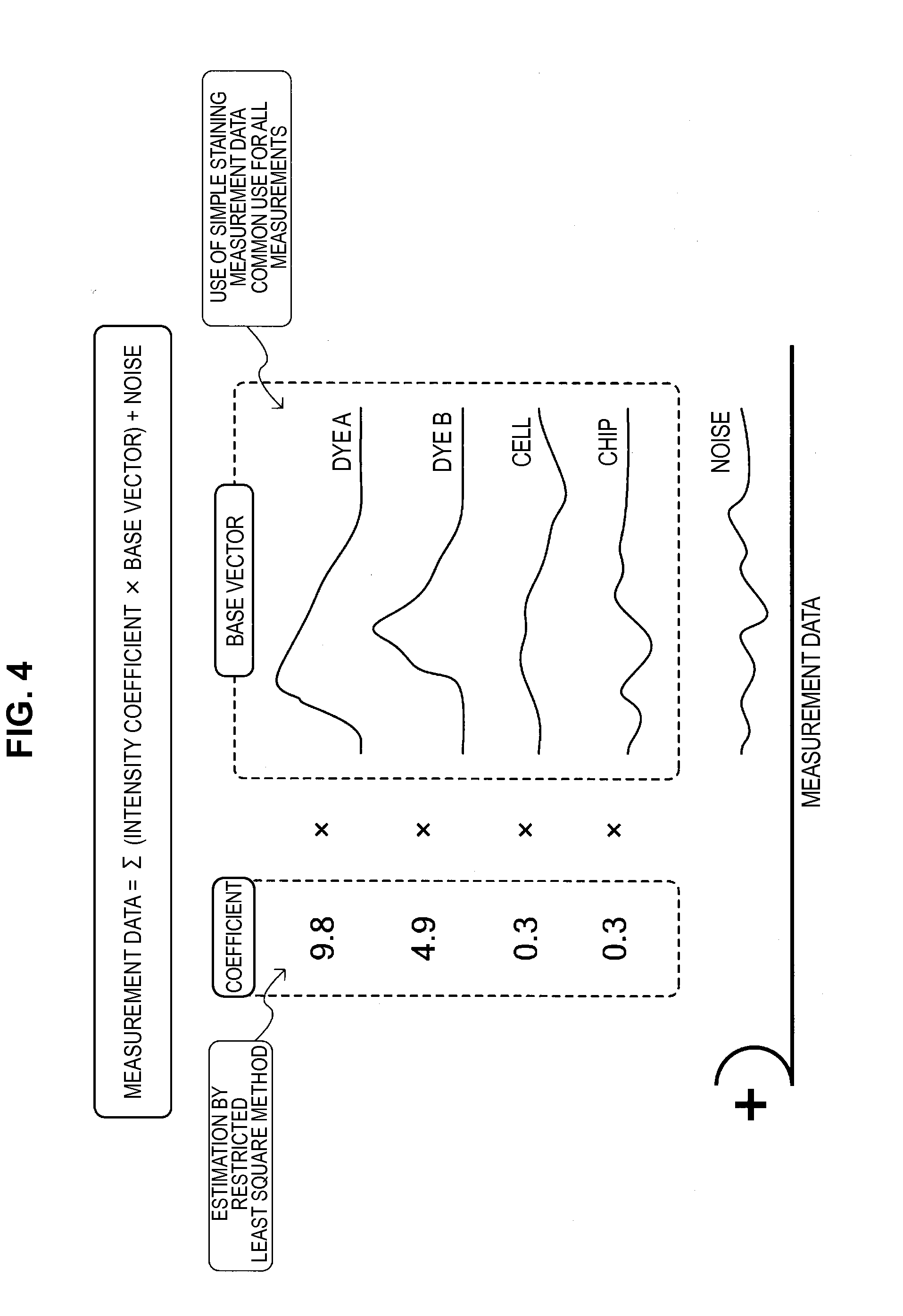
Provided is an information processing apparatus, including a testing section performing statistical testing on simple staining data obtained by performing fluorescence measurement on a particle subjected to simple staining with a staining material having a prescribed fluorescence characteristic and unstaining data obtained by performing fluorescence measurement on an unstained particle for comparison for a frequency band, a masking processing section setting, in a case where there is no significant difference between the simple staining data and the unstaining data for the frequency band, the simple staining data to 0 or a prescribed value, and an estimation section estimating, in a manner that double staining data obtained by performing fluorescence measurement on a particle stained with a plurality of staining materials is represented by a linear combination of base vectors representing a distribution of the simple staining data corresponding to each staining material, a combination coefficient of the linear combination.
Owner:SONY CORP
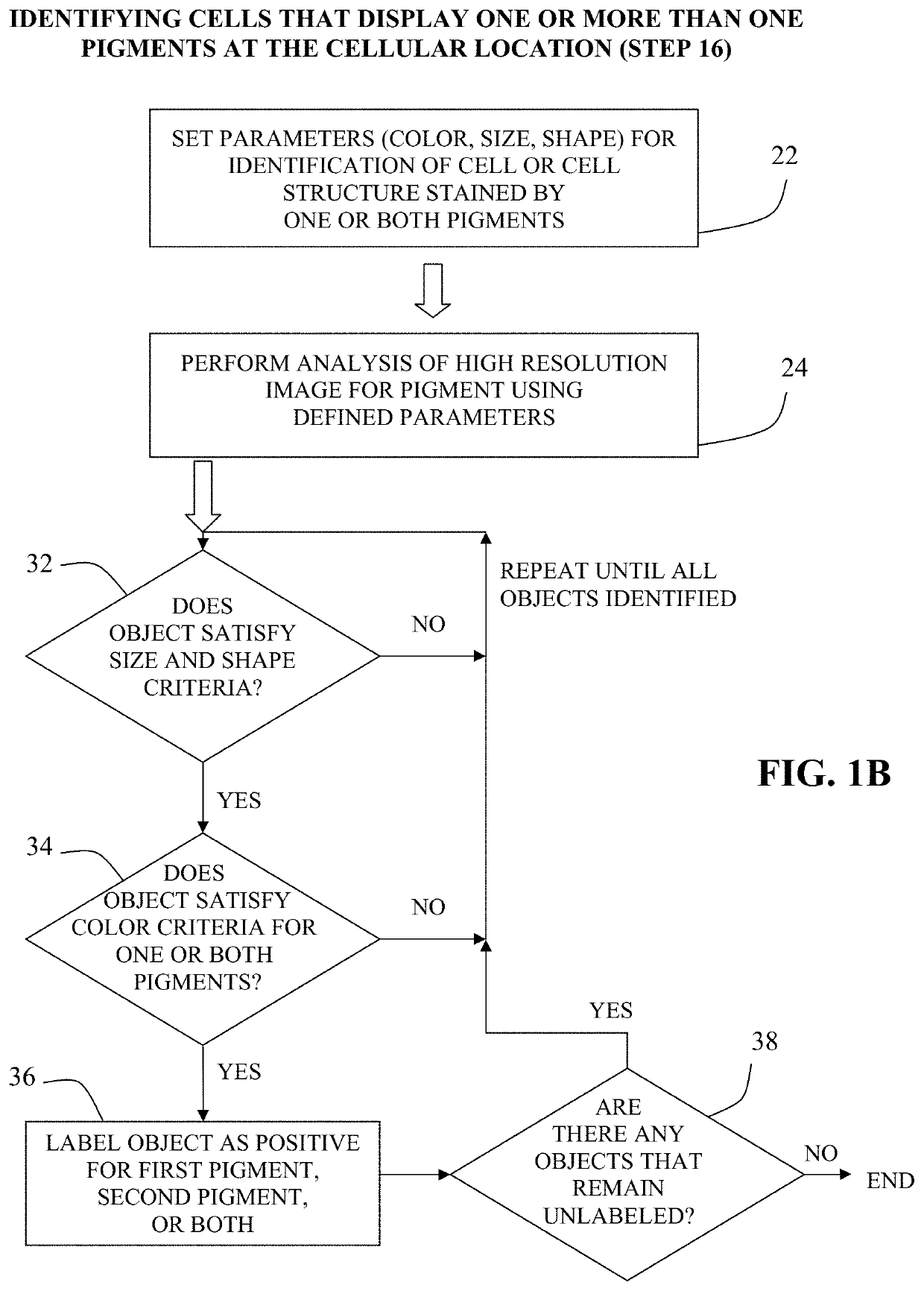
Method for double staining in immunohistochemistry
ActiveUS20100151447A1Measurably differentMicrobiological testing/measurementBiological testingTissue sampleCo localization
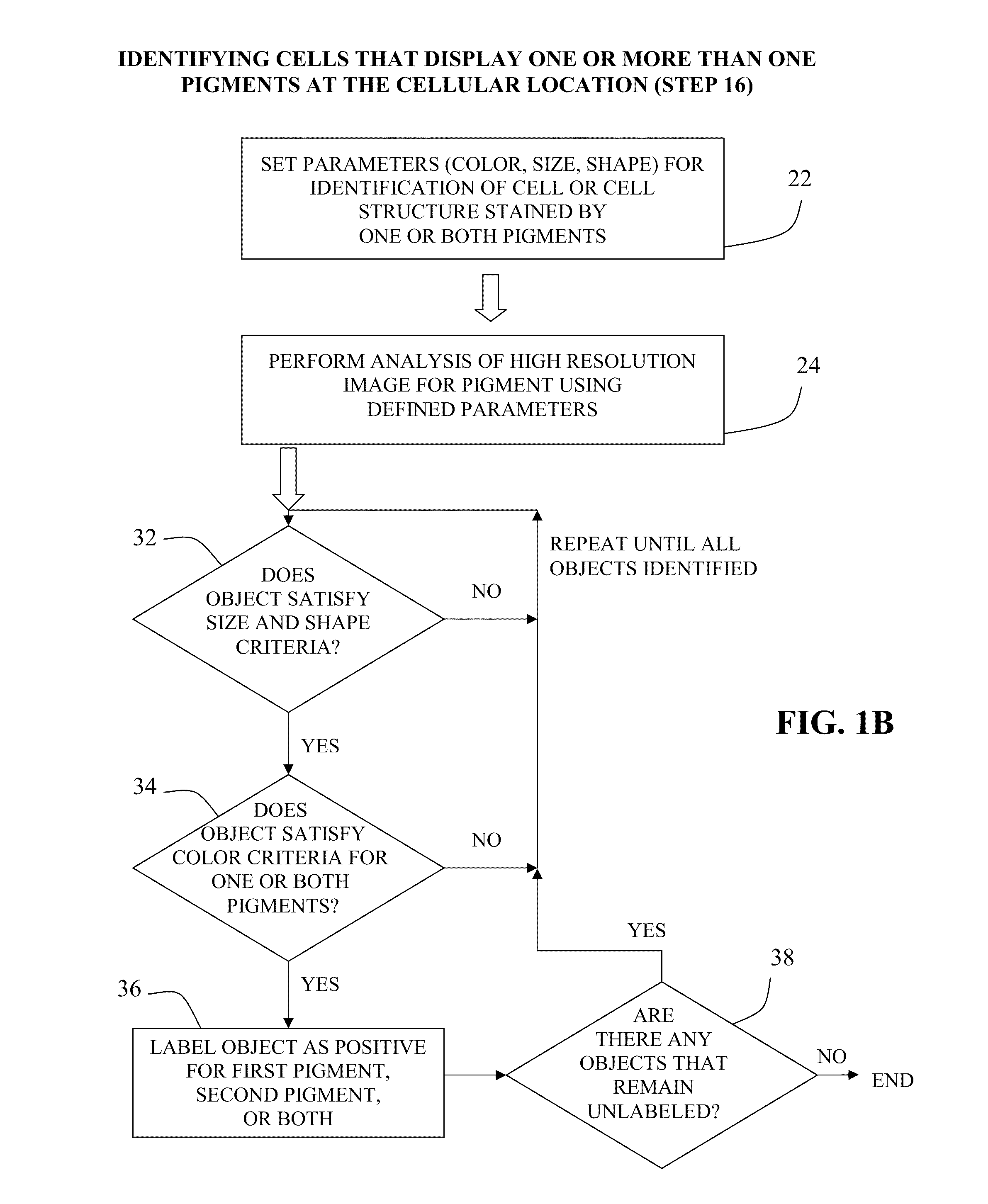
The present invention relates to kits and methods for performing dual-staining immunohistochemistry (IHC) for the detection of specific cell populations in tissue samples containing heterogeneous populations of cells, which can be observed by a light microscope for co-localization of distinct pigments. The method includes providing a tissue sample comprising fixed cells; exposing the sample to first and second ligands that recognize different marker proteins found at the same cellular location, thereby forming a ligand-labeled sample; exposing the ligand-labeled sample to first and second labeling reagents, the first labeling reagent binding to the first ligand and the second labeling reagent binding to the second ligand, the first and second labeling reagents each forming distinct pigments; and identifying the number of cells that display only one particular pigment, or more than one pigment, by the different coloration of the cellular location labeled by the distinct pigment.
Owner:CORNELL UNIVERSITY
Cervical acid phosphatase - papanicolaou (CAP-PAP) test kit, method and accesories, processes for producing and using the same
InactiveUS20040137551A1Improves cell defense mechanismMicrobiological testing/measurementMaterial analysisGynecologyCervical cancer screening
Cervical Acid Phosphatase-Papanicolaou Test Kit (CPK) is an assembly of reagents, controls and instructions for visualization of cervical acid phosphatase on smears or monolayers of cervical specimens, and for performing the CAP-PAP Test (CPT). CPT is a single-slide, double-staining method for demonstration of cervical acid phosphatase activity inside abnormal cervical cells on Papanicolaou stained smears, and a set of criteria for using this test for cervical cancer screening. In previous clinical trials this method was found to enable Pap test screeners to improve test sensitivity (detection of abnormal cells) for more than 10% (from 0.8 to 0.9), and to reduce false negative readings (missing abnormal cells) for more than 50% (from 0.1 to 0.02). Due to better accuracy and the low cost, when approved, CPT may begin to replace current technologies for cervical cancer screening. CPK is designed to meet requirements for testing large series of specimens on regular basis-the usual practice in cytopathology laboratories performing the Pap test. CPK brings consistency for staining and interpretation, makes internal and external controls easier, and improves the test accuracy for lower cost, while increases laboratory productivity for less liability.
Owner:MARKOVIC NENAD S +1
Manufacturing method of daphnia microslide specimen
The invention provides a manufacturing method of a daphnia microslide specimen, which comprises the following steps: firstly fixing the daphnia with a fixative, simple-staining or double-staining, color separating, and stepwise dehydrating with ethanol; then stepwise displacing the ethanol in the dehydrated daphnia with acetone; and finally sucking out the daphnia which is subject to the displacement with acetone, dripping on a glass slide, and dripping prepolymerized polymethacrylate to seal the glass slide to obtain a daphnia microslide specimen. The daphnia microslide specimen manufacturedby the method enables the cell borderlines of the daphnia to be obvious, the intestinal tract, ovary and other structures to be observed more clearly, the transparency is increased, and the drying time is shortened.
Owner:SHANXI UNIV
Method for producing double-staining plastic-sealed specimen of fish bones
InactiveCN107279130AIntegrity guaranteedIncrease interest in learningDead animal preservationEducational modelsEpoxyAlcohol
The invention discloses a method for producing a double-staining plastic-sealed specimen of fish bones. The method comprises the following conditions and steps: cleaning internal organs, scales and skin of fish bodies of fishes with hard and soft bones, fixing by virtue of 10% formalin, soaking with distilled water for 1-2 days so as to completely remove formalin, staining skeletons and soft bones blue by virtue of a dye, namely alcian blue, processing by virtue of alcohols of different concentrations, processing muscles of the fish bodies by virtue of trypsase until the muscles are in a transparent state, simultaneously maintaining the blue of the soft bones, staining the hard bones into red by virtue of alizarin red, carrying out re-transparentization by virtue of different concentrations of mixed solutions of 0.5%KOH and glycerin, plasticizing the fish bodies by virtue of polyvinylpyrrolidone, after the plasticization, carrying out air drying on the specimen, and finally sealing the specimen by virtue of epoxy resin. According to the finished specimen, the skeleton specimen including red hard bones and blue soft bones can be directly seen through the resin and the plasticized muscles.
Owner:DALIAN OCEAN UNIV
Cool-feeling yarn and dyeing and finishing method for spandex tabby fabric of cool-feeling yarn
InactiveCN106149163AFast heat conductionImprove moisture absorption and release performanceDyeing processWoven fabricsYarnPulp and paper industry
The invention discloses a cool-feeling yarn. Nylon and artificial silk serve as raw materials, nanoscale mica powder is added into a spinning solution, nylon cool-feeling silk and artificial-silk cool-feeling silk are obtained, the nylon cool-feeling silk and the artificial-silk cool-feeling silk are subjected to network doubling, and the cool-feeling yarn is obtained. The cool-feeling yarn has the high heat conductivity, and further has the high moisture absorption and moisture liberation effects; the specific heather grey effect is obtained through double staining, the color fastness is high, the cooling feeling performance is lasting, and the moisture absorption and quick drying properties are excellent.
Owner:JIANGSU XINKAISHENG ENTERPRISE DEV
Method for detecting activity of spores of plant pathogenic fungi and plasmodiophora brassicae
InactiveCN103674906AEffectively distinguish between life and deathGood repeatabilityFluorescence/phosphorescenceSpore germinationFluorescence microscope
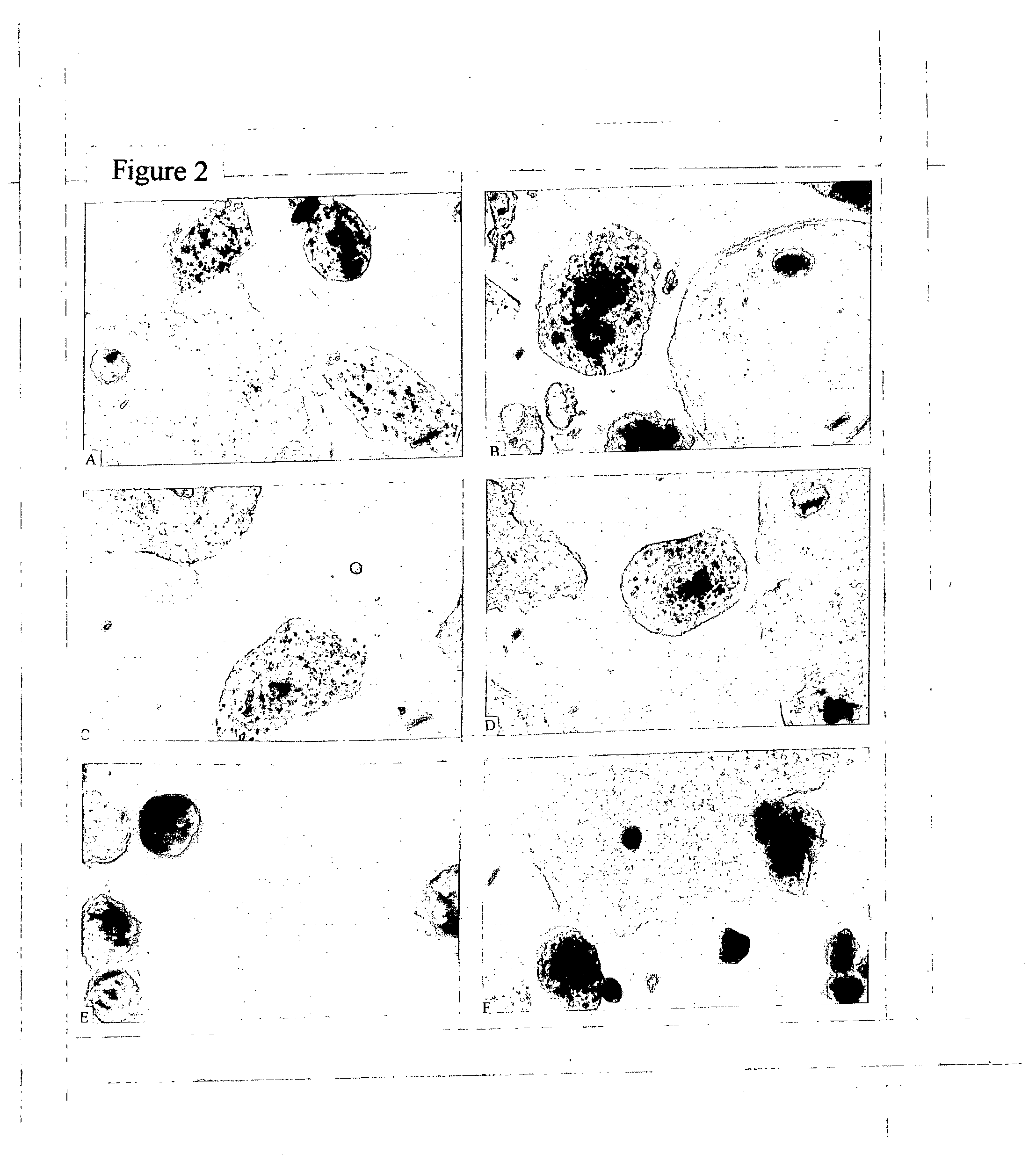
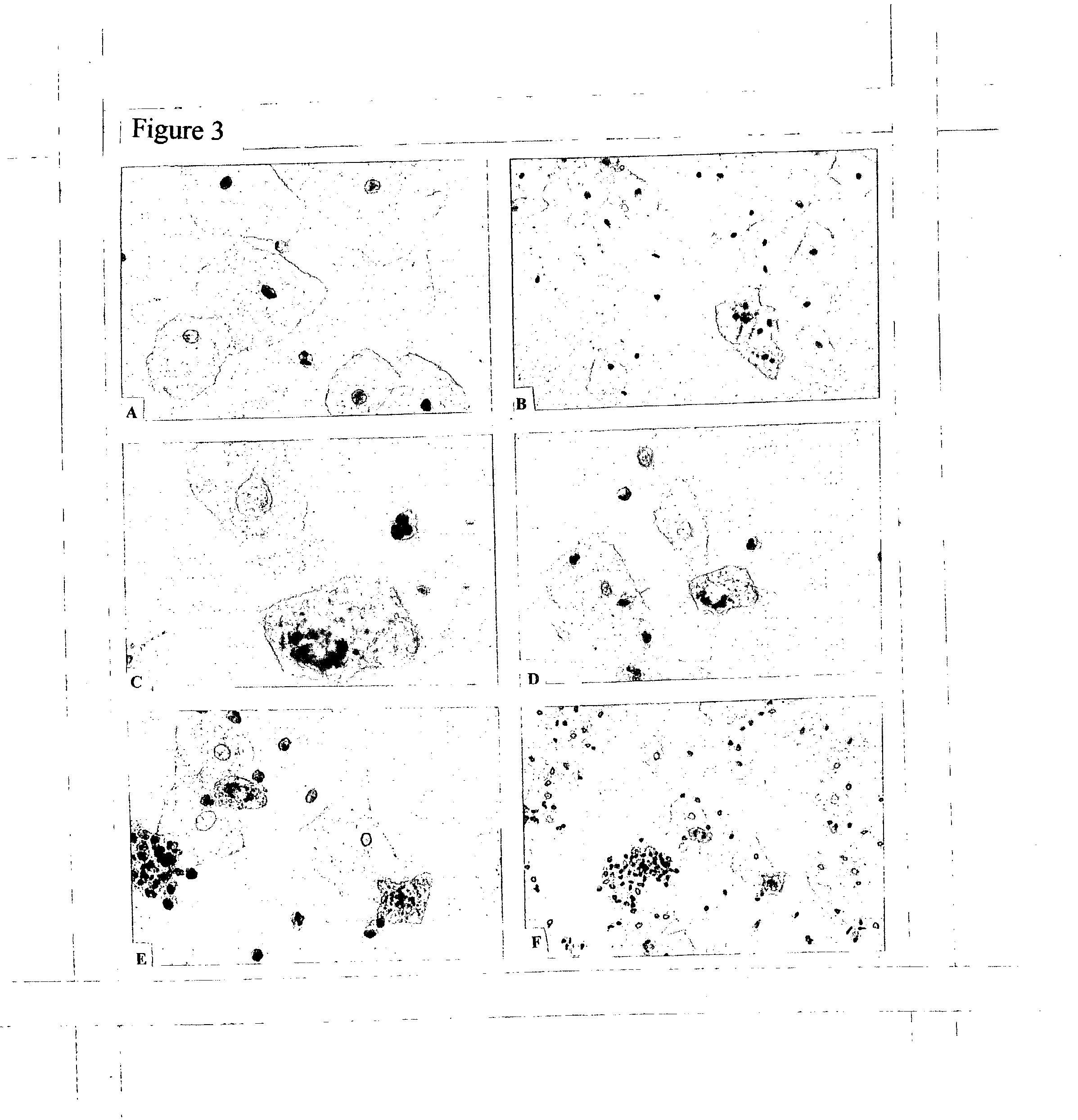
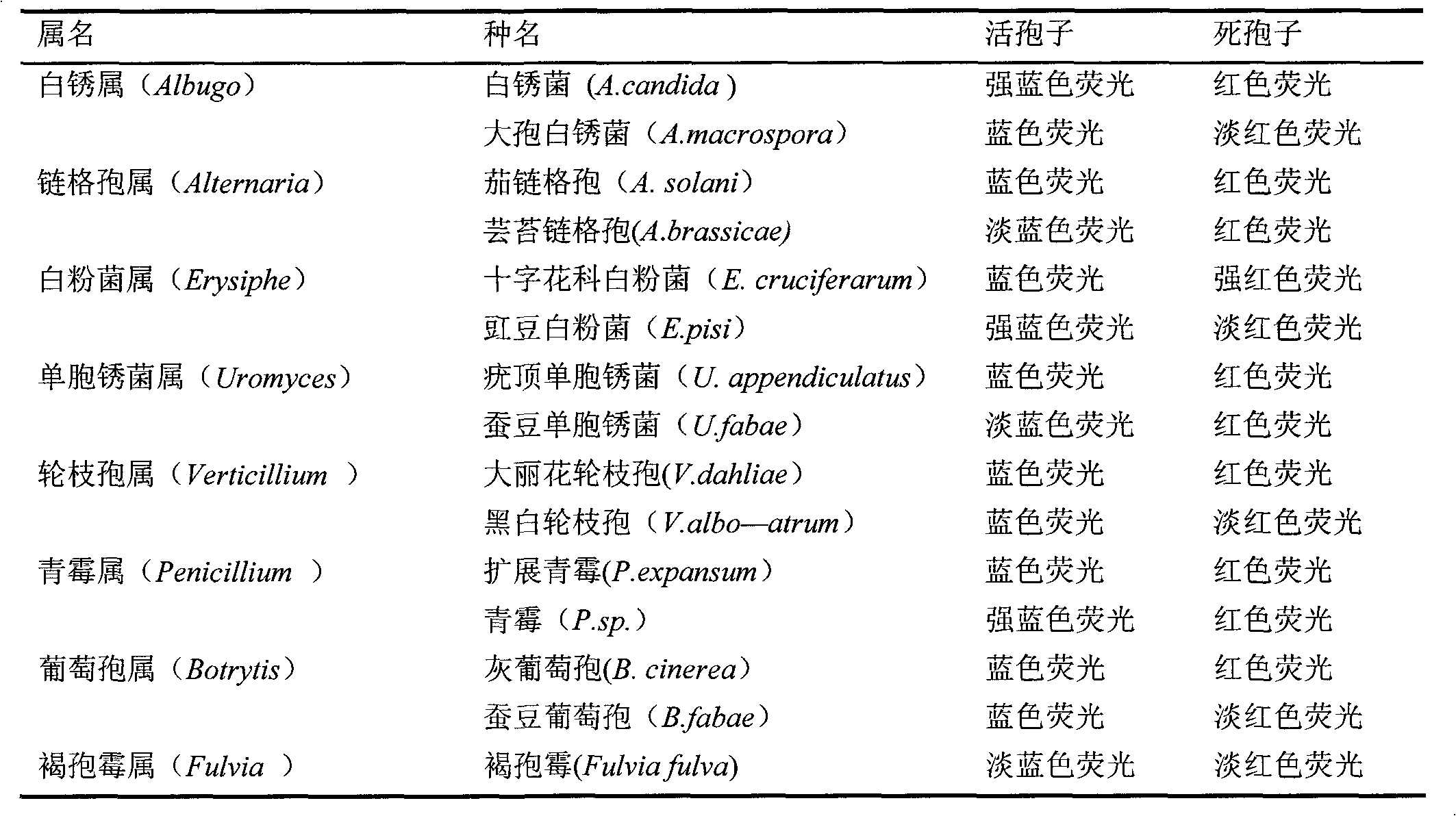
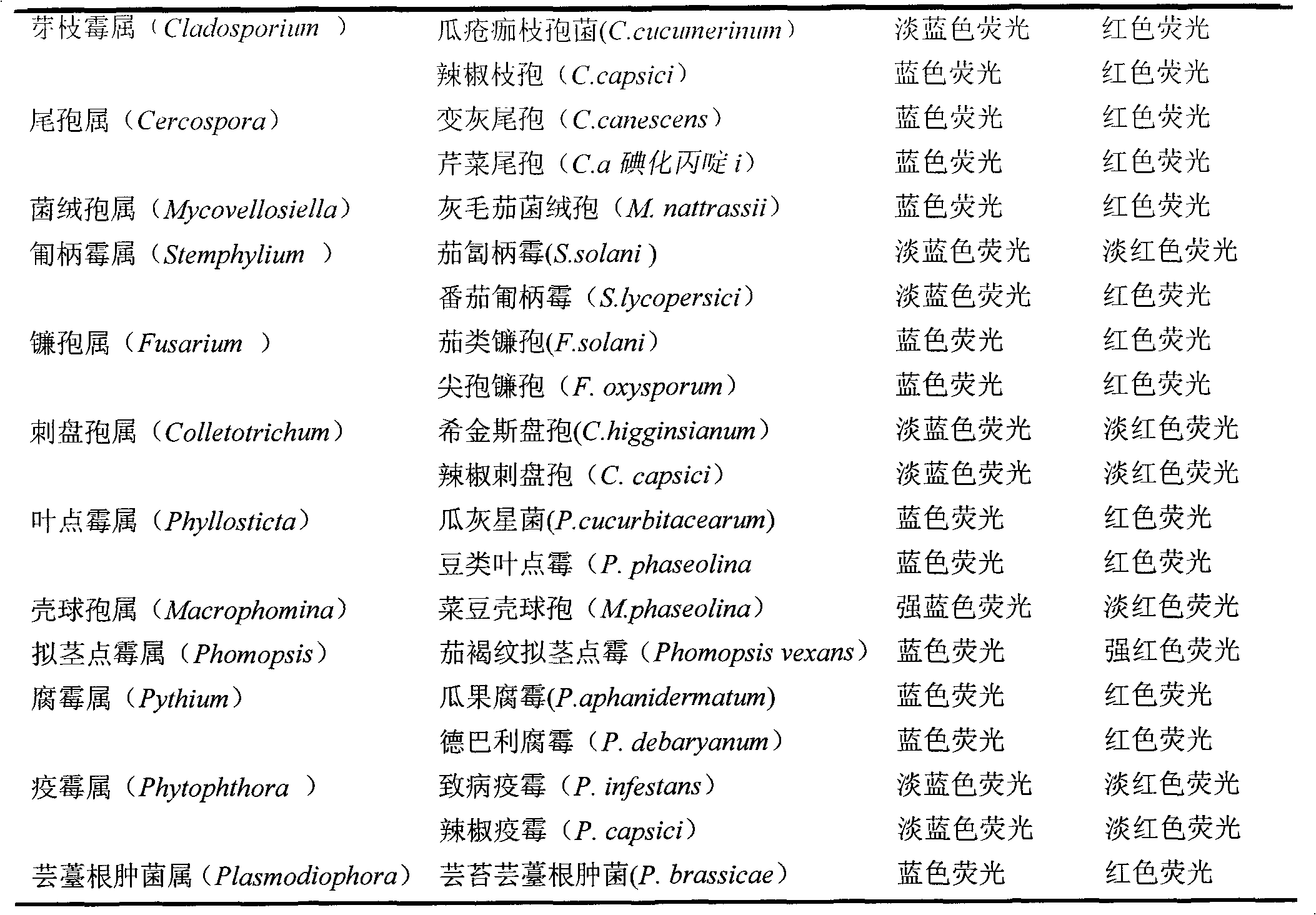
The invention discloses a method for detecting the activity of the spores of plant pathogenic fungi and plasmodiophora brassicae woronin. According to the method, a Hochest 33342 / PI double-staining method is adopted, and a fluorescence microscope is used for detection, so that life or death of the spores of the plant pathogenic fungi and the plasmodiophora brassicae woronin can be rapidly, accurately and sensitively distinguished; a spore germination method is also used for identifying the activity of the spores of the plant pathogenic fungi and the plasmodiophora brassicae woronin, but according to the spore germination method, time-consuming and labor-consuming effects are adopted; the method can be used for replacing the traditional spore germination method and is suitable for departments such as agricultural production, plant protection, etc.
Owner:INST OF VEGETABLE & FLOWERS CHINESE ACAD OF AGRI SCI
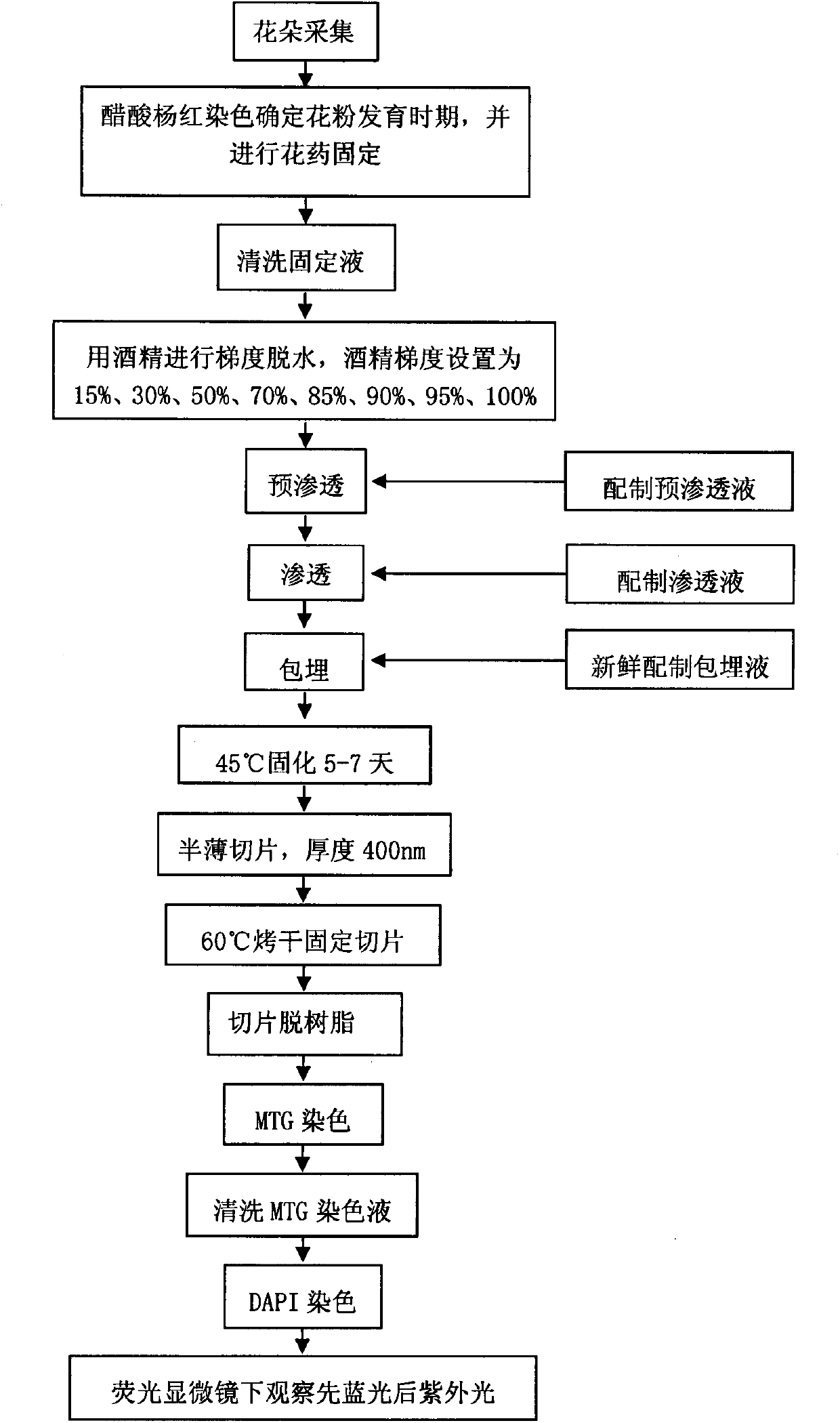
Mitochondria DNA observation through MTG-DAPI double-staining of semi-thin sections of cucumber pollen
Owner:NANJING AGRICULTURAL UNIVERSITY
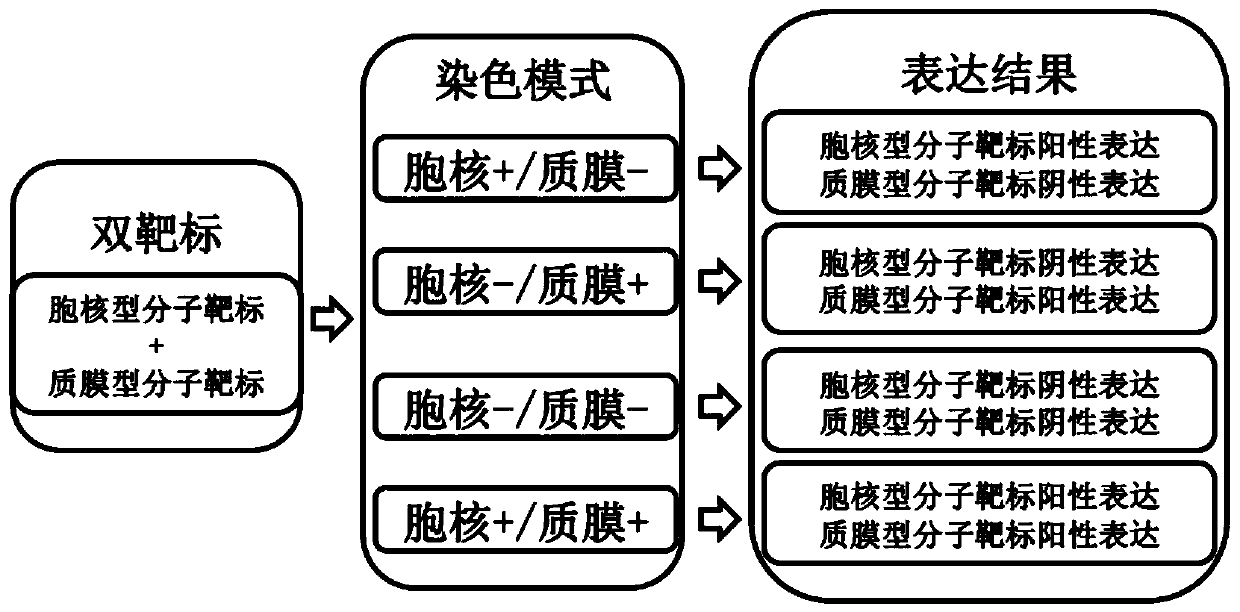
Immunohistochemical double-molecular-target single staining kit as well as using method and application thereof
InactiveCN110850094ASignificant technical advantagesSignificant clinical valueMaterial analysisChromogenic SubstratesAssay
The invention discloses an immunohistochemical double-molecular-target single staining kit as well as a using method and an application thereof. Based on an expression pattern of a molecular target ina sample to be detected, a cell core type molecular target and a cytoplasm or cell membrane or plasma membrane type molecular target are selected as the to-be-detected double-molecular targets; then,specific to the specific antibody of the double-molecular targets, a kit comprising a mixing primary antibody is formed by mixing, wherein the kit can be used in an immunohistochemical staining method such as an immuno-enzyme linked immunosorbent assay or an immune colloidal gold method; the kit and an enzyme labeled secondary antibody and a chromogenic substrate matched with the kit are adopted,so that synchronous detection of two molecular targets can be completed on one slice through one immunohistochemical staining process. The method is obviously superior to the traditional immunohistochemical and immunohistochemical double staining method, and has the characteristics of low cost and high efficiency, and does not need to increase the additional operation steps.
Owner:XI AN JIAOTONG UNIV
Method for detecting activities of cells in fermenting process through adopting flow cytometry (FCM)
InactiveCN103352068AMicrobiological testing/measurementMicroorganism based processesDouble stainingBio engineering
The invention relates to a method for detecting the activities of cells in a fermenting process through adopting FCM. In the invention, the activities of microbial cells (yeast or moulds) cultured through Rh123 / PI double staining are detected in a shaking bottle under normal fermentation or high salt stress through the FCM to obtain an FCM map, an appropriate cross door is adjusted, and a reliable ratio of dead cells to living cells to apoptotic cells can be obtained through quadrant analysis. The method is a simple method for detecting the activities of the cells in the industrial production.
Owner:JIANGNAN UNIV
Method for evaluating biosecurity of 76 nm nano silver on base of intestinal epithelial cells
The invention provides a method for evaluating the biosecurity of 76 nm nano silver on the base of intestinal epithelial cells. According to the method, cell lines of human colon carcinoma epithelial cells Caco-2 are selected as an object of study, nano zinc oxide (90.81 nm) with higher cytotoxicity is taken as a positive control, normal cells without the nano material are taken as a blank control, and the cytotoxicity of the nano material is preliminarily explored by an AO / EB double staining method and a CCK-8 method. The oxidative damage effect and the possible mechanism of nano silver on cells are studied by an oxidative stress method through detecting the influence of emission of ROS, SOD and GSH in the cells Caco-2. The result shows that 76.29nm silver particles (smaller than 200 mu g / ml) don't influence the activity of the cells Caco-2 obviously and cause no oxidative stress damage on the cells Caco-2, but the oxidation resistance of the cells is enhanced and is up to the highest level when the concentration of nano silver is 50 mu g / ml.
Owner:CHINA JILIANG UNIV
Immunofluorescent double staining kit for cervical cancer auxiliary diagnosis
ActiveCN111089964ALow backgroundIncrease signal strengthMaterial analysisImmunofluorescenceMicroscopic observation
The present invention provides an immunofluorescent double staining kit for cervical cancer auxiliary diagnosis. The kit comprises a mixed primary antibody working solution consisting of a glucan labeled Ki67 monoclonal antibody and a glucan labeled P16INK4A monoclonal antibody, and a DAPI staining solution. The kit simultaneously and efficiently excites multi-color fluorescent light, and all colors can be showed by only one kind of excitation light, so that wavebands do not need to be switched and an ordinary microscope is directly used for observation and shooting; a secondary antibody incubation process is removed, thereby shortening the operation time of the whole process; and the operation is simple, and the sensitivity and the accuracy are high.
Owner:江苏美克医学技术有限公司
Preparation and identification method of human leukemia cell cytoplast
InactiveCN102492655AInhibition of mitosisIncrease the denucleation rateMicrobiological testing/measurementTumor/cancer cellsHuman leukemiaColchicine
The invention discloses a preparation and identification method of a human leukemia cell cytoplast. The method comprises the following steps of: treating a purified human leukemia HL-60 cell with a cytochalasin and colchicine, centrifugally denucleating at the temperature of 34 DEG C, collecting a cytoplast component, and purifying with a Percoll density gradient centrifugation method to obtain apurified cytoplast; and identifying the cytoplast with DAPI (4',6-diamidino-2-phenylindole) and CFSE (5,6-carboxyflu-orescein diacetate succinimidyl ester) fluorescent double staining. Due to the adoption of the method, the denucleating rate of a non-adherent human leukemia HL-60 cell can be over 90 percent, the purity of purified cytoplasts is over 95 percent, the quantity of cytoplasts which are more than or equal to 5 mum in diameter is up to 81 percent, and a cytoplast which is chromophilic with a CFSE fluorescent probe can be directly applied to subsequent researches; an HL-60 cell cytoplast obtained with the method can be widely applied to researches of leukemia cell cytoplast components as well as metabolism, functions, cell reconstruction, cell differentiation, apoptosis and the like thereof; and the method is suitable for preparing and identifying all in-vitro non-adherent tumor cell cytoplasts.
Owner:AFFILIATED HOSPITAL OF ZUNYI MEDICAL COLLEGE
Gamma-hydroxy olic acid ester derivatives and use thereof in preparation of antitumor medicines
ActiveCN102304047AReduce volumeGrowth inhibitionOrganic chemistryEster active ingredientsIn vivoBULK ACTIVE INGREDIENT
The invention discloses gamma-hydroxy olic acid ester derivatives having antitumor activity and the use thereof in the preparation of antitumor medicines, and belongs to the field of biochemical technology. The structure of the gamma-hydroxy olic acid ester derivatives is shown in the description. The results of classic methyl thiazolyl tetrazolium (MTT) tumor cell medicine screening experiments, AnnexinV / PI double-staining cell apoptosis measurement experiments, cell periodical detection experiments and in-vivo antitumor experiments prove that the gamma-hydroxy olic acid ester derivatives have proliferation inhibition effects on breast cancer, bladder cancer and liver cancer and can be used in the preparation of antitumor medicines as an active ingredient.
Owner:LANZHOU UNIVERSITY
Method for applying pulse electric field combined carbon nanotube to facilitation of cancer cell apoptosis
PendingCN108866039AExcellent pro-apoptotic effectIncrease apoptosis rateMicrobiological testing/measurementElectrical/wave energy microorganism treatmentElectrical impulseNanotube
The invention discloses a method for applying a pulse electric field combined carbon nanotube to facilitation of cancer cell apoptosis, and relates to application of the pulse electric field combinedcarbon nanotube. The method comprises the following steps: preparing a cell suspension; preparing deionized dispersion liquids of different concentrations of three kinds of carbon nanotubes; uniformlymixing the carbon nanotube deionized dispersion liquids with the cell suspension to obtain a carbon nanotube-cell suspension; adding the cell suspension into an electrode cup to set a blank control group and a pure electric pulse treatment group; adding the carbon nanotube-cell suspension into the electrode cup for setting a pure carbon nanotube treatment group and a pulse electric field combinedcarbon nanotube treatment group; applying a pulse electric field to a positive electrode plate and a negative electrode plate of the electrode cup in an experiment group being subjected to the pulsetreatment; measuring the cell survival rate of each treatment group by using an MTT method after the pulse treatment; analyzing the cell apoptosis of each treatment group through a flow cytometry by an Annexin V-FITC / PI double staining method after the pulse treatment; detecting the change of cell growth and multiplication capabilities through clone formation assay after the pulse treatment.
Owner:XIAMEN UNIV
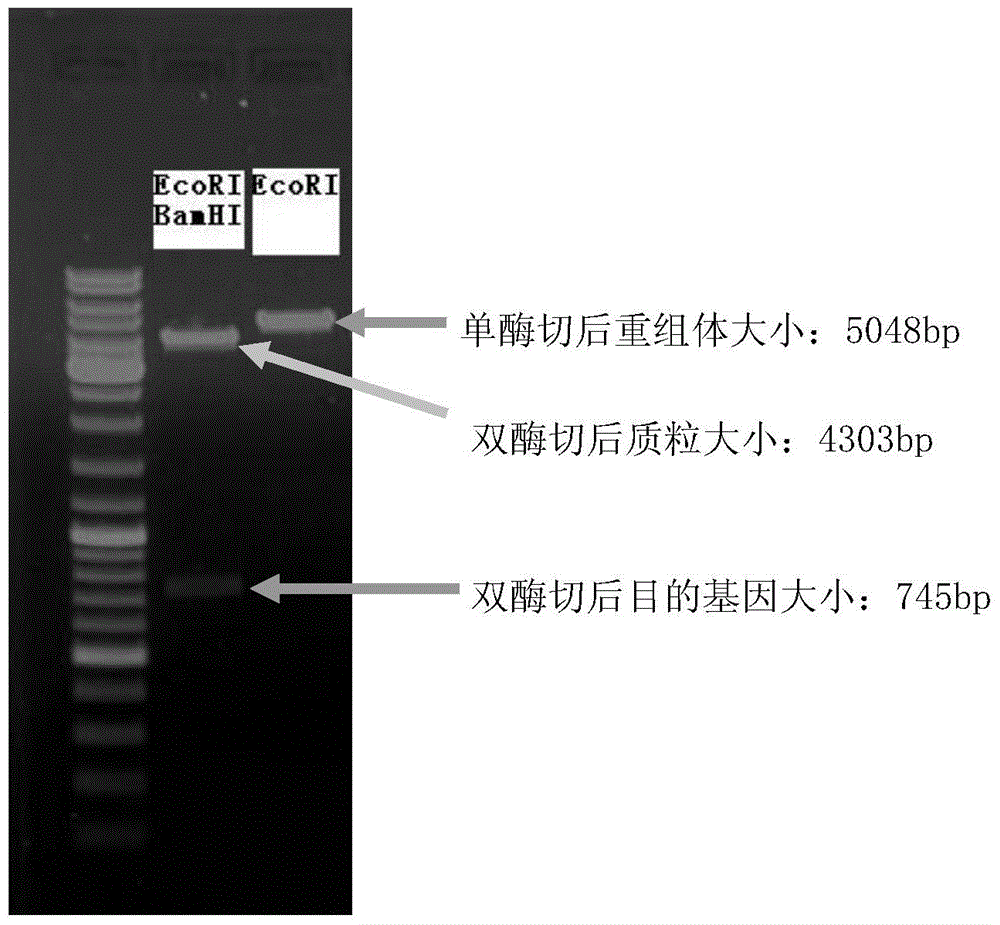
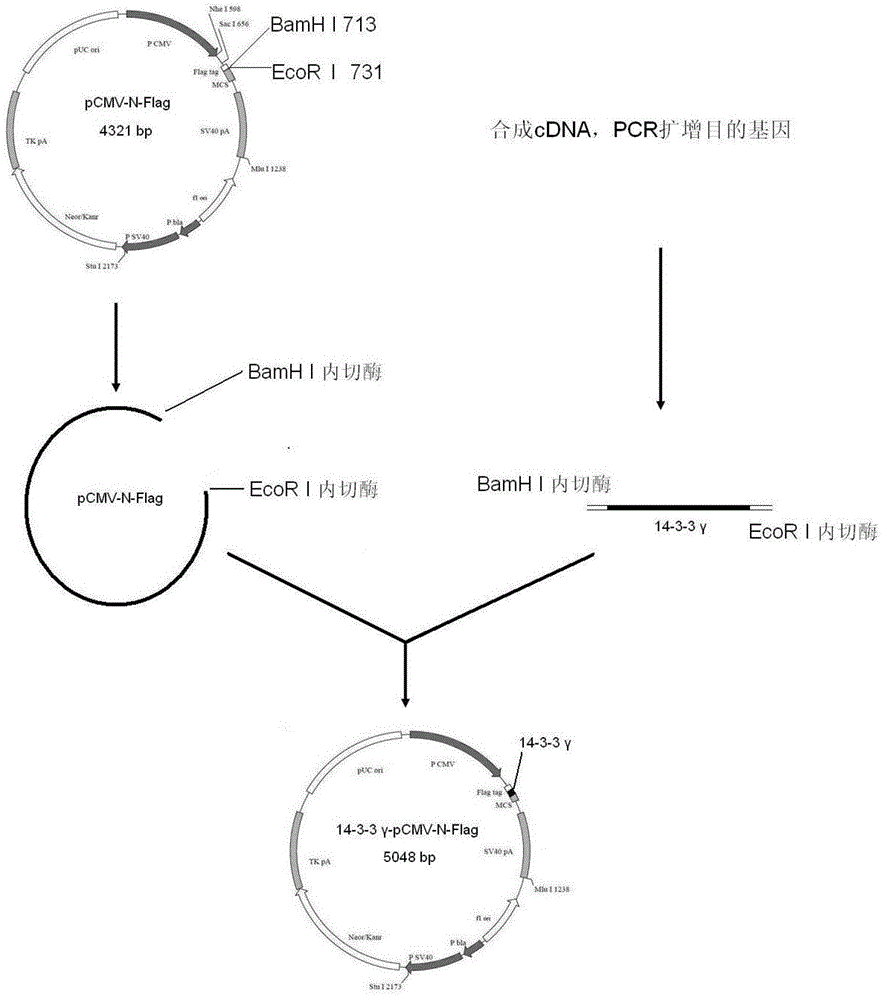
Construction method and application of human hysteromyoma 14-3-3 gamma high expression vector
The present invention discloses a construction method and an application of a human hysteromyoma 14-3-3 gamma high expression vector. The construction method comprises: amplifying and purifying target gene, designing an upstream primer and a downstream primer, extracting hysteromyoma RNA, carrying out reverse transcription to obtain a template, carrying out PCR amplification, cutting the target band, carrying out recovery and purification with a kit, constructing and identifying recombinant plasmid 14-3-3gamma-pCMV-N-Flag, carrying out double digestion on the purified PCR product and the vector pCMV-N-Flag with BamHI and EcoRI, respectively adopting kits to recover and purify the digestion products, linking the fragments, carrying out screening culture, cloning, identifying, sequencing, carrying out WesternBlot to detect protein expression, adopting CCK-8 to detect cell proliferation change, and adopting a AnnexinV-FITC / PI double staining method to detect apoptosis through flow cytometry.
Owner:THE SECOND HOSPITAL AFFILIATED TO WENZHOU MEDICAL COLLEGE
Application of IMCA in preparation of anti-gastric-cancer drug
ActiveCN108078976APrevent proliferationTumor suppressor effectOrganic active ingredientsDigestive systemChemical structureCancer drugs
Owner:HENAN UNIVERSITY
Bivalve mollusc semen quality detection method
InactiveCN108693101ADoes not affect metabolismAccurate determination of survival ratioIndividual particle analysisInner mitochondrial membraneFluorescence
The invention provides a bivalve mollusc semen quality detection method, in which fluorochrome double staining and flow cytometry are combined to detect the bivalve mollusc semen quality. The method includes steps of: adjusting concentration of semen, staining the to-be-detected semen with a membrane-permeable fluorochrome and a membrane-impermeable fluorochrome, and performing room temperature incubation and flow cytometry detection to obtain a detection result. The membrane-permeable fluorochrome can be combined with mitochondrial membrane while the membrane-impermeable fluorochrome can penetrate damaged cell membrane and be specifically combined with intracellular nucleic acids. Through one detection operation, a plurality of parameter results can be obtained. The method not only can clearly distinguish semen in different status and display ratio thereof, and obtain death rate, survival rate, plasma membrane damage rate and ratio of mitochondrial activity, but also can avoid low accuracy due to subjective factors and guarantee the accuracy of the detection results.
Owner:OCEAN UNIV OF CHINA
Method for evaluating biosecurity of 35 nm nano silver on the basis of intestinal epithelial cells
The invention provides a method for evaluating the biosecurity of 35 nm nano silver on the basis of intestinal epithelial cells. According to the method, cell lines of human colon carcinoma epithelial cells Caco-2 are selected as an object of study, nano zinc oxide (26.21 nm) with higher cytotoxicity is taken as a positive control, normal cells without the nano material are taken as blank control, and the cytotoxicity of the nano material is preliminarily explored by an AO / EB double staining method and a WST-1 method. The oxidative damage effect and the possible mechanism of nano silver on cells are studied by an oxidative stress method through detecting the influence of emission of ROS, SOD and GSH on the cells Caco-2. The experimental result shows that a low dose (smaller than 50 mu g / ml) of 35.48 nm silver particles cannot influence the activity of the intestinal epithelial cells, and causes no oxidative stress damage; and 75 mu g / ml of 35.48 nm silver particles and 100 mu g / ml of 35.48 nm silver particles have a dose-effect relation and a time-effect relation with the cytotoxicity of intestinal epithelial cells (Caco-2), and cause minor oxidative stress damage on the cells.
Owner:CHINA JILIANG UNIV
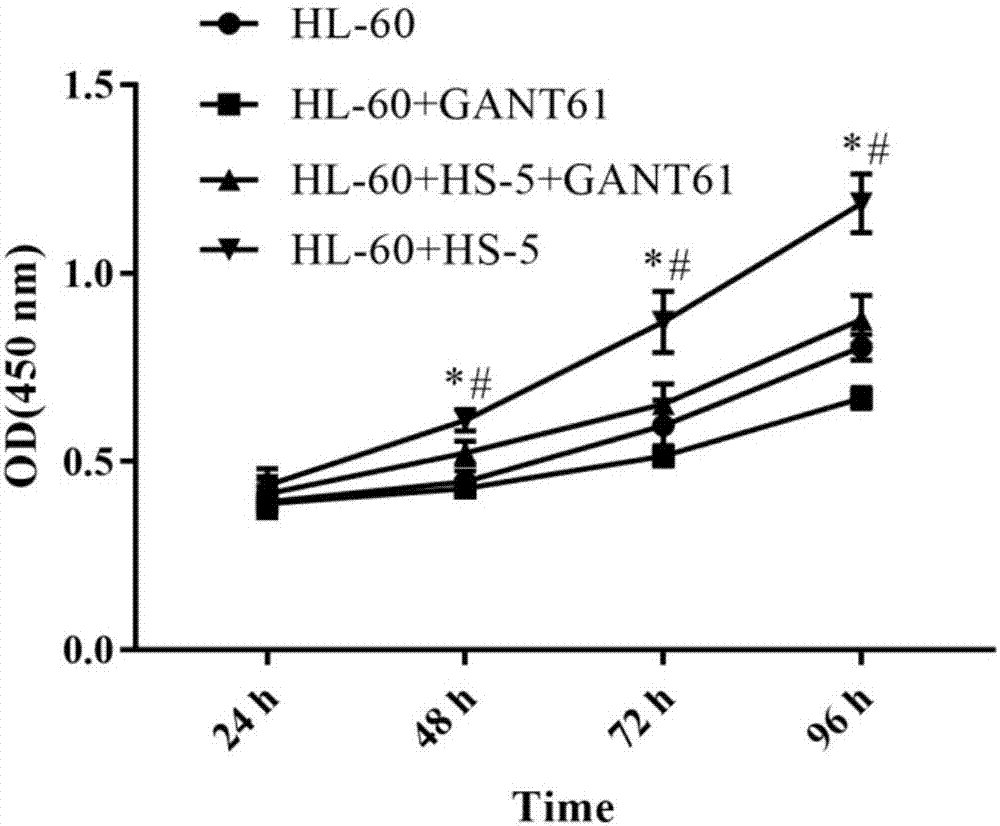
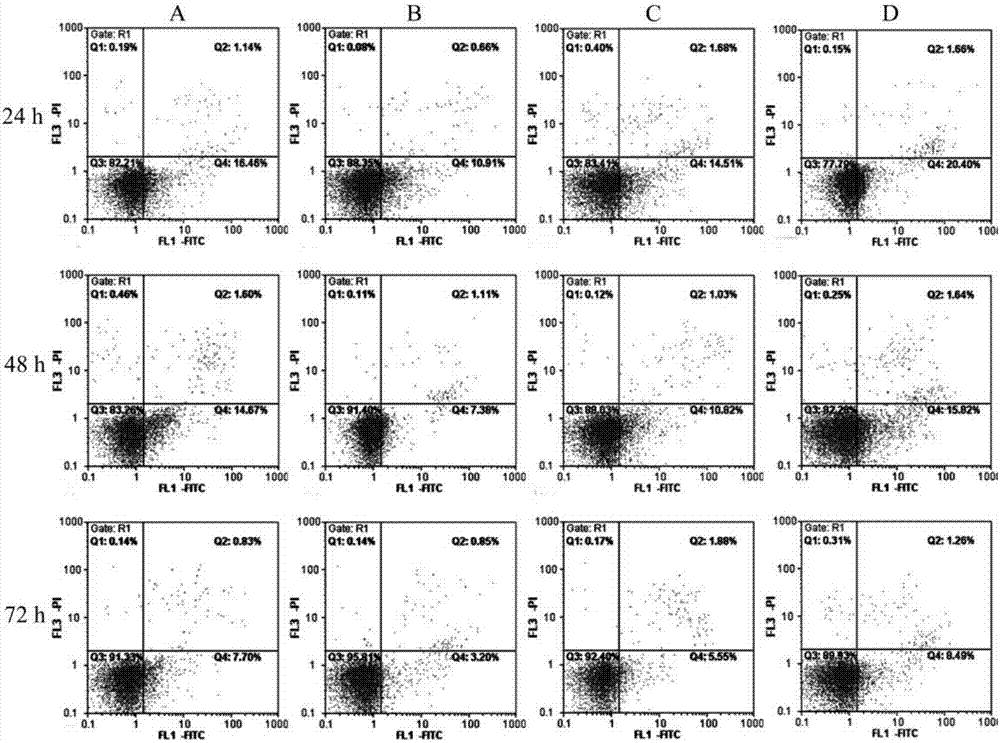
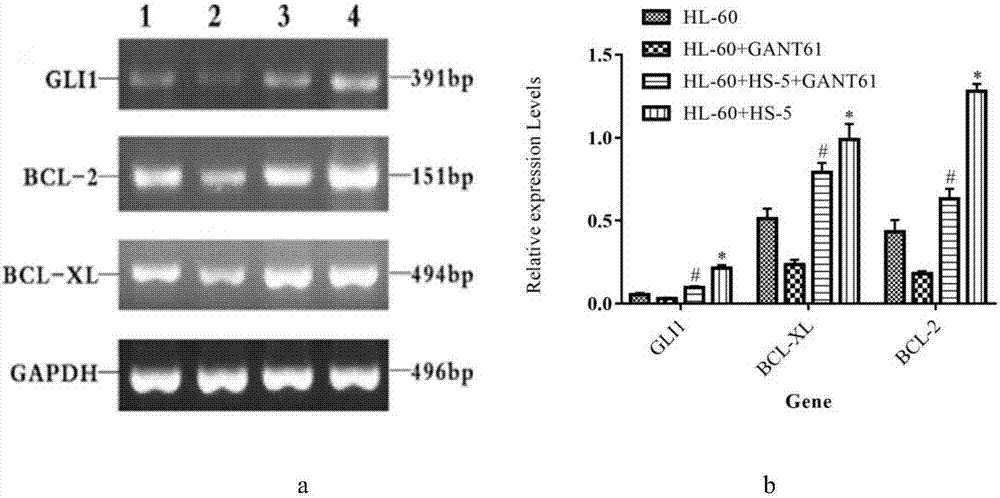
Detection method for regulating Hh signal channel to inhibit HL-60 cells
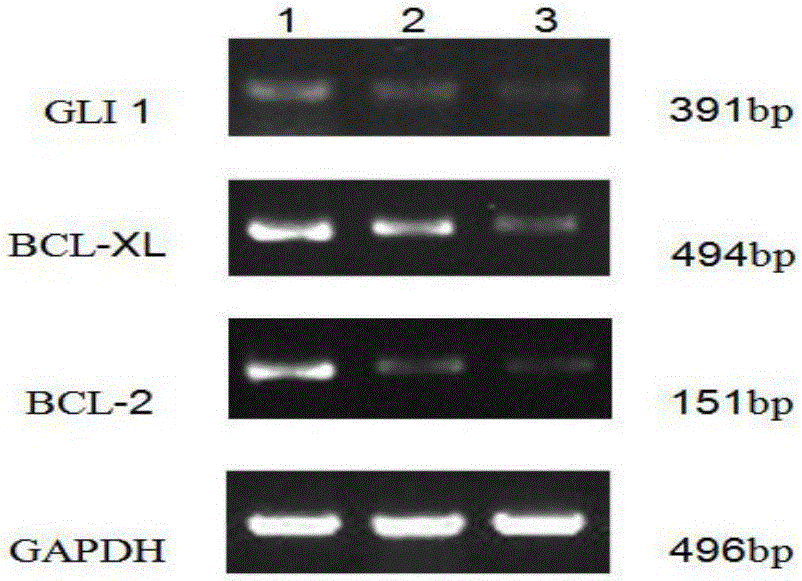
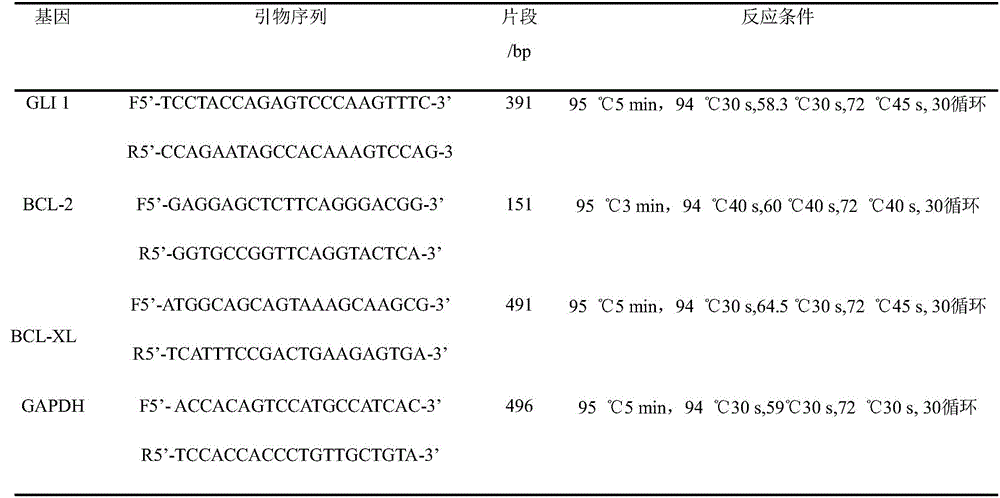
The invention discloses a detection method for regulating an Hh signal channel to inhibit HL-60 cells. The detection method comprises the steps as follows: proliferation of HL-60 cells among different experiment groups is detected with a kit, the HL-60 cell apoptosis rate of each group is detected through double staining, expression of Hedgehog signal channel components, namely, GLI1 genes and apoptosis genes BCL-2 and BCL-XL, of each experiment group is detected with a semi-quantitative RT-PCR method, and expression of GLI1 protein is detected with an immunofluorescence method. A co-culture mode is established in vitro by use of human acute myelocytic leukemia cell strains HL-60 and HS-5 cells, the interaction of in-vivo leukemia cells and hemopoietic microenvironment around the leukemia cells is simulated, the possible mechanism of the effect and action of the hemopoietic microenvironment on leukemia cell apoptosis is researched, and a theoretical foundation is laid for studying leukemia treatment drugs targeted at blocking the interaction of the hemopoietic microenvironment and the leukemia cells.
Owner:SHIHEZI UNIVERSITY
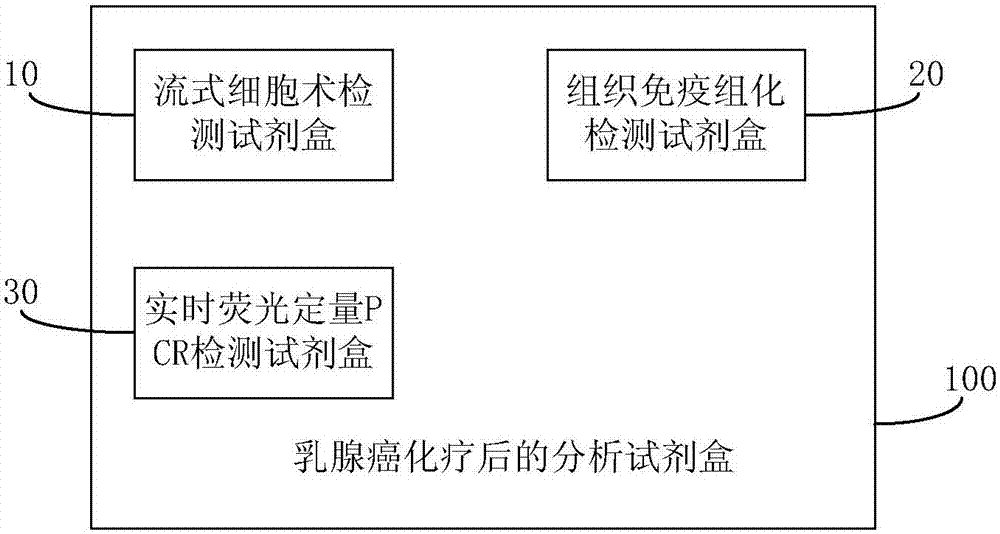
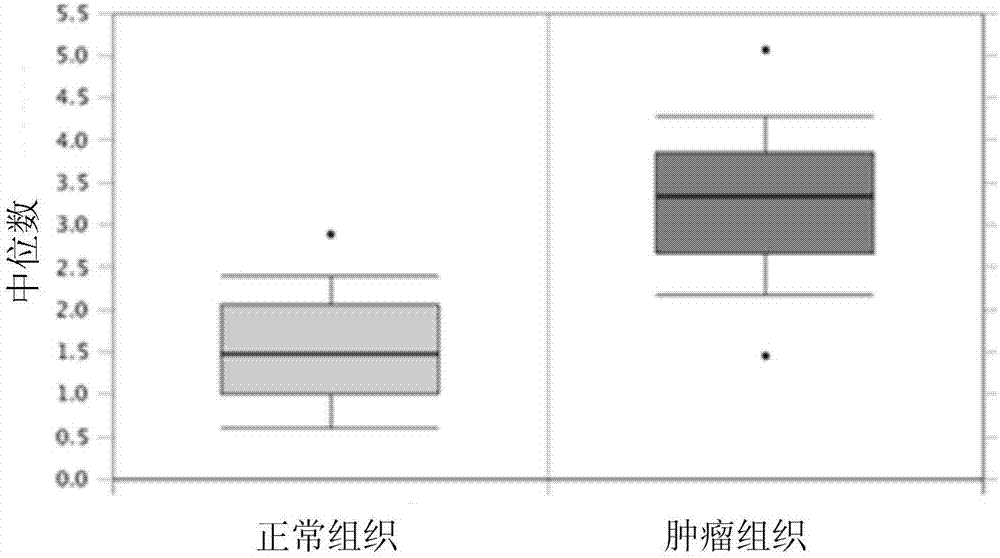
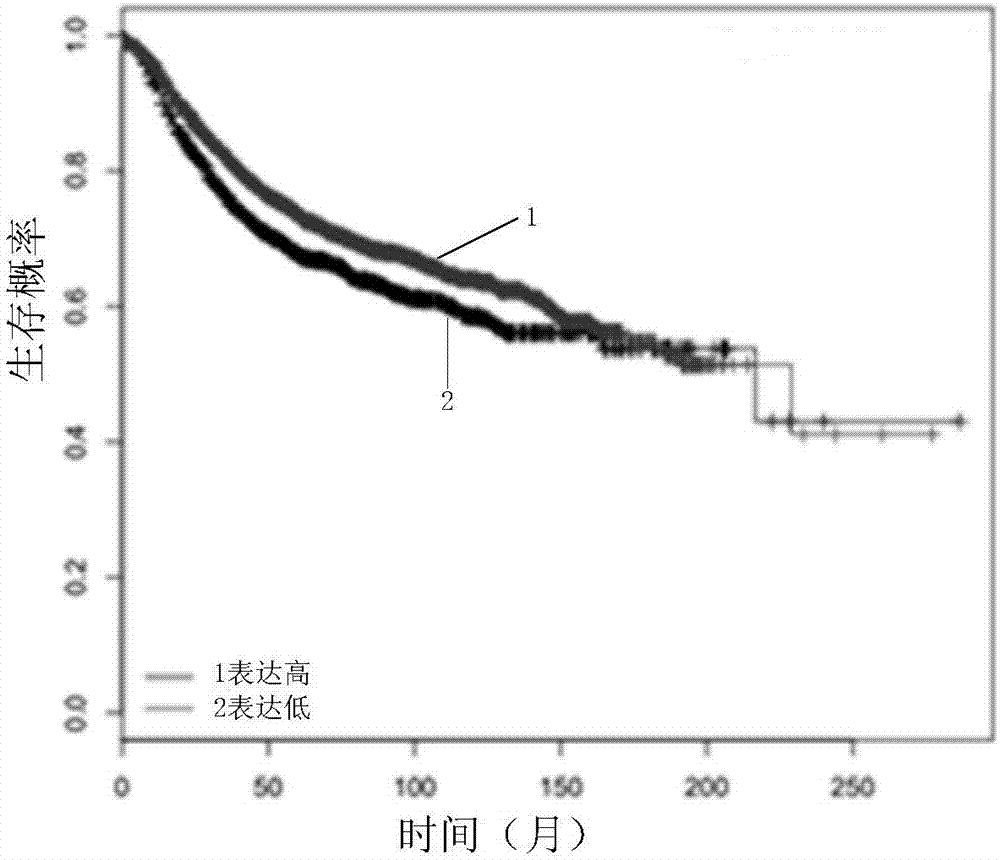
Analytic kit for breast cancer chemotherapy
InactiveCN107884330AMicrobiological testing/measurementIndividual particle analysisIV ChemotherapyTissue sample
The invention discloses an analytic kit for breast cancer chemotherapy. The kit comprises a flow cytometry detection kit, a tissue immunohistochemical detection kit and a real-time fluorescent quantitative PCR detection kit, wherein the flow cytometry detection kit is used for counting the number of cells of a clinical tissue sample after chemotherapy of a breast cancer patient to obtain a first drug resistance assessment coefficient of the breast cancer patient after chemotherapy; the tissue immunohistochemical detection kit is used for performing double staining on the clinical tissue sampleto obtain a second drug resistance assessment coefficient of the breast cancer patient after chemotherapy; performing quantitative amplification for the clinical tissue sample with a real-time fluorescent quantitative PCR detection kit to obtain a third drug resistance assessment coefficient of the breast cancer patient after chemotherapy; and calculating the drug resistance index of the breast cancer patient after chemotherapy through the first drug resistance assessment coefficient, the second drug resistance assessment coefficient and the third drug resistance assessment coefficient. By adopting the method, the product support is provided for comprehensive and complement assessment on the drug resistance situation of the breast cancer patient after chemotherapy.
Owner:NANTONG UNIVERSITY
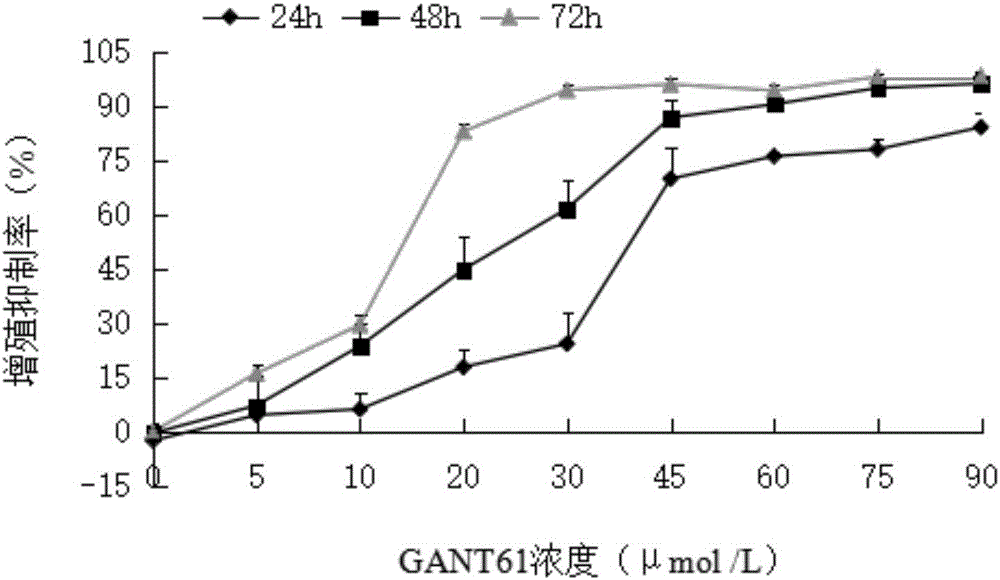
Detection method for effects of inhibitor GANT61 on multiplication and apoptosis of HL-60
The invention discloses a detection method for effects of an inhibitor GANT61 on multiplication and apoptosis of HL-60. The detection method comprises the following steps: detecting the growth and multiplication conditions of HL-60 cells with inhibitors of different concentrations through a CCK-8 (Cell Counting Kit-8) method, detecting the apoptosis condition of the HL-60 cells with an inhibitor through double staining, detecting the expression condition of the HL-60 cells with inhibitors of different concentrations through RT-PCR, and detecting the expression condition of proteins of the HL-60 cells with inhibitors of different concentrations through an immunofluorescence technology. According to the detection method, expression of a Hedgehog-GLI passageway is inhibited by a Hedgehog-GLI signal passageway specific inhibitor taking GLI as a target, and when the Hedgehog-GLI passageway is inhibited, the multiplication and apoptosis conditions of acute myeloid leukemia cells are observed; and a new treatment strategy is supplied to treatment of leucocythemia.
Owner:SHIHEZI UNIVERSITY
Application of triptonide in preparation of medicine for treating acute monocyte leukemia
PendingCN111407766ALimit concentrationAchieve inhibitionOrganic active ingredientsAntineoplastic agentsInducer CellsAcute leukemia
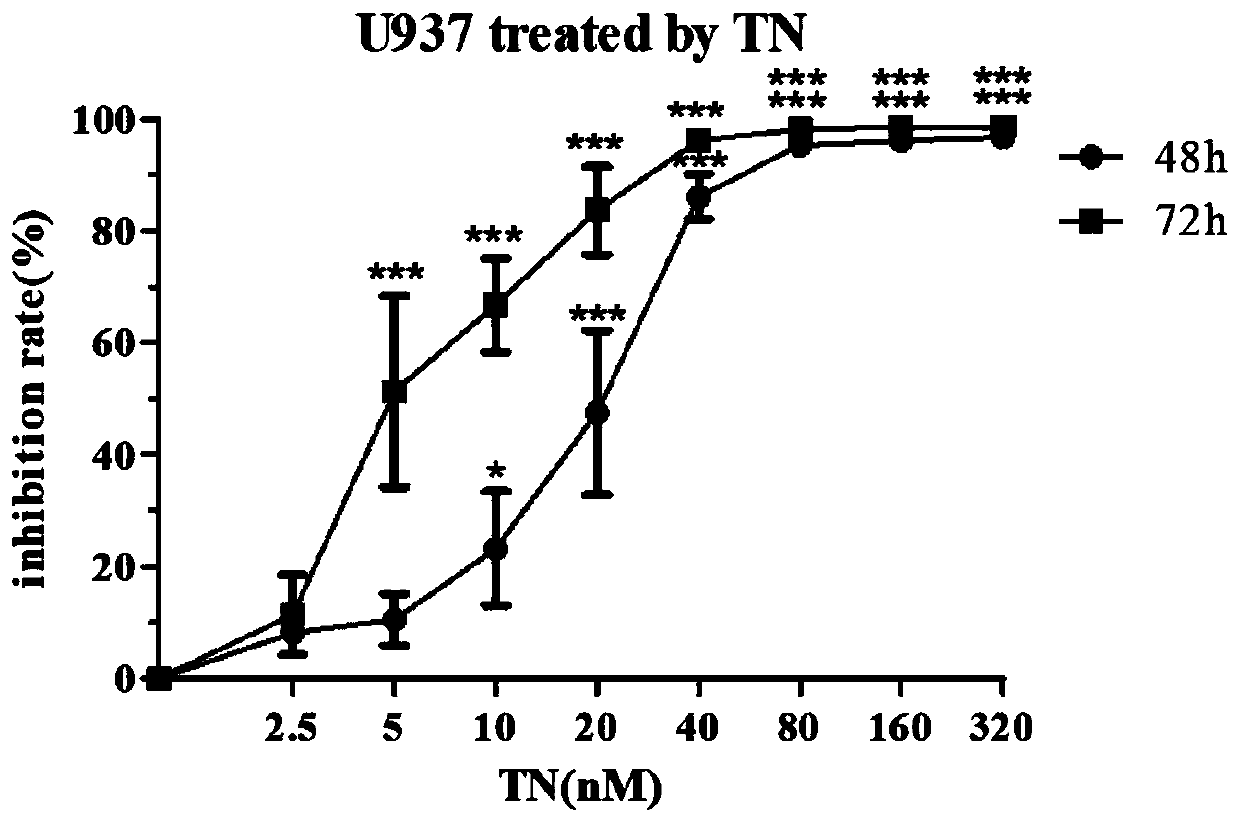
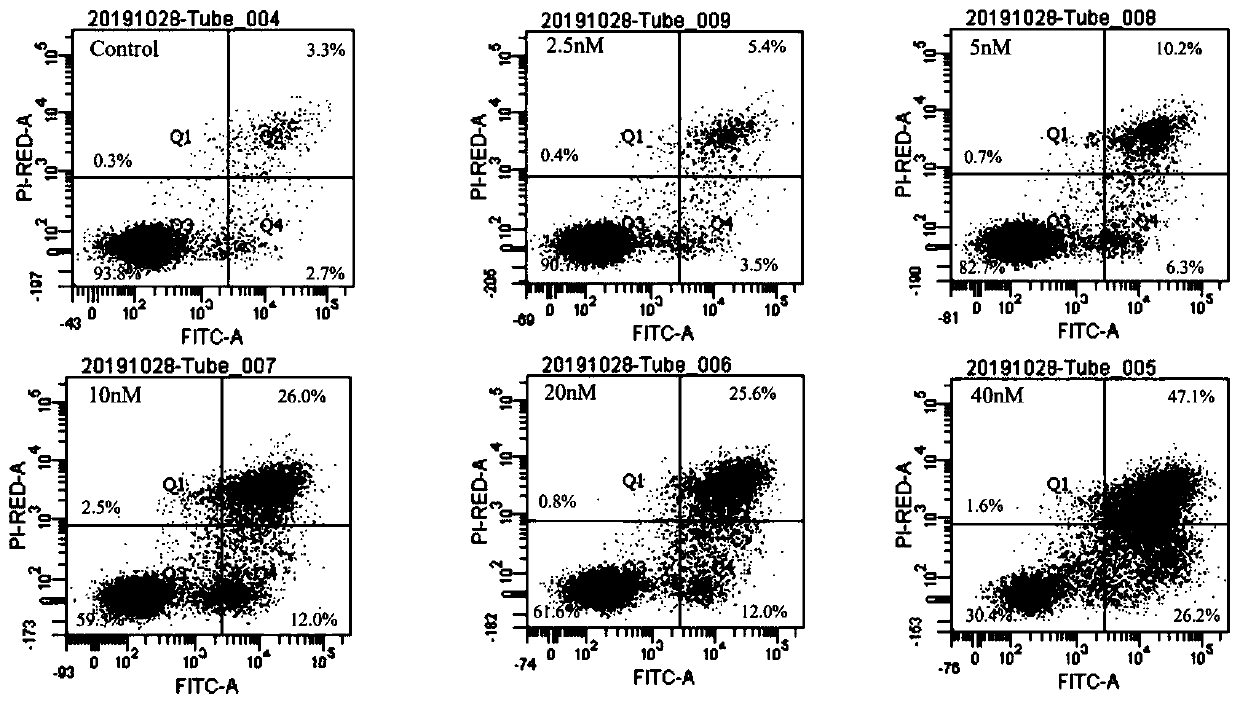
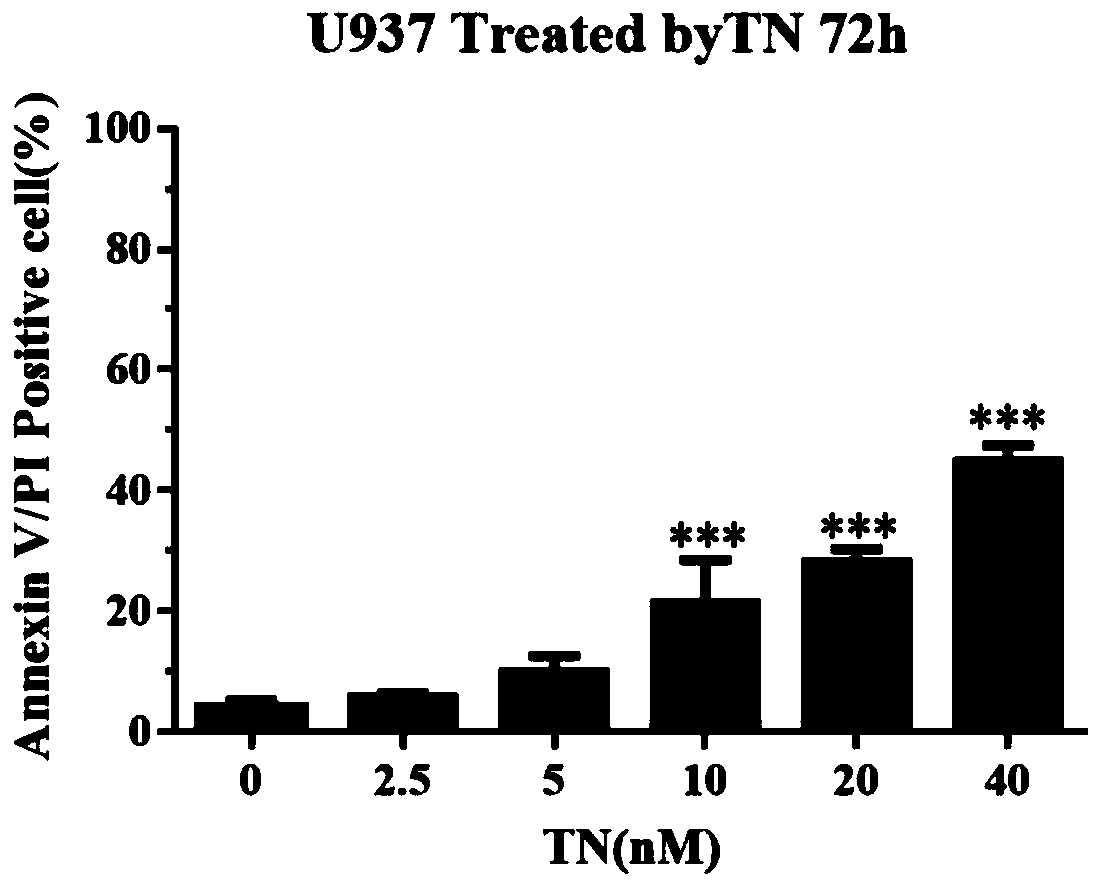
The invention provides application of triptonide in preparation of a medicine for treating acute monocyte leukemia, and belongs to the technical field of leukemia medicines. According to the application of the triptonide in preparation of the medicine for treating the acute monocyte leukemia, acute monocyte leukemia cells U937 are taken as research objects, U937 cells are treated by a triptonide solution, the cell activity of the U937 cells is detected by an MTT method, and the result shows that the triptonide has an effect of inhibiting proliferation of the U937 leukemia cells. The influenceof the triptonide on apoptosis of the U937 cells is detected by an Annexin V / PI double staining method, and the result shows that the triptonide can induce apoptosis of the U937 cells, and the apoptosis is more obvious along with increase of concentration. Therefore, the triptonide can inhibit proliferation of the acute leukemia cells and induce apoptosis, so that treatment of the acute monocyte leukemia is realized.
Owner:INST OF CHINESE MATERIA MEDICA CHINA ACAD OF CHINESE MEDICAL SCI
Rapid double staining solution for freehand sections of main stems of tobaccos
ActiveCN108918236AAchieve color renderingColoring effect does not fadePreparing sample for investigationNicotiana tabacumDistilled water
The invention relates to a staining technology of freehand sections of plants, and in particular relates to rapid double staining solution for freehand sections of main stems of tobaccos, and a staining method. The invention aims to provide the rapid staining method for increasing the staining efficiency of the freehand sections of the main stems of the tobaccos. The adopted technical scheme is asfollows: a rapid double staining method for the freehand sections of the main stems of the tobaccos comprises the steps of: (1), preparing rapid double staining solution and eluant; (2), putting a freehand section sample of the main stems of tobaccos on a glass slide, and removing excess distilled water by absorption through a straw; (3), dropping 1-2 drops of rapid double staining solution ontothe surface of the sample, and waiting for about one minute till the xylem of the sample has macroscopic red; and (4), removing the rapid double staining solution by absorption through the straw, dropping 1-2 drops of eluant onto the surface of the sample, decolouring till the sample becomes reseda and the xylem of the sample becomes purple, and then, performing microscopy shooting. According to the method in the invention, double staining of the freehand sections of the main stems of the tobaccos can be completed for about one minute.
Owner:TOBACCO RES INST CHIN AGRI SCI ACAD
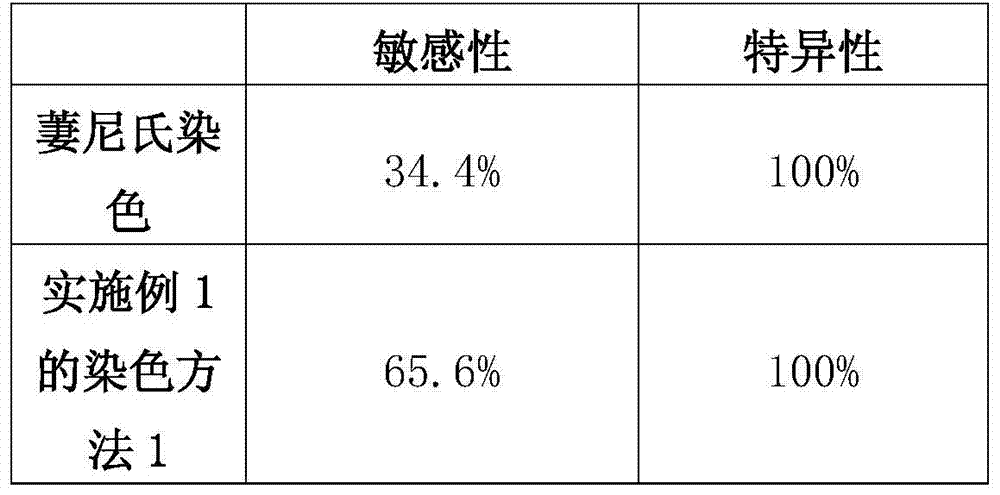
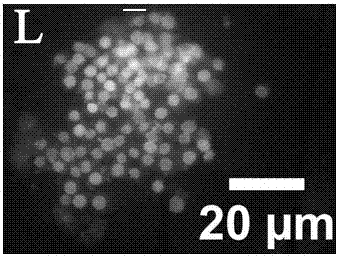
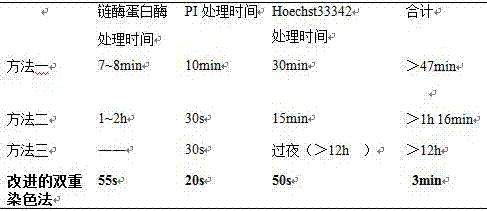
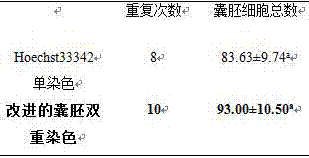
Double staining method for bovine in-vitro fertilization blastocyst
InactiveCN105259007AShorten the timeGuaranteed dyeing effectPreparing sample for investigationAnimal scienceUltraviolet lights
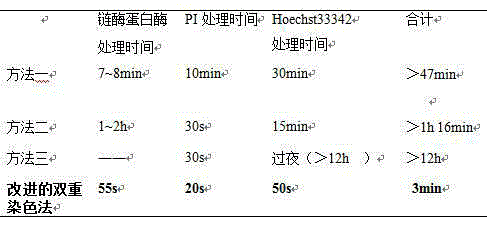
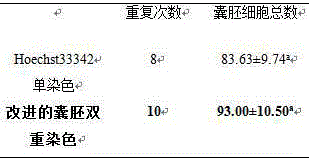
The invention discloses a double staining method for a bovine in-vitro fertilization blastocyst. The method comprises the steps that the bovine in-vitro fertilization blastocyst which is cultured for 7-9 days is processed with 0.5% pronase for 55 seconds, washed in a PBS for 2-3 times, stained in PI (1% TritonX-100 / PBS) with the concentration of 100 mug / ml for 20 seconds, washed in the PBS for 2-3 times, transferred into Hoechst33342 with the concentration of 45 mug / ml to be stained for 50 seconds, washed in the PBS for 2-3 times and then processed through preforming; the bovine in-vitro fertilization blastocyst is placed under an inverted fluorescence microscope after preforming is finished, observing and photographing are performed under ultraviolet light, cell nucleuses of an inner cell mass (ICM) are blue, and cell nucleuses of trophoderm cells are red. According to the double staining method, staining can be completed in 3 minutes, but at least 47 minutes are needed in previous reports; accordingly, the time needed by double staining of the blastocyst is greatly shortened, the efficiency is improved, and the bovine blastocyst quality can be quickly evaluated.
Owner:天津市农业科学院
Preparation method of compound alisol A and application thereof causing renal toxicity
InactiveCN102675396ANephrotoxicSteroidsColor/spectral properties measurementsExperimental methodsMedicine
The invention provides a preparation method of compound alisol A causing renal toxicity and an experimental method of the compound alisol A, aiming at solving the problem that the modern clinical practice and the pharmacology experiment are only limited to the description of the intoxicating phenomenon and are undefined in the material basis of the renal toxicity causing effect. The invention has the key points of: (1) preparing rhizoma alismatis ethanol extracting solution; (2) preparing chloroform extractant; (3) purifying compound alisol A; (4) detecting the proliferate inhibiting effect of the alisol A to the HKC (renal tubular epithelial cell) by an MTT (microwave theory and techniques) method; and (5) observing cytomorphology change by the Hoechst33342-PI fluorescent double staining method. The invention has the benefit effects that the basis is provided for the reasonable application of the traditional Chinese medicine rhizoma alismatis and the prevention and cure of the causing of the renal injury.
Owner:SHENYANG PHARMA UNIVERSITY
A kit and method for double staining of Mycobacterium tuberculosis and its antigen
ActiveCN104596827BEasy to useIncreased sensitivityPreparing sample for investigationAntigenPathology diagnosis
The invention discloses a kit and a method for staining Mycobacterium tuberculosis and its expressed protein. The present invention provides a method for staining Mycobacterium tuberculosis and its expressed protein, comprising the following steps: 1) sequentially incubating a sample containing Mycobacterium tuberculosis with a primary antibody and a secondary antibody to obtain an antibody binding product; 2) ) The antibody-binding product is sequentially stained with DAB staining solution, phenolic fuchsin staining solution and hematoxylin staining solution to achieve staining of Mycobacterium tuberculosis and its expressed protein. The experiments of the present invention prove that the present invention has developed a new kit for pathological diagnosis of tuberculosis, which can simultaneously detect Mycobacterium tuberculosis cells and their expressed proteins in a tissue specimen slice, Thereby improving the sensitivity of pathological diagnosis of tuberculosis. The kit is easy to use, suitable for routine detection in pathology department, and has good clinical promotion value.
Owner:BEIJING CHEST HOSPITAL CAPITAL MEDICAL UNIV
A method for double staining of bovine in vitro fertilized blastocysts
InactiveCN105259007BShorten the timeGuaranteed dyeing effectPreparing sample for investigationProteinase activityUltraviolet lights
The invention discloses a double-staining method for bovine in vitro fertilized blastocysts, which comprises treating the bovine in vitro fertilized blastocysts cultivated to 7-9 days with 0.5% pronase for 55 seconds, and then washing them in PBS for 2 to 3 times. Stain in 100µg / ml PI (1%TritonX‑100 / PBS) for 20s, wash in PBS for 2-3 times, transfer to 45µg / ml Hoechst33342 for staining for 50s, wash in PBS for 2-3 times, and press. After pressing, the tablets were observed and photographed under an inverted fluorescent microscope under ultraviolet light. The nuclei of the blastocyst inner cell mass (ICM) are blue, and the nuclei of trophoblast (TE) cells are red. The staining method can be completed in 3 minutes, while the previous report required at least 47 minutes. Therefore, the time required for double staining of blastocysts is greatly reduced, and the efficiency is improved, so that the quality of bovine blastocysts can be quickly evaluated.
Owner:天津市农业科学院
Method for double staining colocalized nuclear-markers in histological lymphoid or bone marrow tissue sample
The present invention relates to kits and methods for performing dual-staining immunohistochemistry (IHC) for the detection of specific cell populations in tissue samples containing heterogeneous populations of cells, which can be observed by a light microscope for co-localization of distinct pigments. The method includes providing a tissue sample comprising fixed cells; exposing the sample to first and second ligands that recognize different marker proteins found at the same cellular location, thereby forming a ligand-labeled sample; exposing the ligand-labeled sample to first and second labeling reagents, the first labeling reagent binding to the first ligand and the second labeling reagent binding to the second ligand, the first and second labeling reagents each forming distinct pigments; and identifying the number of cells that display only one particular pigment, or more than one pigment, by the different coloration of the cellular location labeled by the distinct pigment.
Owner:CORNELL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com