Gamma-hydroxy olic acid ester derivatives and use thereof in preparation of antitumor medicines
A kind of hydroxyalkynoate, antitumor drug technology, applied in the field of biochemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
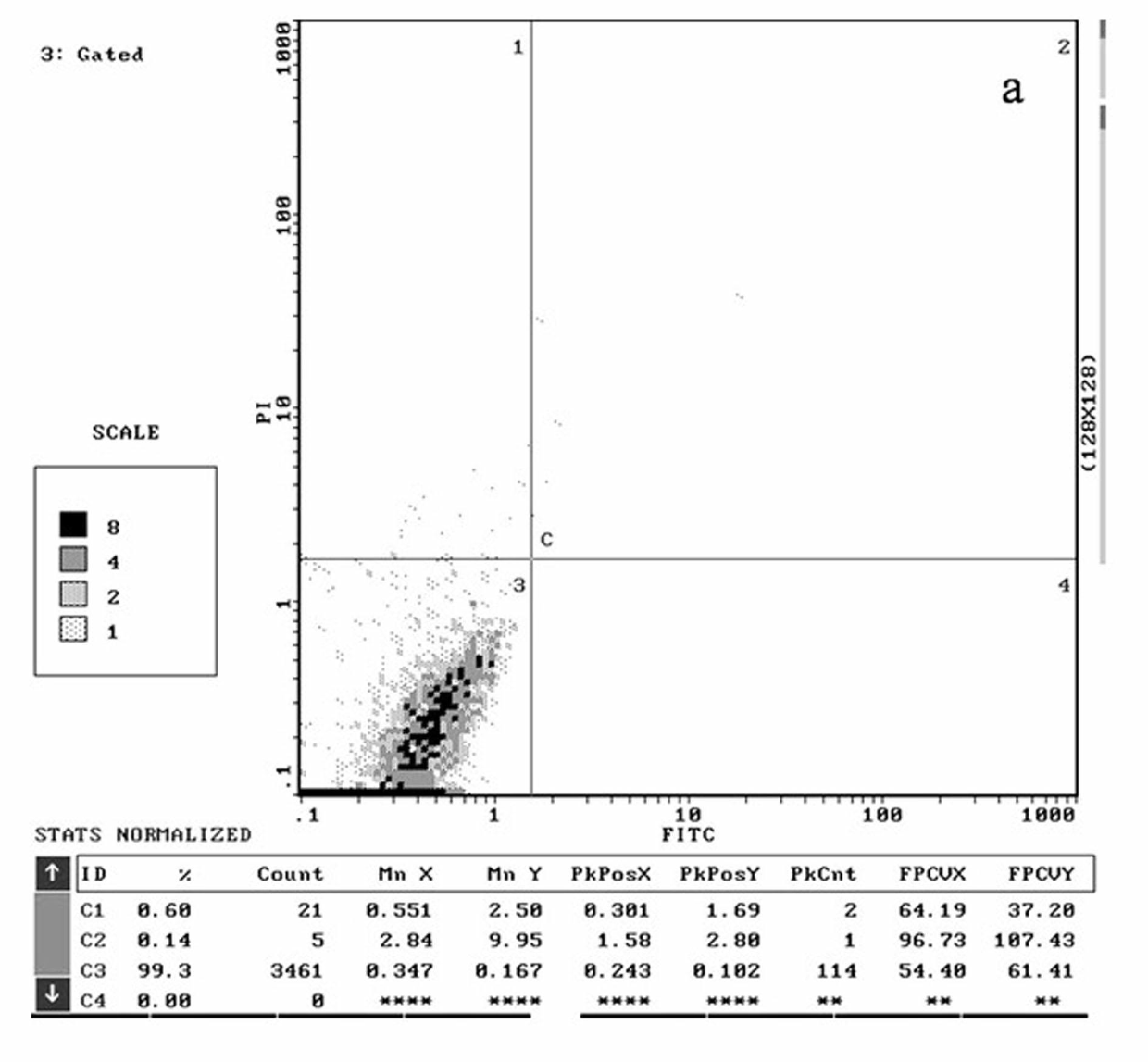
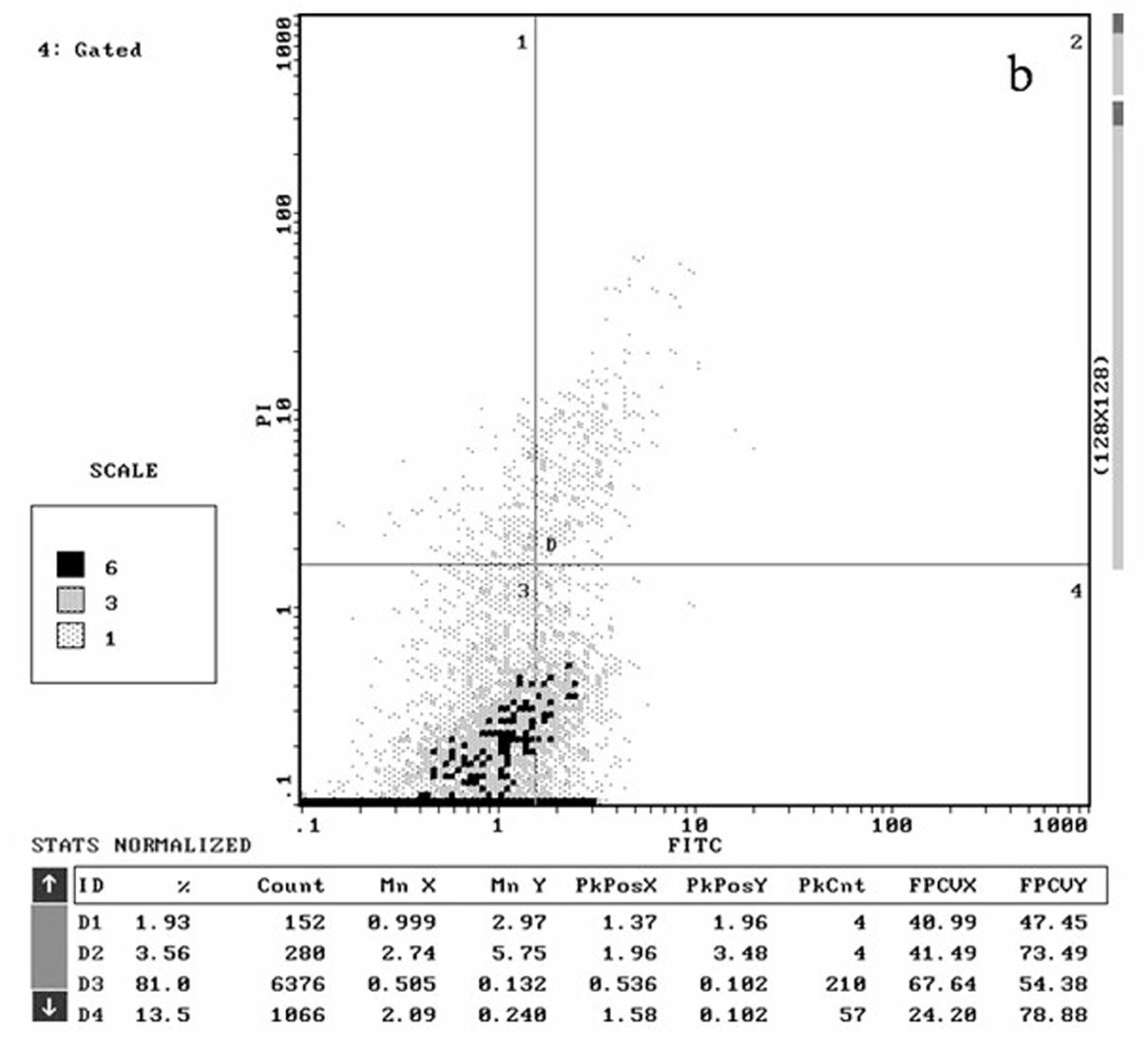
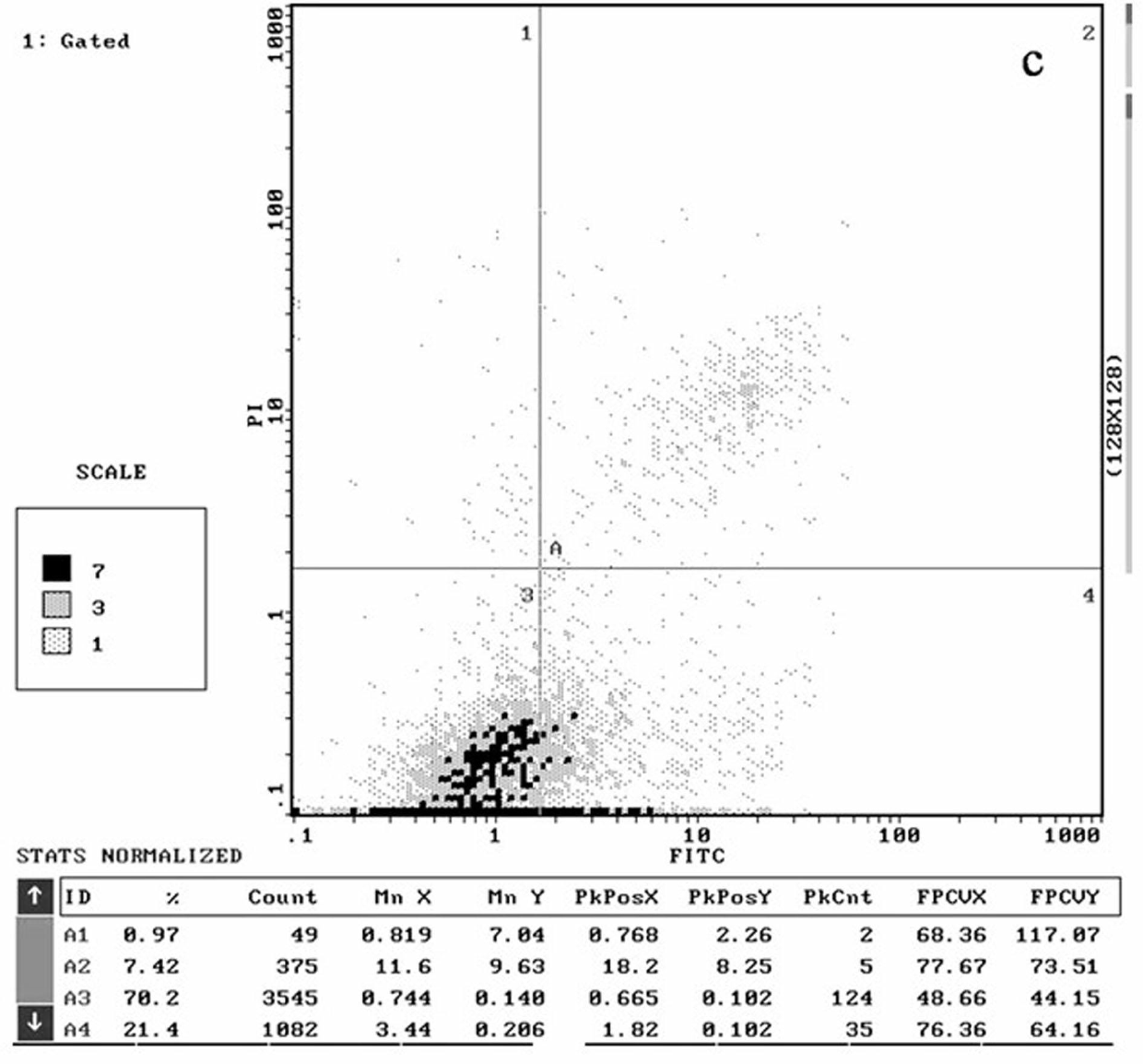
[0052] The structure, synthesis and antitumor activity of the γ-hydroxyalkynoate derivatives of the present invention will be described in detail below through specific examples.
[0053] In Examples 1 to 43, the structure of the gamma-hydroxy alkynoate derivative is as follows:
[0054]
[0055] Its synthetic method can select the synthetic method of aforementioned chiral (R)-γ-hydroxy alkynoate and the synthetic method of chiral (S)-γ-hydroxy alkynoate according to different configurations, and according to different compounds, in Pay attention to the selection of corresponding aldehydes during the synthesis process. In Examples 1-43, the configuration, characterization and other characteristics of the γ-hydroxyalkynoate derivatives are shown in Table 1.
[0056] Table 1.
[0057]
[0058]
[0059]
[0060]
[0061]
[0062]
[0063]
[0064]
[0065]
[0066] In Examples 44 to 60, the structures of gamma-hydroxy alkynoate derivatives are as f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com