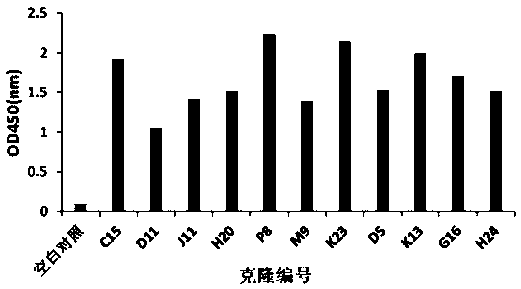
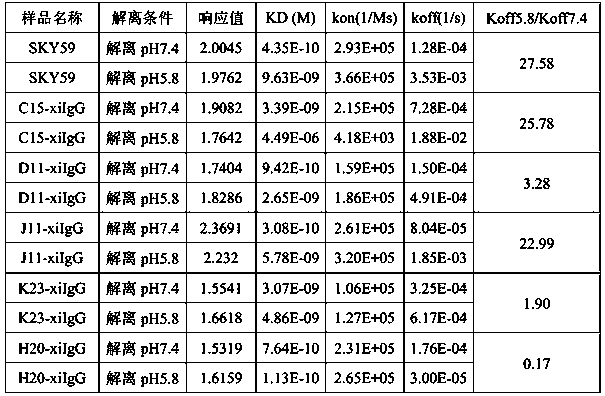
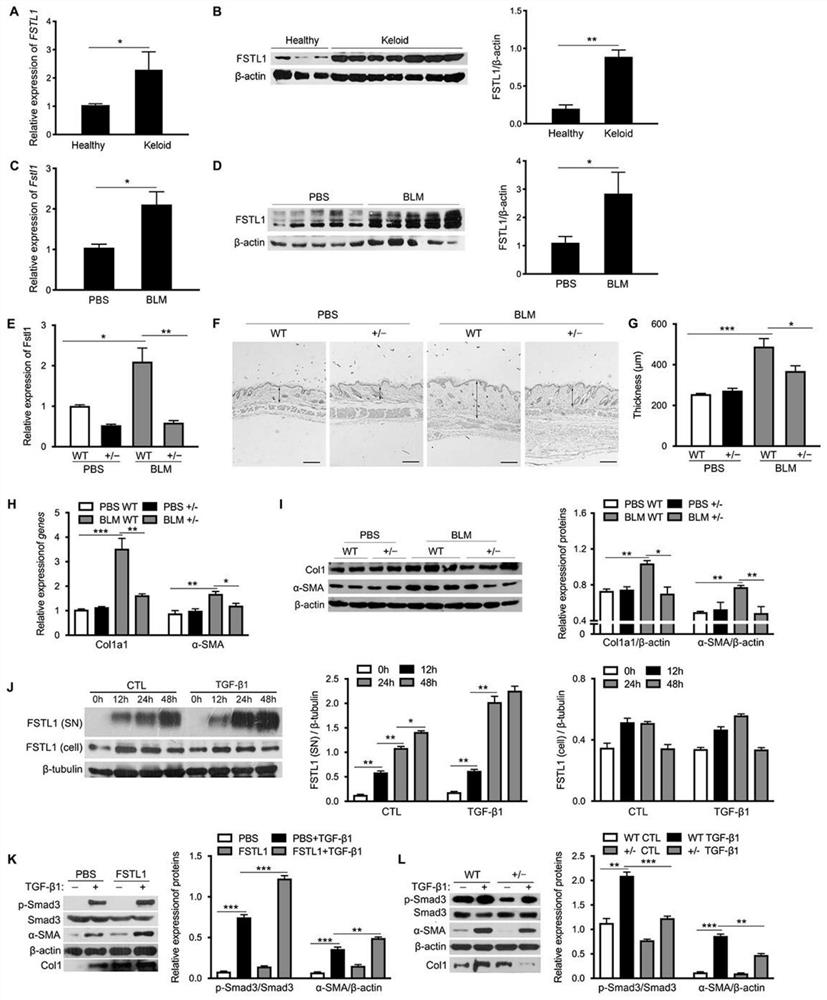
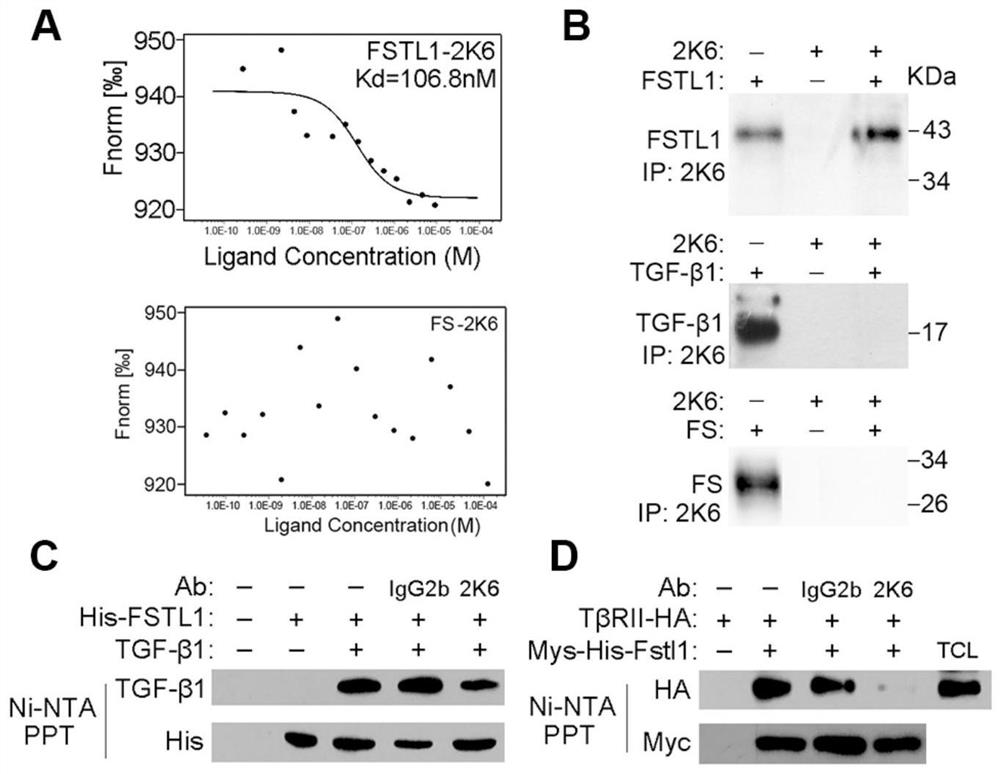
Patents
Literature
87 results about "Treatment and control groups" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
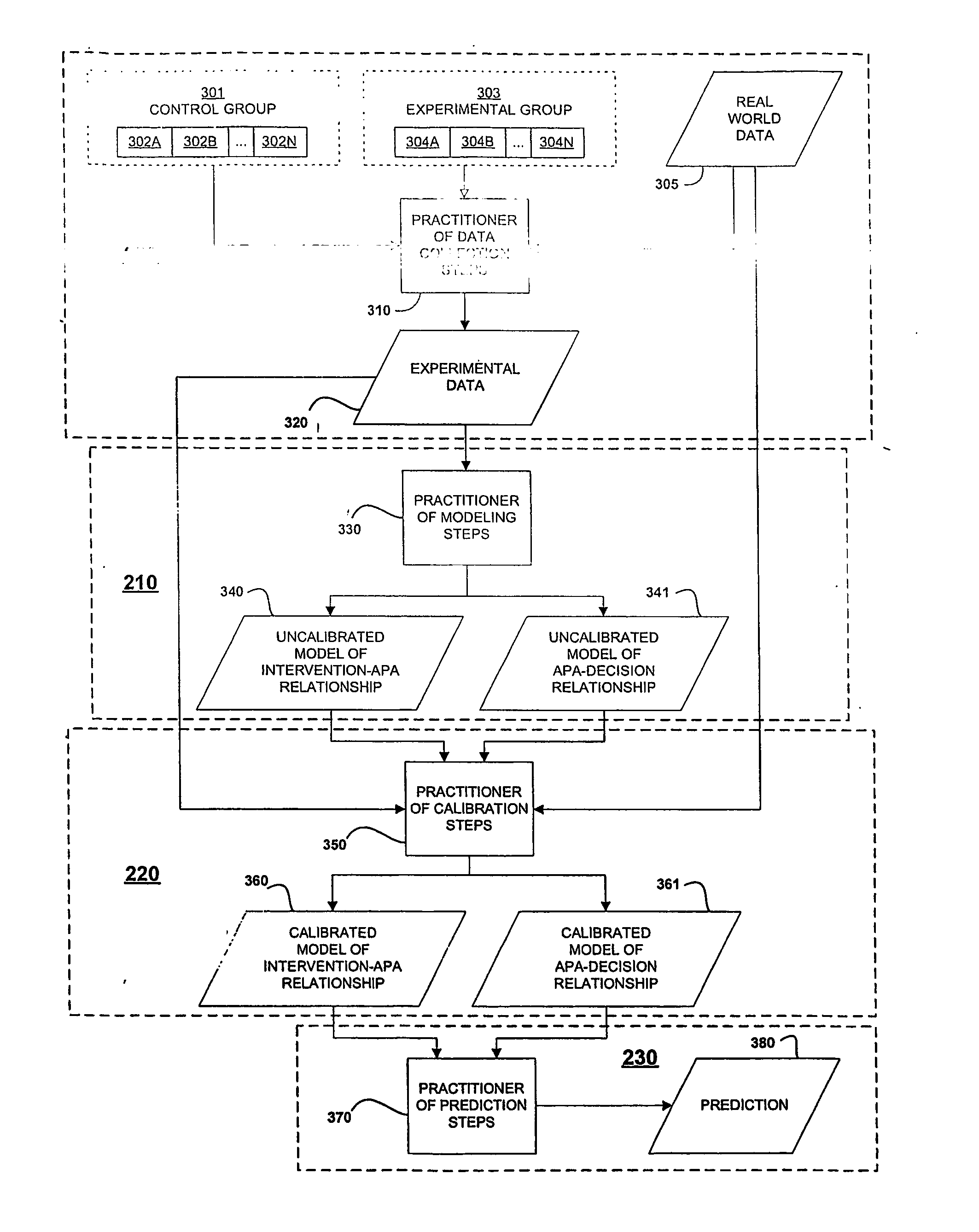
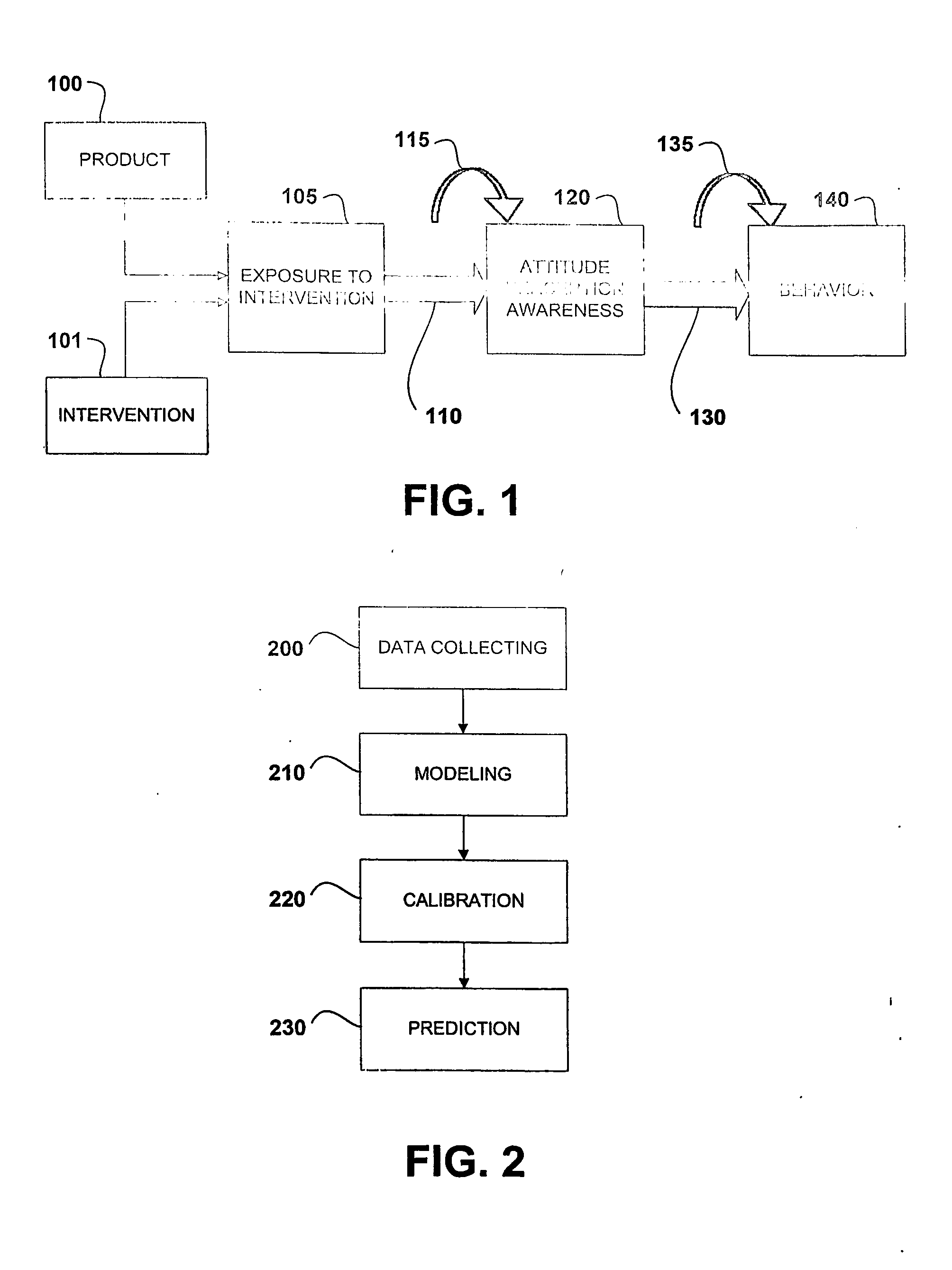
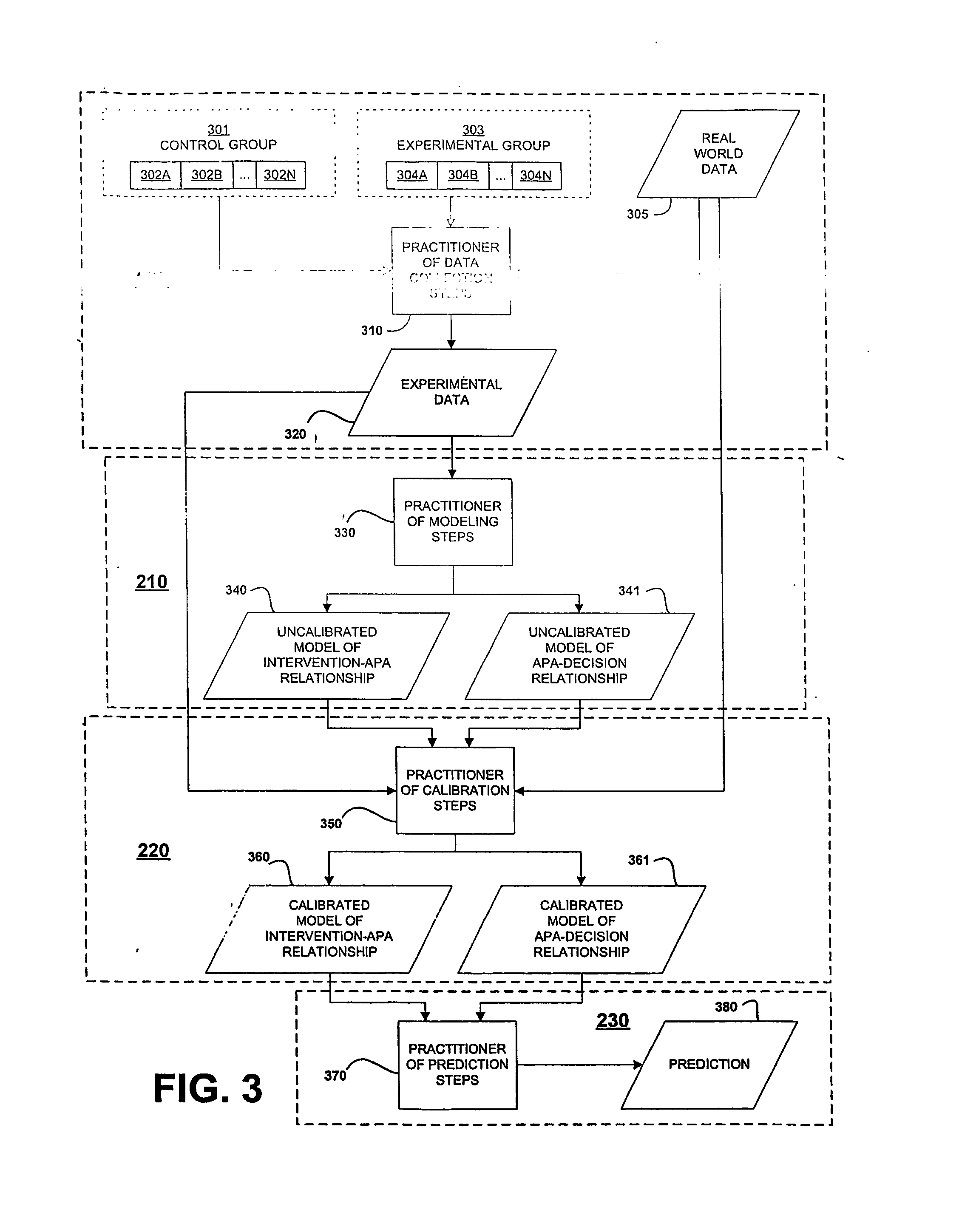
In the design of experiments, treatments are applied to experimental units in a treatment group. In comparative experiments, members of a control group receive a standard treatment, a placebo, or no treatment at all. There may be more than one treatment group, more than one control group, or both.
Risk environment modeling for predicting decisions
Owner:R SQUARED ANALYTICS
Systems and methods for performing computer-simulated evaluation of treatments on a target population
ActiveUS10991465B2Medical simulationPathological referencesSpecific modelTreatment and control groups
Owner:HEARTFLOW
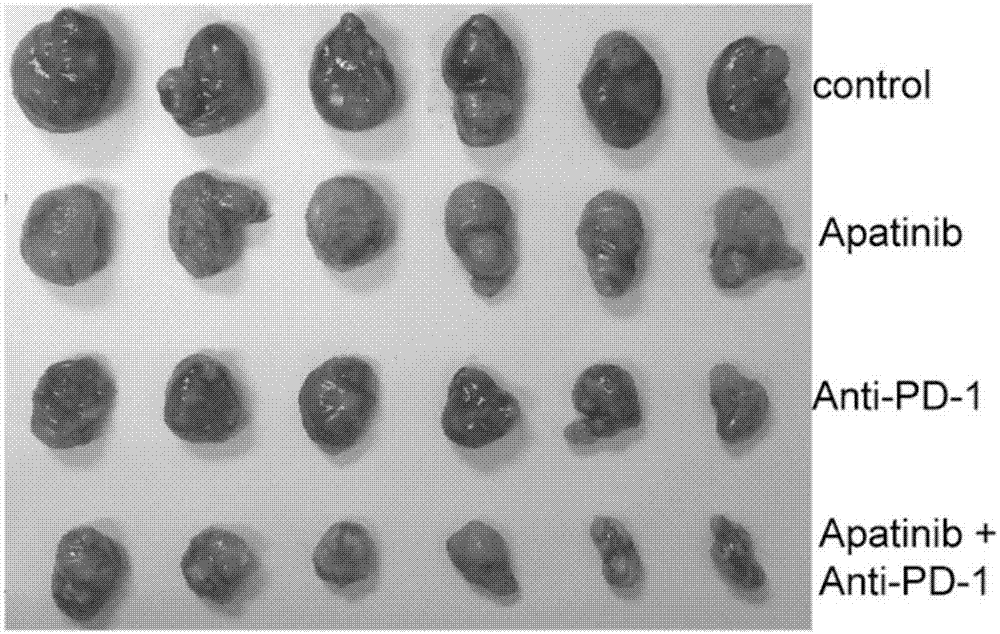
Application of apatinib and anti-PD-1 antibody combination to preparation of colon cancer medicines
InactiveCN106963948AHigh tumor inhibition rateIncreased area of necrosisOrganic active ingredientsAntibody ingredientsWilms' tumorCancer research
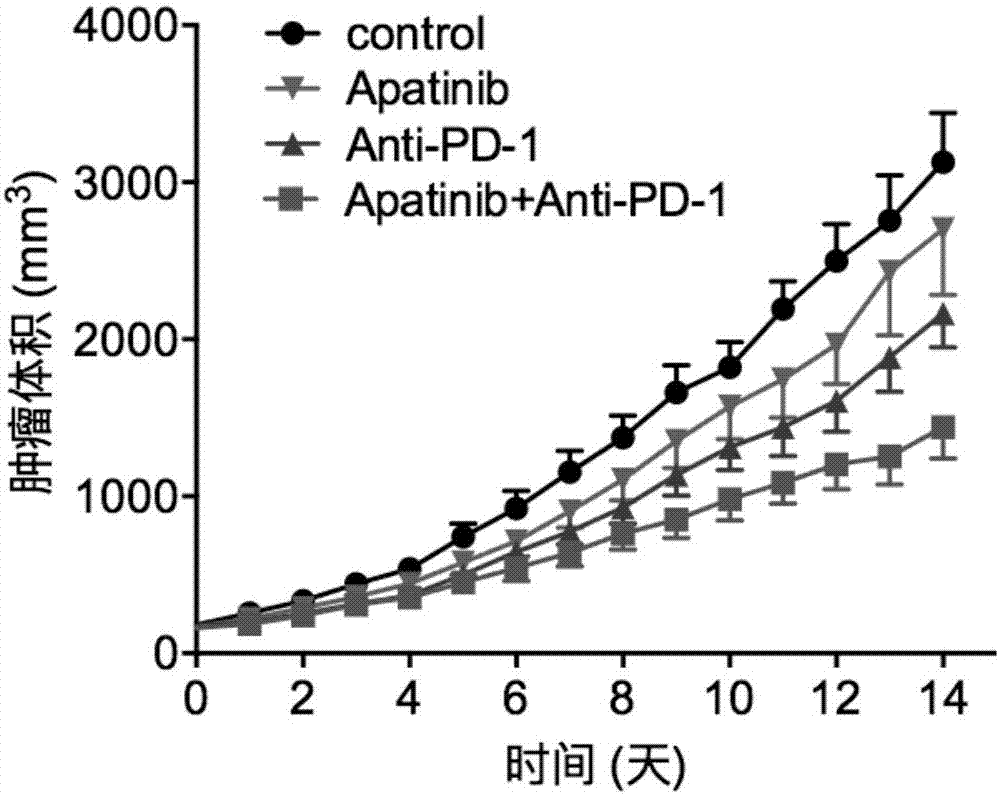
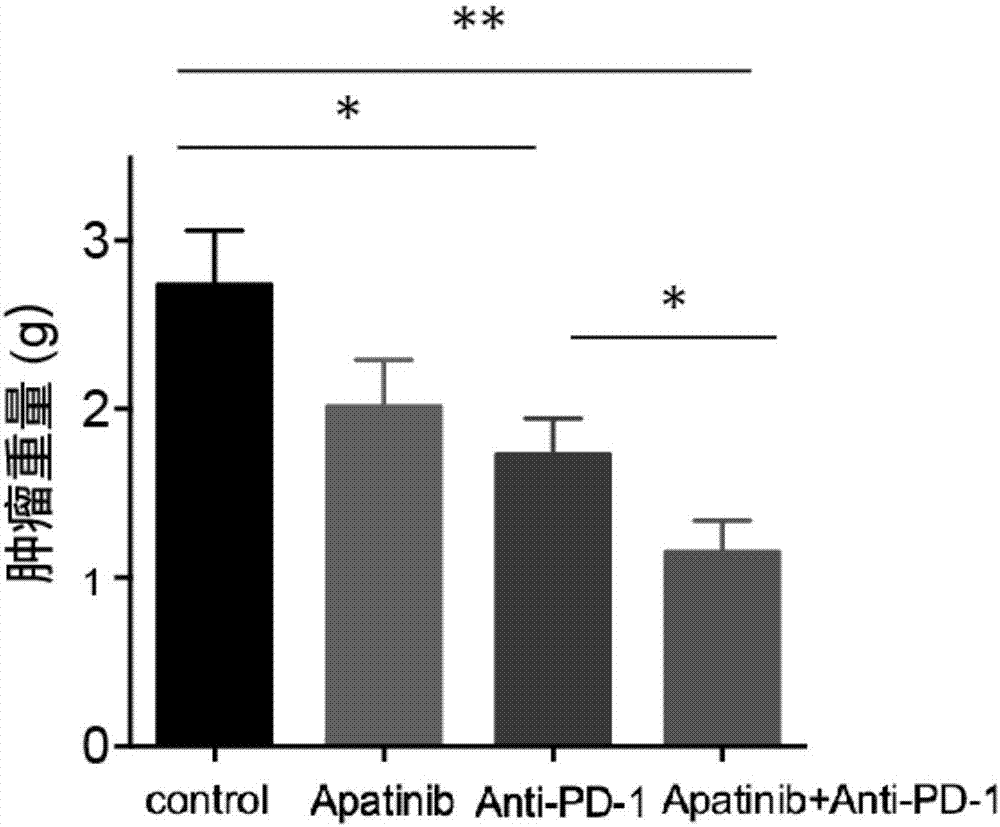
The invention discloses application of apatinib and anti-PD-1 antibody combination to preparation of colon cancer medicines. The colon cancer is treated through combined medication of apatinib and an anti-PD-1 antibody; through experiments, the tumor inhibition rate of apatinib and anti-PD-1 antibody combination is 53.97 percent, the tumor inhibition rate is increased by 40.4 percent as compared with that of a single apatinib group (53.97 percent vs 13.57 percent, P is less than 0.05, Mann-Whitneytest), and the tumor inhibition rate is increased by 23.17 percent as compared with that of a single anti-PD-1 antibody group (53.97 percent vs 13.57 percent, P is less than 0.05, Mann-Whitneytest) (the tumor inhibition rate is equal to 1-expreiment group tumor volume / control group tumor volume). After the apatinib and the anti-PD-1 antibody are combined, the tumor necrosis area can be obviously increased as compared with that of single groups.
Owner:顾艳宏
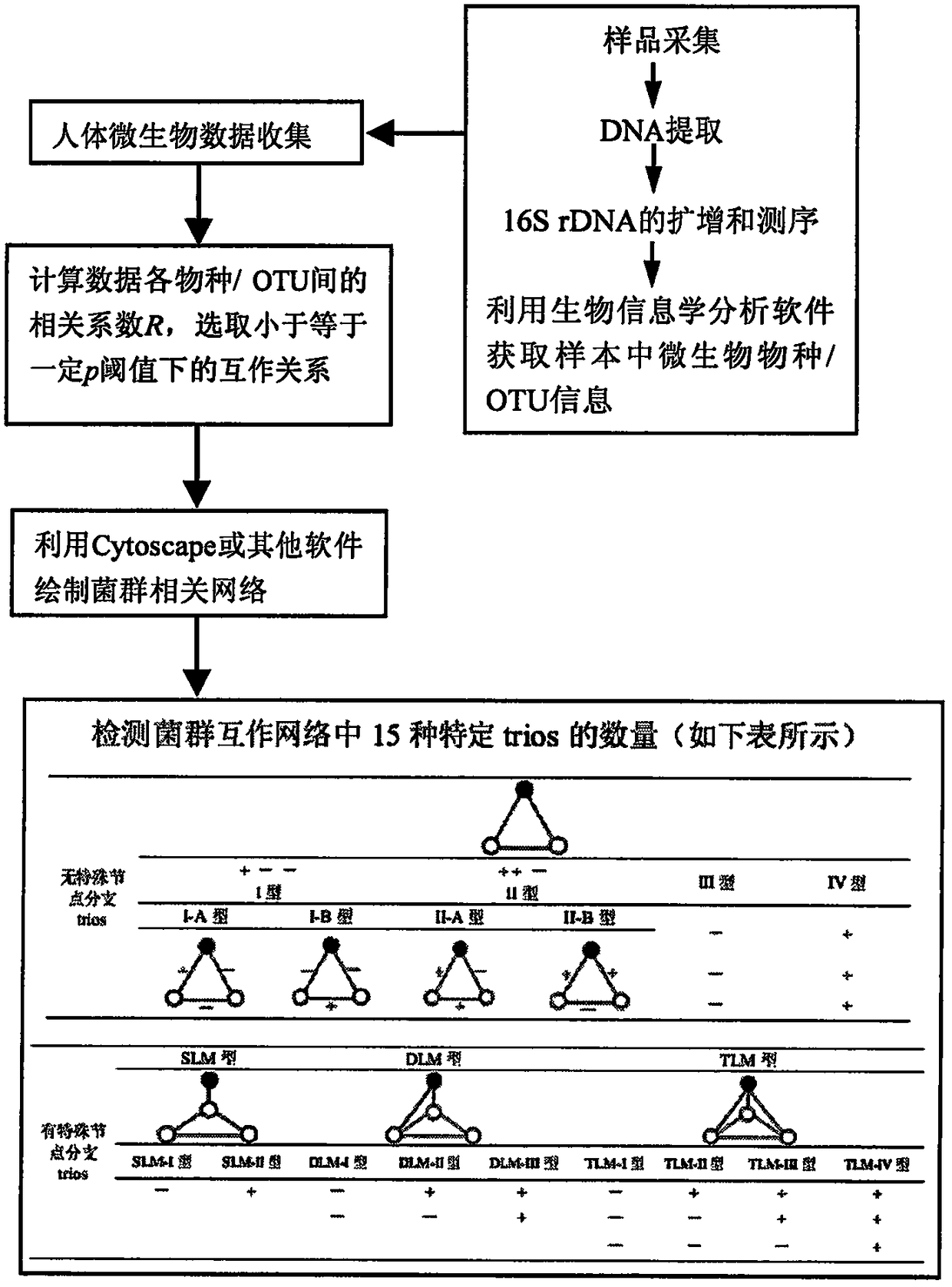
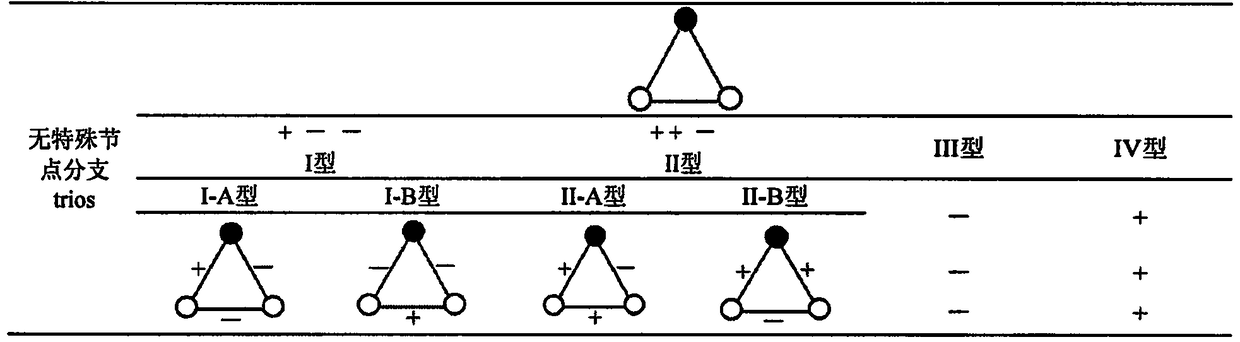
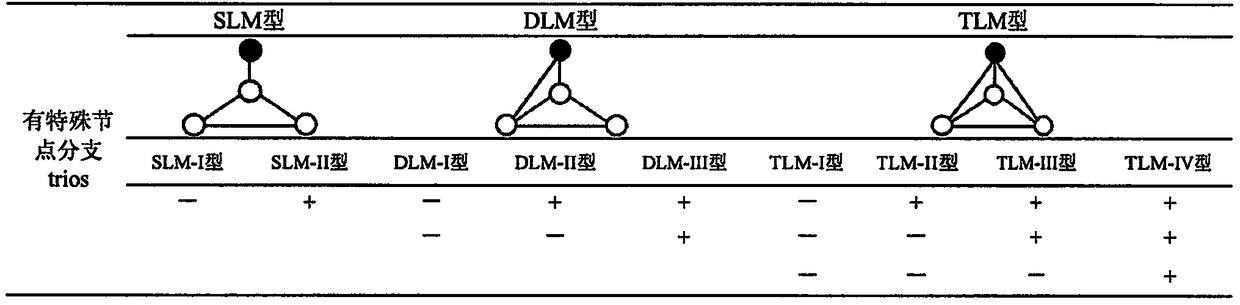
Method for humanbody health assessment anddiseasediagnosis based on human body flora interaction network analysis
Owner:KUNMING INST OF ZOOLOGY CHINESE ACAD OF SCI
Experimental method based on tensile stress lasting time serving as variable, and impact experiment device
InactiveCN105954121ASame collision speedThe same collision speed is the same, and the efficiency of the experiment is improved at the same timeMaterial strength using single impulsive forceExperimental methodsTotal thickness
The invention provides an experimental method based on the tensile stress lasting time serving as a variable, and relates to the field of impact dynamics. The experimental method includes S1, producing a plurality of flying sheets; S2, producing a plurality of sample targets, wherein each sample target comprises a first sample layer and a second sample layer, the first sample layers are thinner than the second sample layers, the total thicknesses and textures of the sample targets are the same, and the thicknesses of the first sample layers of the target samples are different; S3, corresponding each flying sheet to the sample targets to form an impact experiment group, wherein the sample targets in each impact experiment group are arranged adjacently; S4, performing impact experiments on the impact experiment groups. The experimental method has the advantages that the tensile stress lasting time is taken as the single variable successfully, intermediate experiment information and data in the same damage evolution path are acquired, and the influence of taking the tensile stress lasting time as the single variable on the slabbing phenomenon is predicted accurately.
Owner:INST OF FLUID PHYSICS CHINA ACAD OF ENG PHYSICS
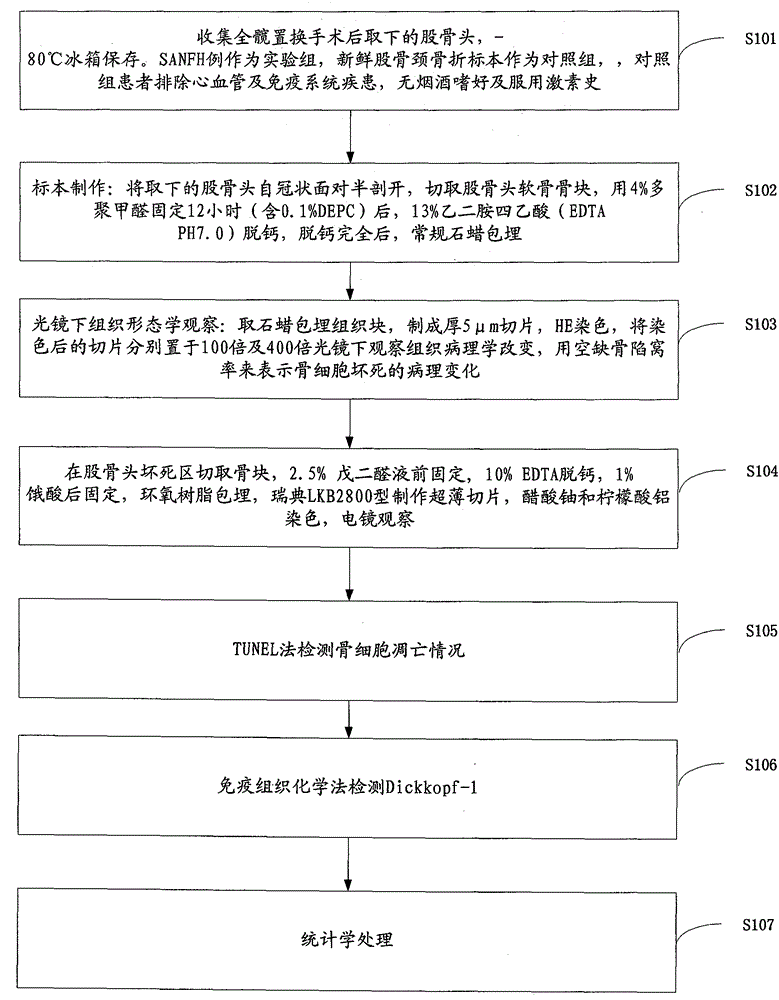
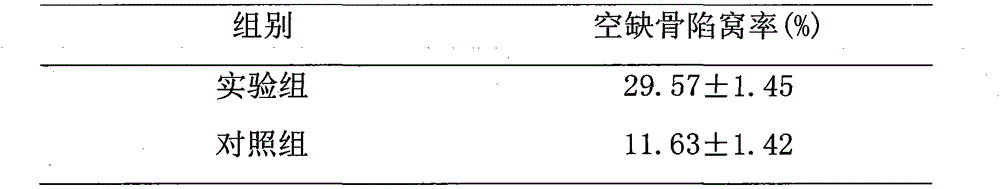
Method for researching effects of Dickkopf-1 and cell apoptosis in steroid-induced avascular necrosis of femoral head (SANFH)
InactiveCN105043981AMaterial analysis by optical meansMaterial analysis by transmitting radiationStaining techniqueRight femoral head
The invention discloses a method for researching the effects of Dickkopf-1 and cell apoptosis in steroid-induced avascular necrosis of femoral head (SANFH). The method comprises the following steps: by taking the femoral head sample of SANFH taken through total hip replacement as an experiment group and taking the sample after the hip replacement for fracture of neck of femur as a contrast group, cutting the femoral head sample, fixing and decalcifying the cut femoral head sample to prepare slices, observing the morphologic change of osteocytes by an electronic microscope, observing the pathological change of the osteocytes by a light microscope, counting the vacant bone lacuna, detecting the expression of Dickkopf-1 by an immunohistochemical staining technique, thus exploring the effects of Dickkopf-1 and cell apoptosis in SANFH. The method can be used for providing assistance for early diagnosis and curing of SANFH and also providing a theoretical basis for clinical prevention and curing of SANFH in future.
Owner:刘万林
Method for evaluating efficacy of anti-tumor drug at cellular level
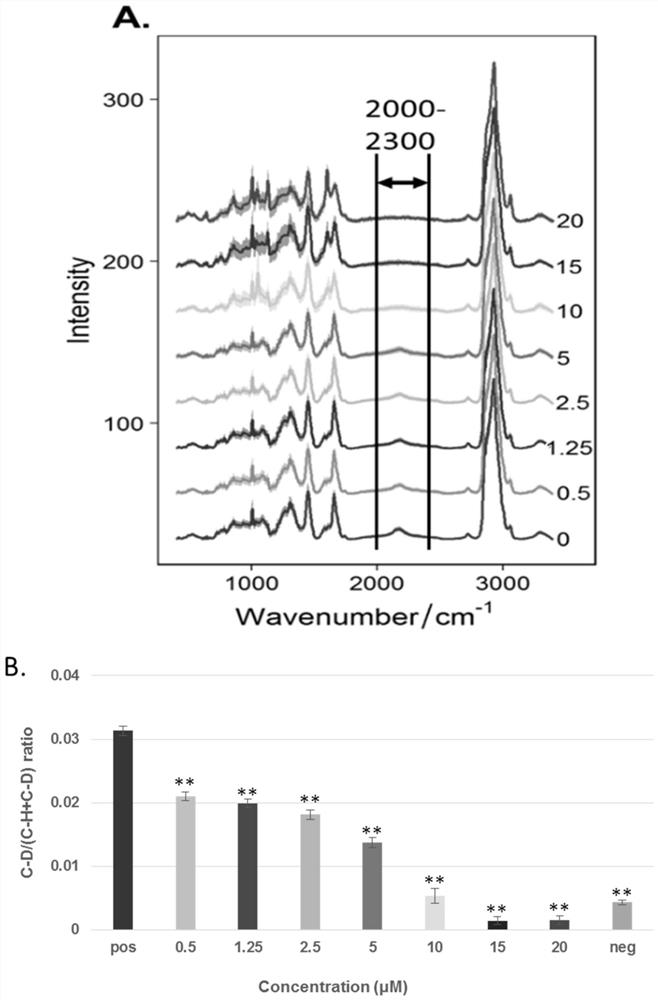
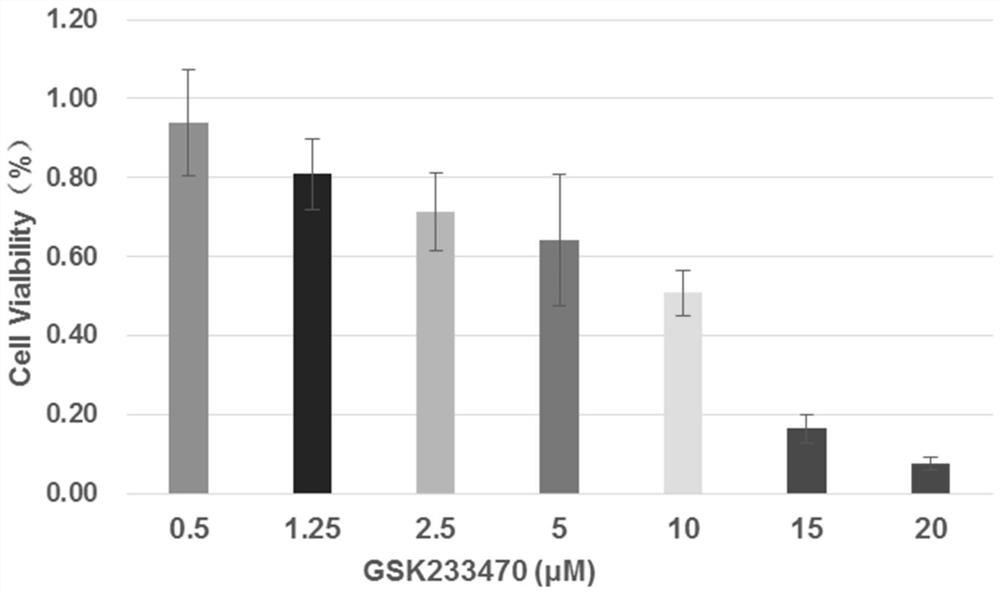
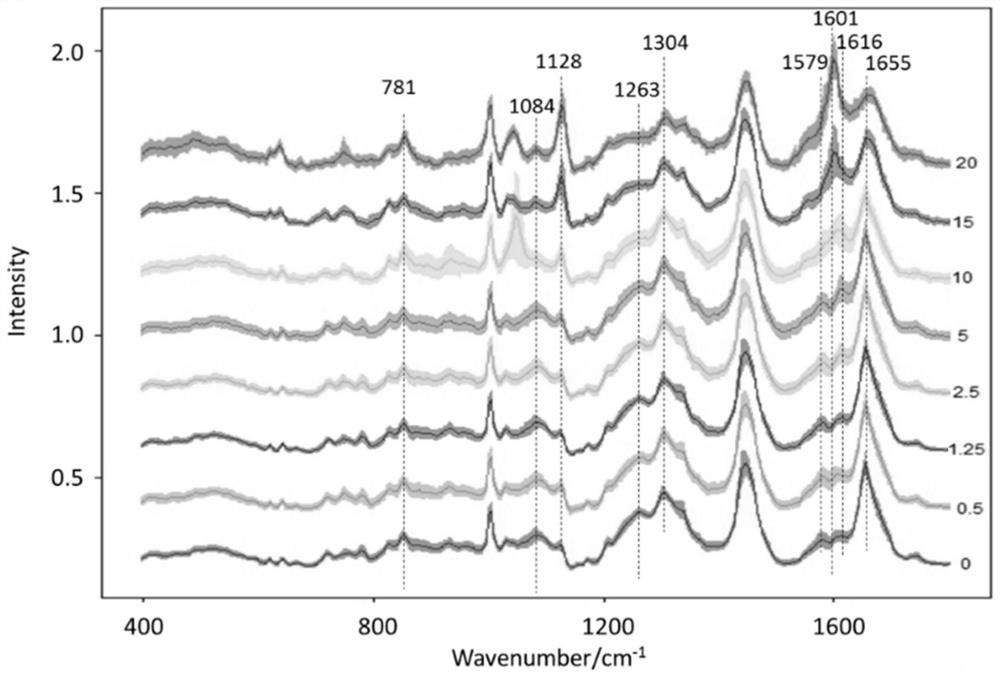
PendingCN111912826AAccurate assessmentMicrobiological testing/measurementRaman scatteringEfficacyTreatment and control groups
The invention relates to a method for evaluating the efficacy of an anti-tumor drug at a cellular level. The method comprises the following steps of setting anti-tumor drug experiment groups and control groups with different concentrations, taking a group with the anti-tumor drug concentration of 0 and added with heavy water as a positive control group (pos), and taking a group with the anti-tumordrug concentration of 0 and not added with heavy water as a negative control group (neg); digesting the tumor cells cultured in the experimental group and the control group, centrifugally cleaning and dropwise adding to a low-Raman background chip for Raman detection, calculating the CD / (CD + CH) of the experimental group and the control group according to the obtained Raman spectra, and determining whether the anti-tumor drug to be detected is effective to the tumor cells or not according to the CD / (CD + CH) of the experimental group and the control group. Compared with the prior art, the method has the advantages that the effectiveness of the anti-tumor medicine is evaluated from the cell metabolism level, compared with a traditional method for evaluating the effectiveness of the medicine by calculating the number of cells, the anti-tumor medicine which only inhibits cell growth but does not inhibit cell metabolism can be evaluated more accurately, and then medication is guided.
Owner:上海氘峰医疗科技有限公司
Method for extracting exosome derived from human hair follicle dermal papilla cells
PendingCN109852578AHelp regulate growthHelp regulate developmentMicrobiological testing/measurementArtificial cell constructsGrowth phaseCD63
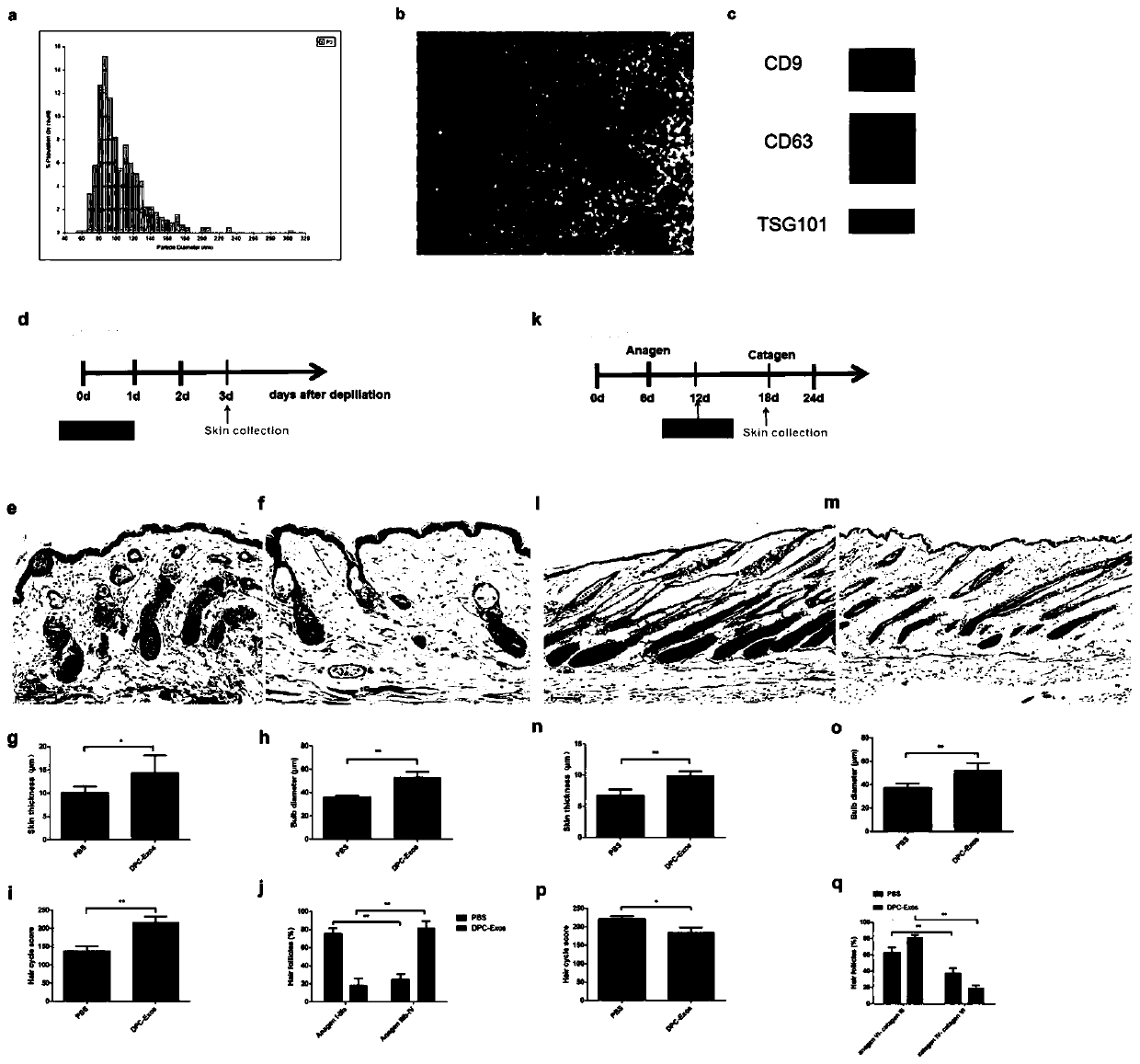
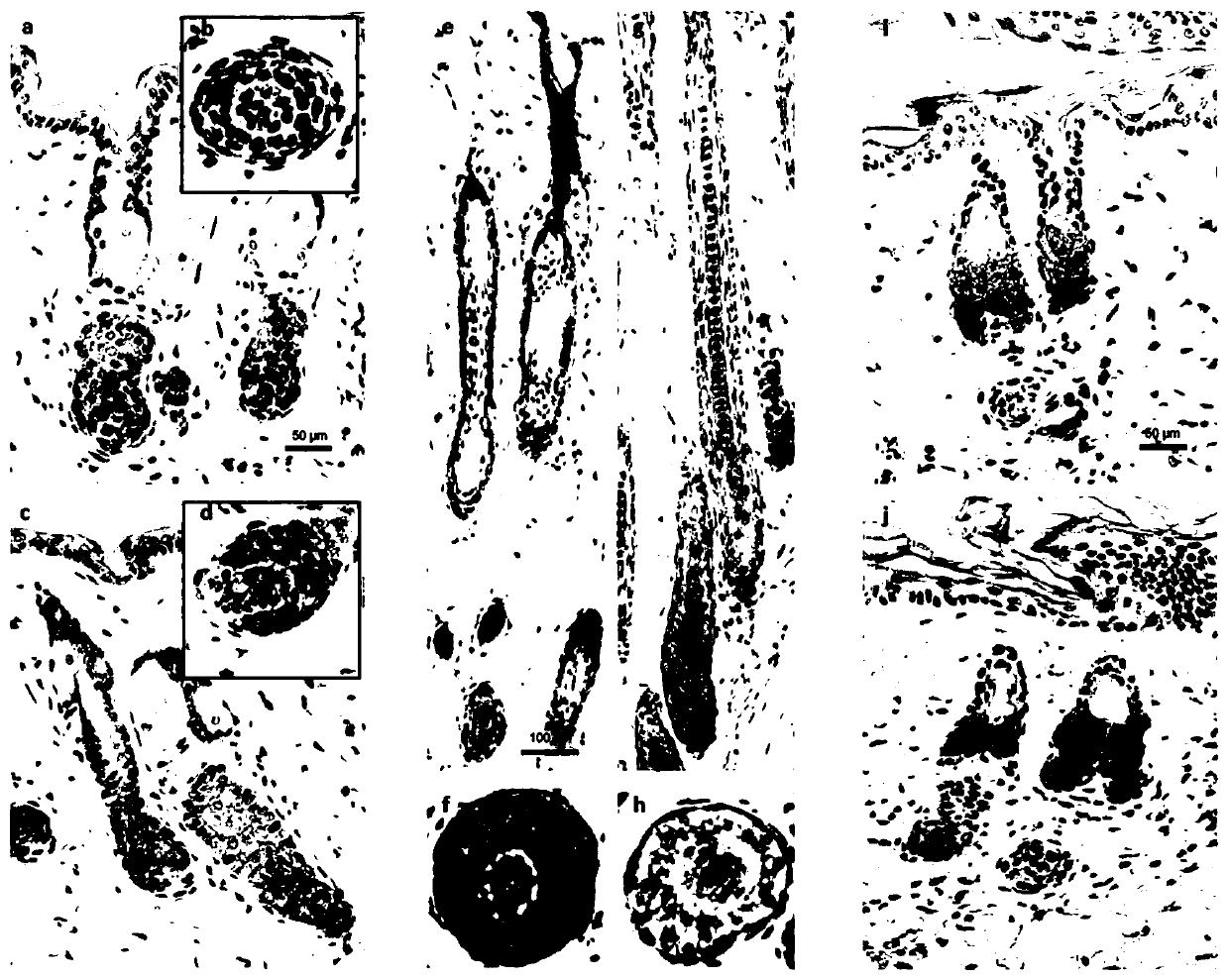
The invention belongs to the technical field of cell secretion extraction, and discloses a method for extracting exosome derived from human hair follicle dermal papilla cells (DPC-Exos). The method isas below: identifying DPC-Exos by controlled resistance pulse sensing (TRPS) analysis, electron microscopy and Western Blot; and adding the DPC-Exos to a culture medium of human hair follicle outer root sheath cells (ORSCs) cultured in vitro. The DPC-Exos are identified to be about 105 nm in diameter and expressing tumor susceptibility gene (TSG) 101, differentiation cluster (CD) 9 and CD63; DPC-Exos promote proliferation and migration of human ORSCs, and mRNA and protein levels of beta-catenin and Sonichedgehog (Shh) in ORSCs are detected to be up-regulated after treatment; transdermal injection of DPC-Exos accelerates an HF growth phase of mice and delays the occurrence of regression. Immunohistochemical analysis shows that the expression levels of beta-catenin and Shh are up-regulatedin the hair follicles of an experimental group. DPC-Exos help regulate the growth and growth cycle of the HF and provide a new potential for the treatment of clinical hair loss.
Owner:ZHEJIANG UNIV
Treating method for viscous fluid specimen
InactiveCN105506085AShorten the liquefaction timeReduce viscosityMicrobiological testing/measurementPreparing sample for investigationSpecimen HandlingViscosity
The invention discloses a treating method for a viscous fluid specimen, and relates to the technical field of biochemistry. The method includes the steps that the viscous fluid specimen is added into pre-prepared treating agents with the volume equal to that of the viscous fluid specimen, and repetition is carried out to obtain experimental groups; the experimental groups are treated according to the detected type of the viscous fluid specimen to obtain viscous fluid specimen treating fluids, and then the viscous fluid specimen is treated. By means of the method, the liquefaction time of the specimen is significantly shortened, the viscosity of the specimen is reduced, and the diagnosis precision is not affected; the method can be flexibly applied according to diagnosis purposes; meanwhile, medical cost is not increased.
Owner:北京澳利文生物科技有限公司
Analysis method for eliminating exogenous components in metabonomics data processing
The invention provides an analysis method for eliminating exogenous component data from massive metabonomics data. The method is characterized in that experiment groups are combined and are divided into two groups; one group comprises all groups which do not supply medicines, namely generally comprises a blank group and a model group, and the other groups only comprise groups which supply medicines; the groups are processed by a multi-variable counting method; marks which only exist in the groups which supply the medicines and do not exist in other groups and serve as exogenous components, and then the data of the exogenous components are eliminated; and after the data are updated, the next mode identification analysis is executed. By the method, the interference of the exogenous component data can be easily, quickly and completely eliminated, so that the integrated change of endogenous metabonomics capable of reflecting the whole biological effect of the medicines (in particular traditional Chinese medicines) can be really displayed and scientifically evaluated.
Owner:刘树民
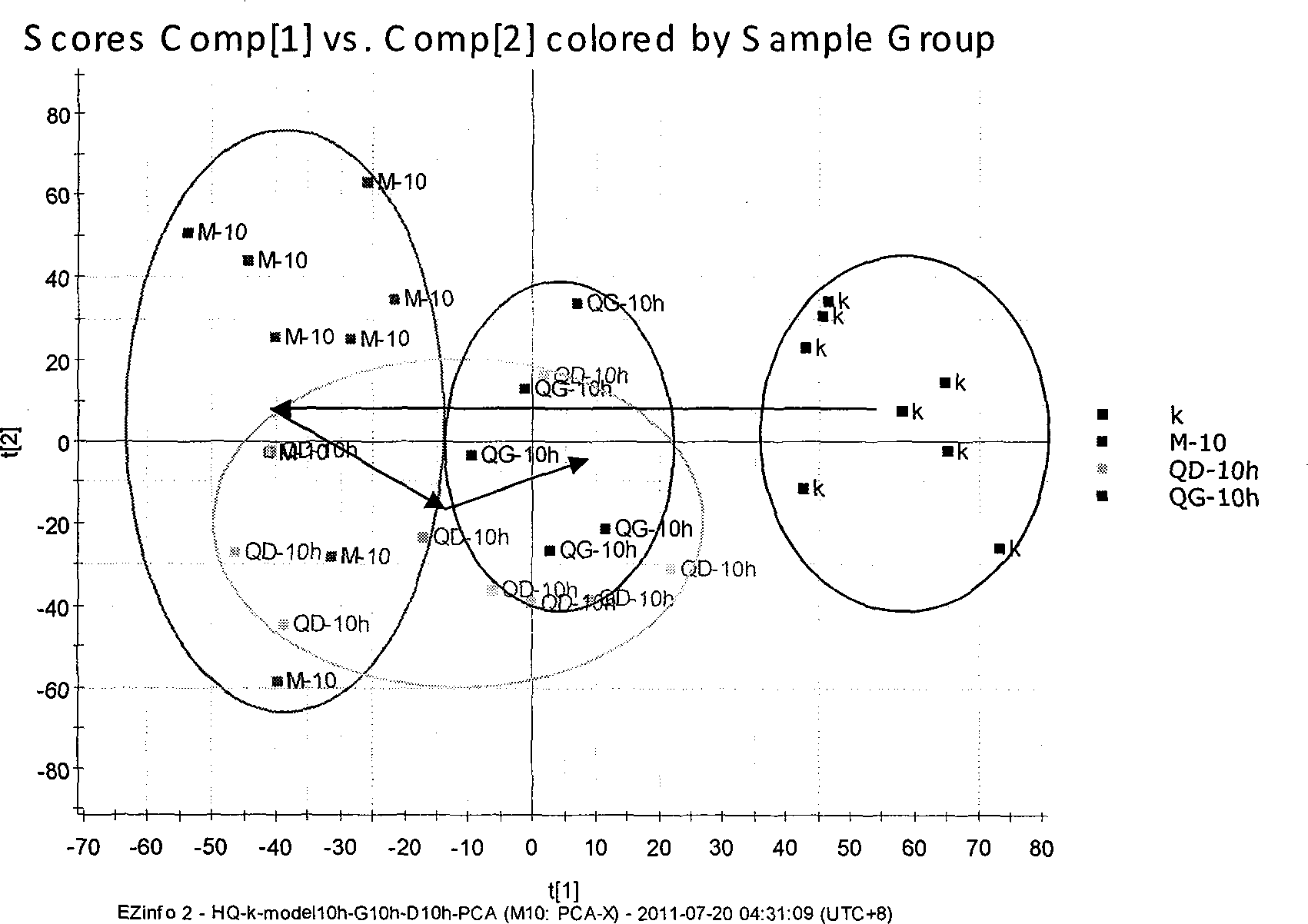
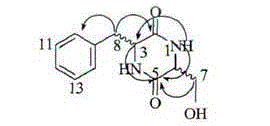
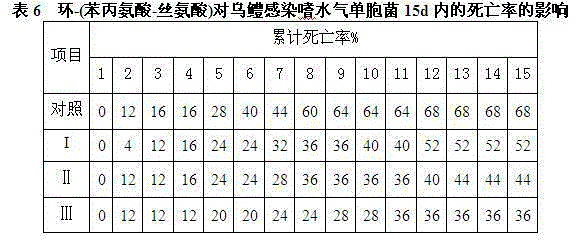
Disease-resistant immunoenhancement cyclo-(phenylalanine-serine) for channa argus
InactiveCN105076861AImprove balanceGrow fastFood processingClimate change adaptationDiseaseMetabolite
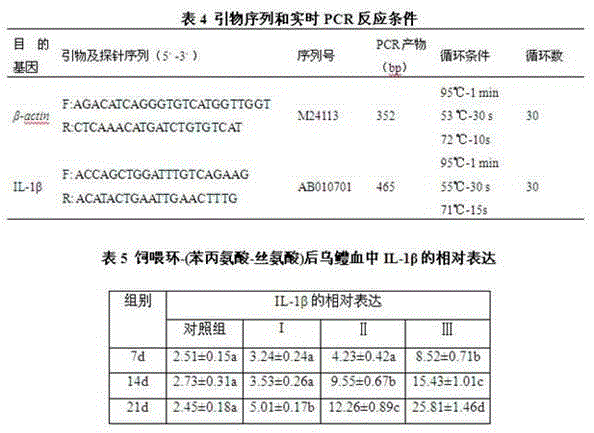
The invention discloses a disease-resistant immunoenhancement cyclo-(phenylalanine-serine) for channa argus, provides a composite feed for channa argus, in which pseudomonas putida metabolin cyclo-(phenylalanine-serine) is added so as to improve the disease-resistant ability of the channa argus and promote growth. After the channa argus is fed with the feed, through immunological detection, the serum lysozyme activity, serum phagocytosis ability and serum bactericidal ability of channa argus in an experimental group are obviously improved respectively up to 78.26U / mL, 63.52U / mL and 75.69U / mL, which are higher than those of a control group (respectively 17.23U / mL, 20.96U / mL and 39.36U / mL). Relative expression of IL-1beta gene of channa argus is up-regulated to 25.81, while relative expression of IL-1beta gene of channa argus in the control group is only 2.45; and by injecting medial lethal concentration of bacteria into fish belly to detect the disease resistance, the accumulative death rate of the experimental group after 15 days is 36% minimally, while that of the control group is 68%, and the survival rate of the experimental group is higher than that of the control group obviously. A comprehensive breeding method provided by the invention, matched with cultivation, makes full use of a water body, and an immunoenhancement is added, thus improving immunity and promoting growth, and enhancing economic benefits.
Owner:JILIN AGRICULTURAL UNIV
Method for screening cotton drought-resistant related genes
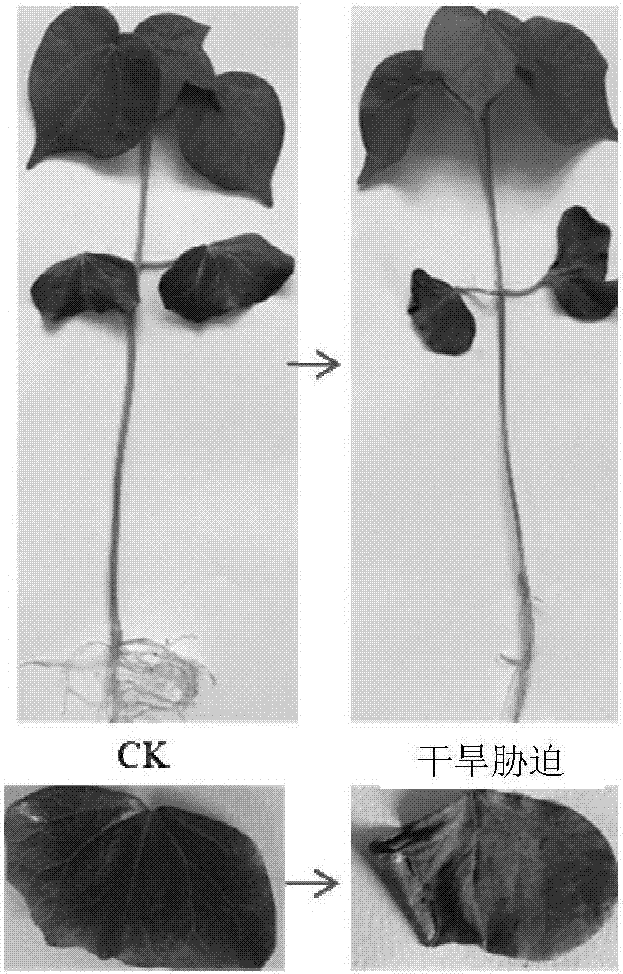
The invention discloses a method for screening cotton drought-resistant related genes. The method comprises the steps that 1, an experimental group for conducting drought stress treatment on cotton to be detected and a control group for conducting no drought stress treatment on the cotton to be detected are arranged; 2, the methylation levels and / or modes of genomes DNA of the cotton to be detected in the experimental group and the control group are detected and compared, and genes different in methylation level and / or mode of genome are found in the two groups and are the cotton drought-resistant related genes or serve as cotton drought-resistant related gene candidates. The methylation level and / or mode change differences of upland cotton seedlings under drought stress are analyzed by utilizing an MSAP technology, the methylation levels and / or mode changes of CCGG locus cytosine before and after stress are analyzed, the drought-resistant related genes are searched by utilizing methylated difference fragments, and a basis can be provided for cotton drought-resistant epigenetic regulation mechanism research and drought-resistant related gene screening.
Owner:INST OF COTTON RES CHINESE ACAD OF AGRI SCI
Metabolomic analysis method of safflower against scleroderma based on liquid chromatography-mass spectrometry
ActiveCN108508123BHigh detection sensitivityExpand the scope of detectionComponent separationMetaboliteLiquid chromatography mass spectroscopy
The invention discloses a safflower scleroderma-resisting metabonomics analysis method based on liquid chromatography-tandem mass spectrometry. The safflower scleroderma-resisting metabonomics analysis method comprises the following steps: 1) dividing healthy mice into three experimental groups including a control group, a scleroderma model group and a safflower treatment group; subcutaneously injecting a bleomycin solution into the mice of the scleroderma model group and the safflower treatment group every day to replicate a mouse scleroderma model; feeding safflower water decoction into themice of the safflower treatment group every day and treating; modeling for 28 days and judging whether modeling succeeds or not by physiological changes and pathological changes; 2) after the modelingsucceeds, taking blood serum samples of the mice of the three experimental groups and detecting by adopting the liquid chromatography-tandem mass spectrometry to obtain a fingerprint spectrum of theblood serum samples of the mice of the three experimental groups; 3) analyzing the fingerprint spectrum by adopting multivariate statistics to obtain a differential metabolite; identifying to obtain abiomarker. According to the method disclosed by the invention, the biomarker of safflower scleroderma-resisting metabonomics is obtained and a safflower scleroderma-resisting multi-component and multi-target acting mechanism is analyzed and identified.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGXI MEDICAL UNIV
Proteome mass spectrometric data processing method and device
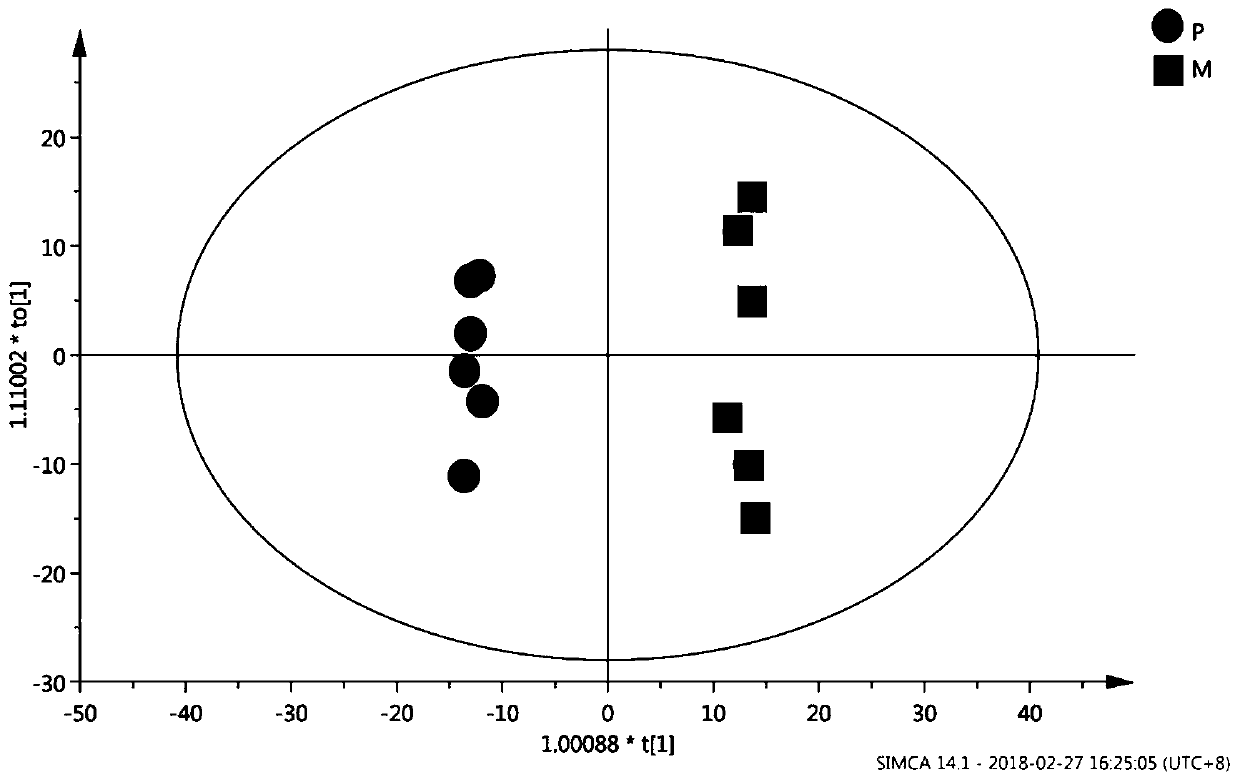
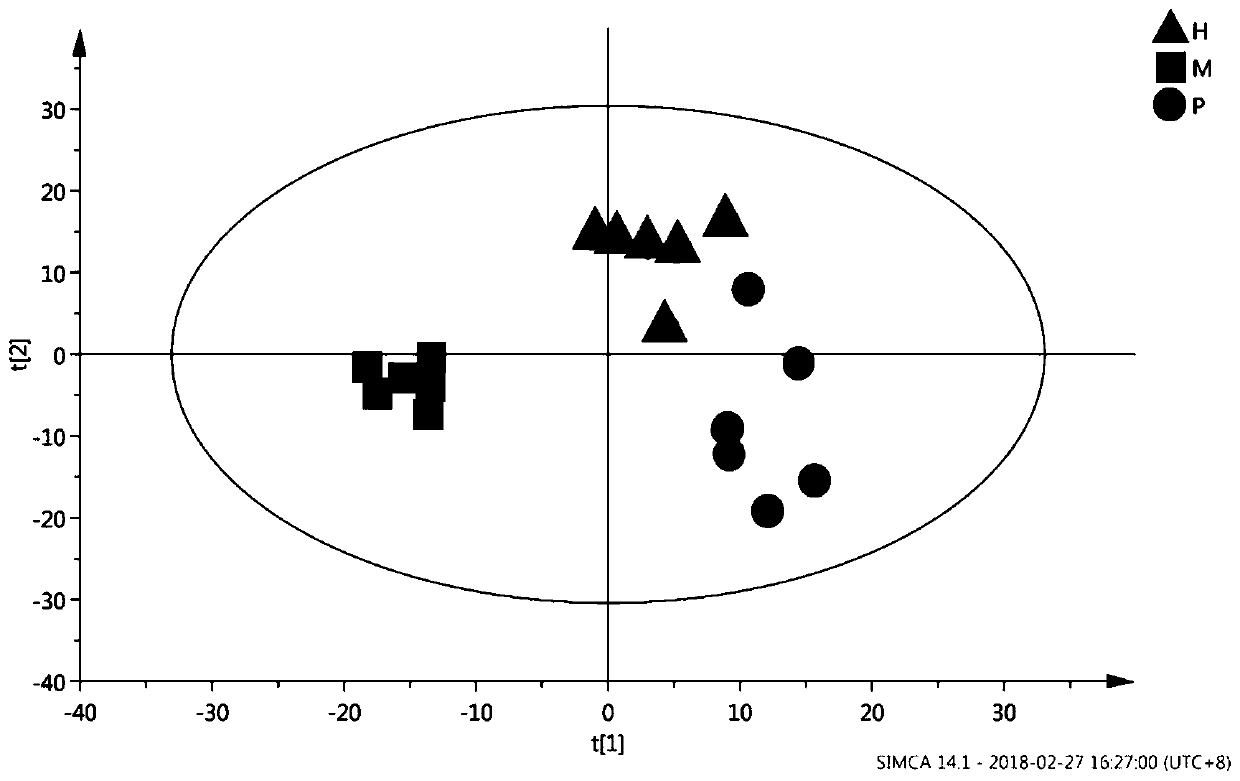
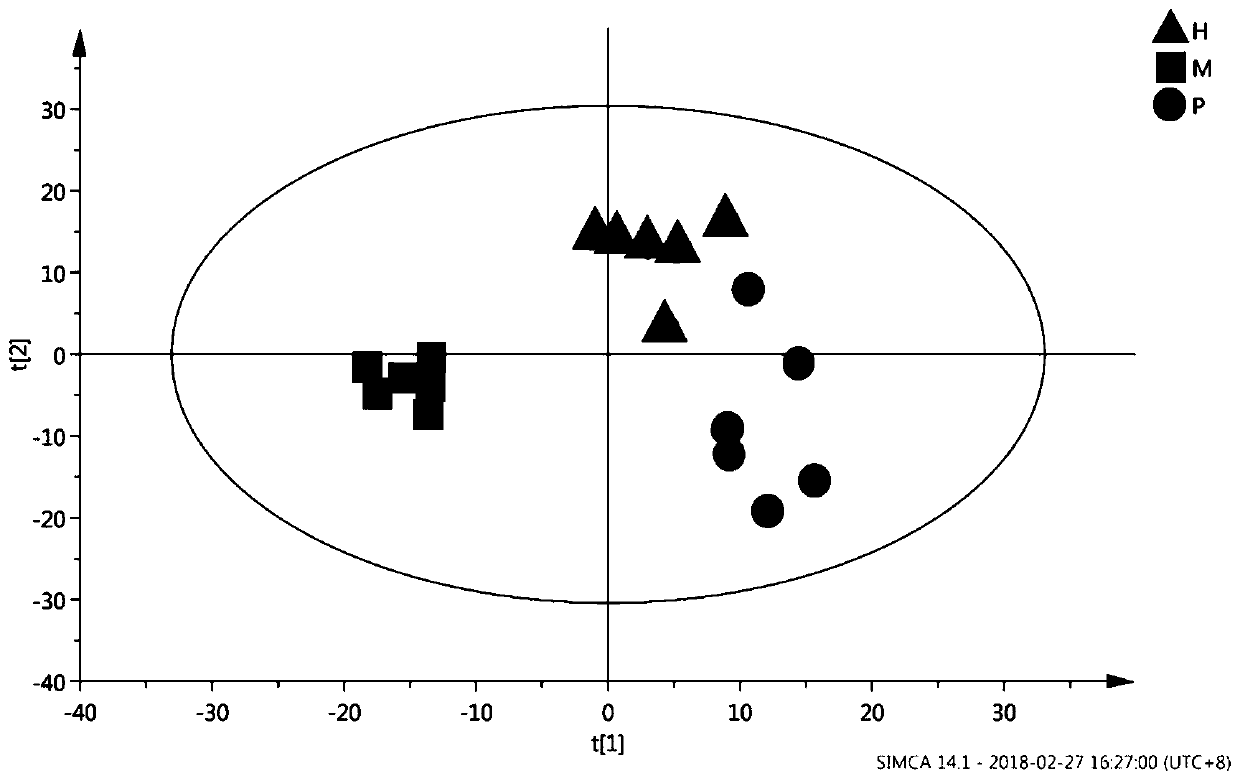
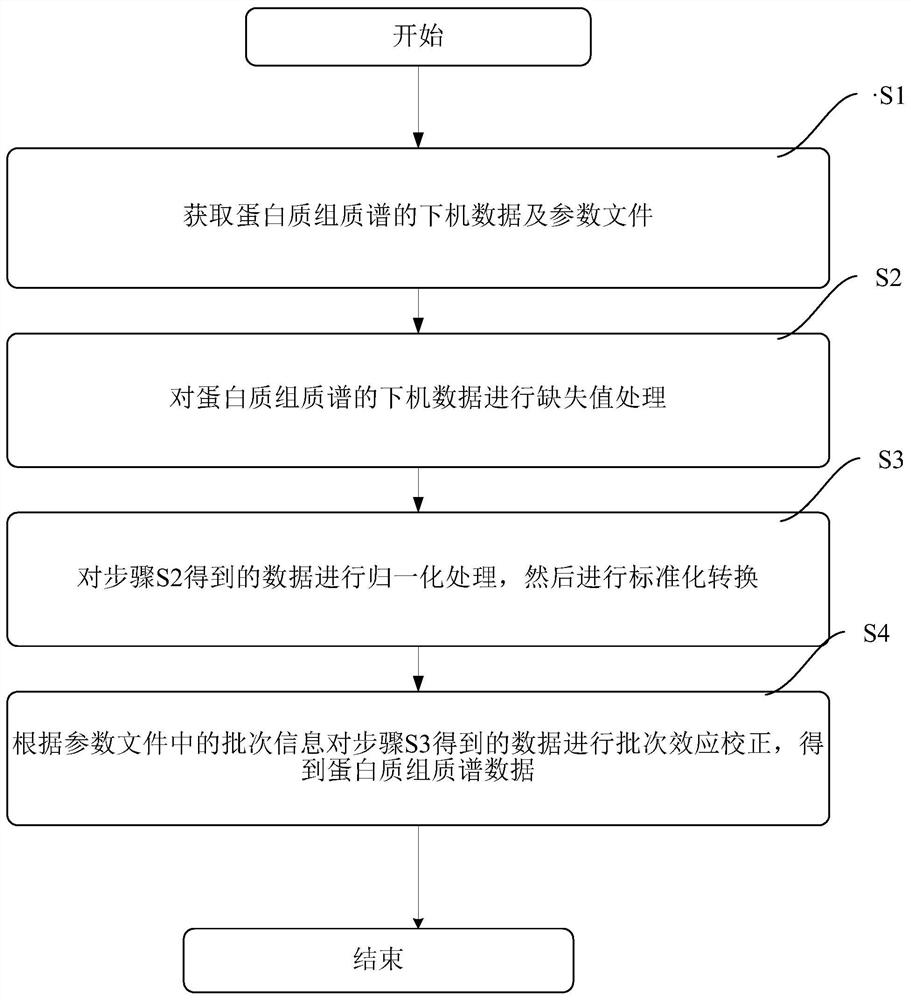
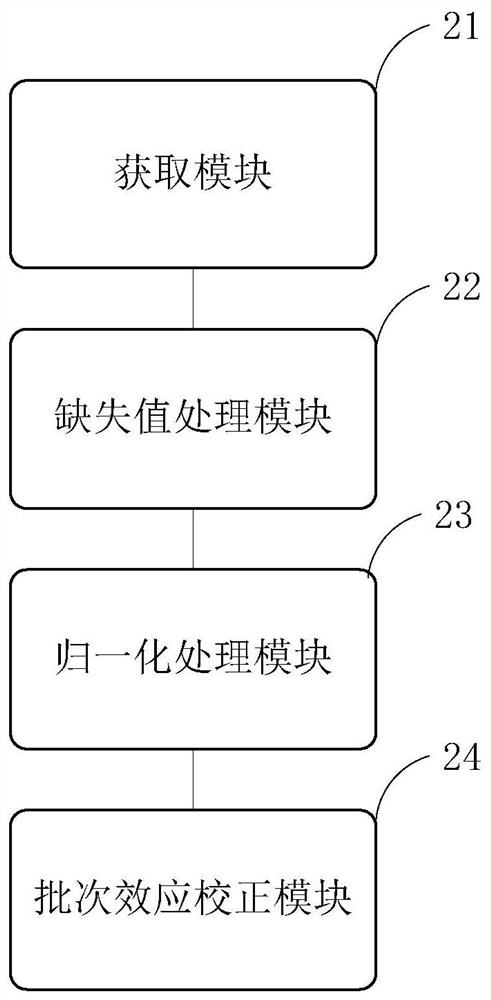
InactiveCN111796095AConform to objective realityAccurately reflectBiostatisticsBiological testingTreatment and control groupsMass spectrometric
The invention provides a proteome mass spectrum data processing method. The method at least comprises the following steps: acquiring offline data and a parameter file of proteome mass spectrum; performing missing value processing on the offline data of proteome mass spectrometry; S2, performing normalization processing on the data obtained in the step S2, and then performing standardized conversion; and S4, performing batch effect correction on the data obtained in the step S3 according to batch information in the parameter file to obtain proteome mass spectrum data. The invention discloses the proteome mass spectrometric data processing method and device, the change of protein expression under different experimental conditions can be reflected more accurately, and then through enrichmentanalysis based on hyper-geometric distribution, different biological functions and biological pathways of different experimental groups under different experimental treatments are obtained, so that the method has important significance in combined analysis with other omics data.
Owner:苏州扇贝生物科技有限公司
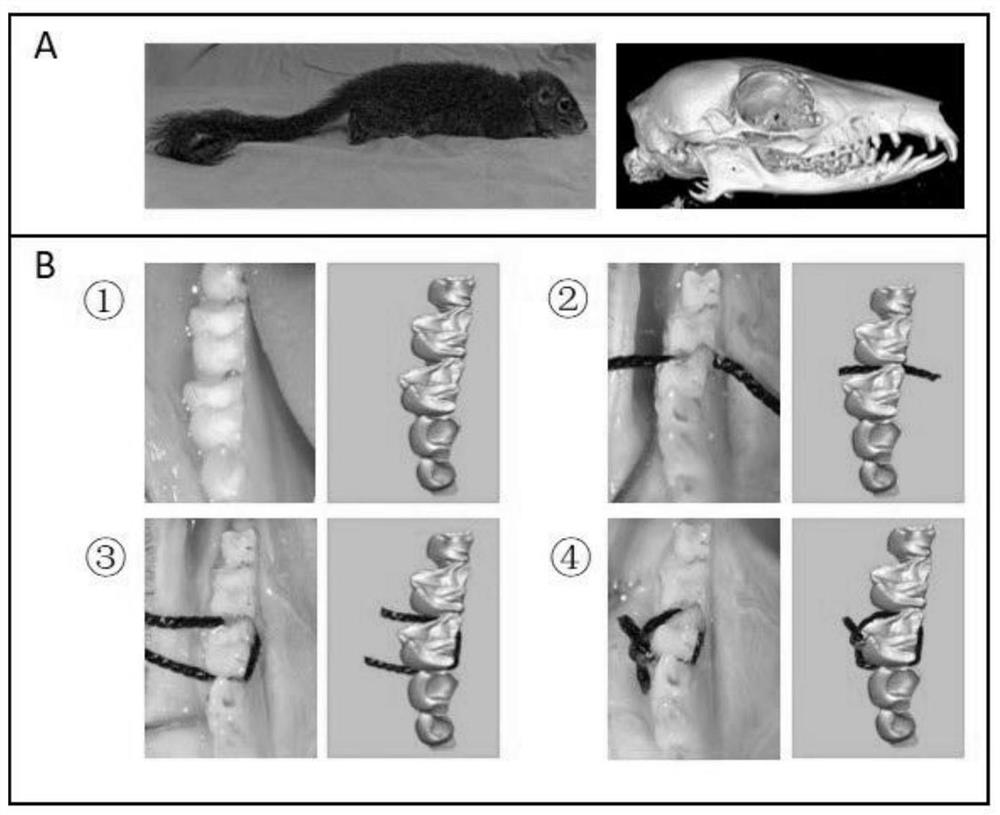
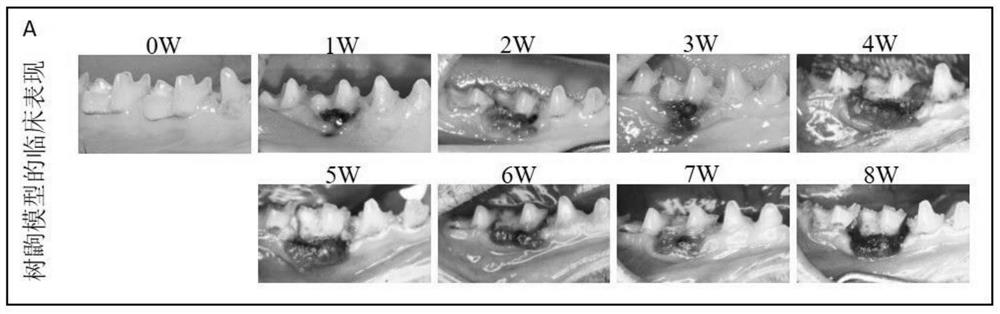
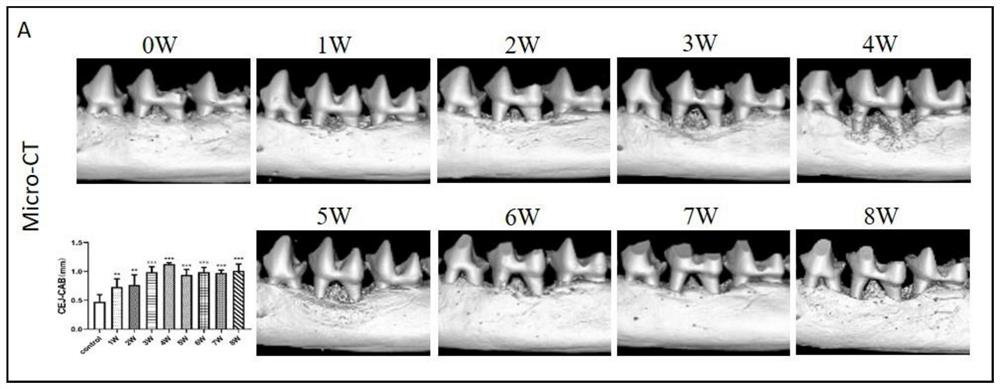
Method for establishing tree shrew periodontitis model
PendingCN113237902AMaterial analysis using wave/particle radiationPreparing sample for investigationCathepsin KStaining
The invention discloses a method for establishing a tree shrew periodontitis model. The method comprises the following steps of (1) selecting an adult healthy male tree shrew for modeling, the weight of the male tree shrew being 130-200g; a random control method being adopted, 40 tree shrews being divided into a normal control group and an experimental group, observation being carried out for 1-8 weeks, and 3-8 tree shrews being arranged in each group at each time point; the control group being not subjected to any treatment, and the other tree shrews being subjected to silk thread ligation on first molars of the lower jaws on the right sides of the tree shrews by using 2-0 silk threads; (2) 1-8 weeks after ligation, observing and recording the clinical manifestation of the tree shrew at the ligation position at the time point of each week, killing the tree shrew, and collecting the lower jawbone of the tree shrew; and (3) detecting the absorption degree of bone tissues by Micro-CT scanning, detecting the infiltration degree of periodontal tissue inflammatory cells by HE staining, detecting the infiltration degree of osteoclasts by TRAP staining, and detecting the expression conditions of inflammation-related proteins TNF-alpha, IL-1alpha and MPO and osteoclast-related protein Cathepsin K by IHC staining.
Owner:昆明医科大学附属口腔医院
Establishment of stress cardiomyopathy animal model
The invention relates to the field of animal model establishment, and discloses a method for establishing an animal model of stress cardiomyopathy, including preoperative preparation, operation, vagus nerve stimulation and postoperative suturing, by studying the blank group, experimental group, ARB class Drug interference, ACEI drugs, studied the induction of cardiomyopathy under drug-induced and electrical stimulation, and performed pathological characterization to verify the successful establishment of animal models to solve the problem that there is currently no suitable animal model of stress cardiomyopathy available problem in research.
Owner:郭瑞威
Method for constructing DC vaccine modified by Ad-NK4
PendingCN111773382APrevent proliferationInduces apoptosisCancer antigen ingredientsBlood/immune system cellsIn vivo experimentTreatment and control groups
The invention provides a method for constructing a DC vaccine modified by Ad-NK4. According to the technical scheme, firstly, a human multiple myeloma cell lysate is prepared, meanwhile, DC is separated from peripheral blood of a patient with multiple myeloma and transfected with Ad-NK4, and finally, the human multiple myeloma cell lysate is used to sensitize the DC transfected by Ad-NK4 so as toobtain the DC vaccine modified by the Ad-NK4. On the basis, the immune efficacy of the vaccine is investigated through in vivo and in vitro experiments. The results confirm that the DC vaccine constructed in the invention can significantly inhibit the proliferation of MM cells in vitro, and has the effect of inducing apoptosis, and has obvious changes in cell proliferation, cell cycle, apoptosis and the expression of Akt-mTOR related proteins; and in an in vivo experiment part, in an experimental group administered with the vaccine, the tumor volume is obviously smaller and the tumor is obviously inhibited, thus confirming the technical effects of the DC vaccine.
Owner:阙文忠
Screening method of pH-dependent antibody targeting complement protein C5
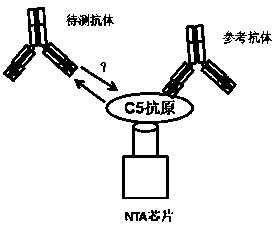
ActiveCN111484553AEfficient screeningShorten the development cycleImmunoglobulins against animals/humansBiological material analysisBio moleculesComplement S-Protein
The invention discloses a screening method of a pH-dependent antibody targeting complement protein C5 and an antibody obtained by the screening method. The screening method disclosed by the inventionis carried out on a biomolecule interaction analysis system; the method comprises the following steps of: setting an experiment group 1, a control group 1, an experiment group 2 and a control group 2,and enabling each group of chips to be combined with antigen complement protein C5 (A) as a reference antibody (Ab1) and a to-be-detected antibody (Ab2) of a pH-dependent C5 monoclonal antibody: theexperiment group 1 A-Ab1-Ab2; the control group 1 is A-Ab1-0; the experimental group 2 is A-0-Ab2; the control group 2 is A-0-0; and detecting the binding signal and calculating the ratio of (experimental group 1-control group 1) / (experimental group 2-control group 2) to judge whether the antibody to be detected is a pH-dependent C5 antibody or not. According to the method, the pH-dependent C5 monoclonal antibody development period can be shortened, and the labor capacity and the time cost are saved; a hybridoma cell culture supernatant can be used as a to-be-detected sample for direct identification, so that the antibody screening efficiency is further improved.
Owner:SHANGHAI PUREMAB BIO TECH CO LTD
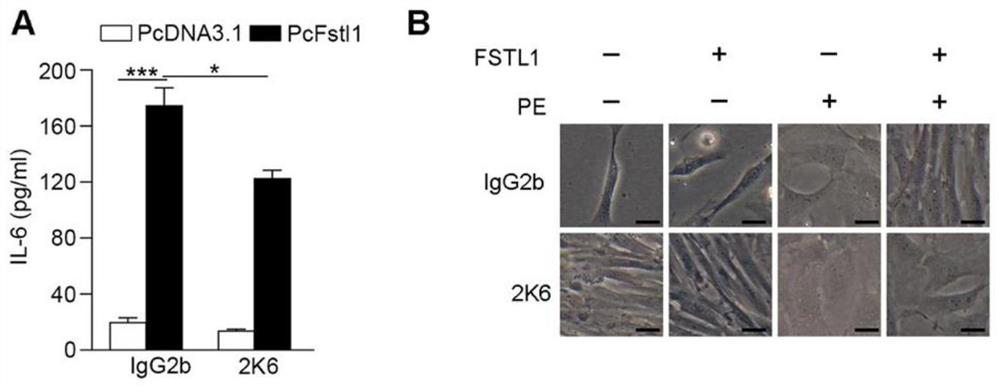
Neutralizing antibody of follistatin-like 1 and application thereof
The invention relates to a neutralizing antibody of follistatin-like 1 and an application thereof. The antibody is a murine FSTL1 neutralizing antibody 2K6. The invention also discloses a heavy chainvariable region and a light chain variable region of the antibody or an antigen binding fragment thereof. The invention also relates to an application of the FSTL1 in promoting the treatment of skin fibrosis diseases, the application of the murine FSTL1 neutralizing antibody 2K6 in treating or remitting pulmonary fibrosis, skin fibrosis and arthritis diseases or symptoms, and a pharmaceutical composition prepared from the murine FSTL1 neutralizing antibody 2K6. Research results show that the murine FSTL1 neutralizing antibody 2K6 can treat or remit pulmonary fibrosis, skin fibrosis and arthritis diseases; and compared with an antibody 22B6 which is researched and developed by the research and development team in the earlier stage and used for treating pulmonary fibrosis, the antibody 2K6 has better prevention and treatment effects on mouse pulmonary fibrosis than the antibody 22B6, that is to say, the prevention and treatment remission rates of a 2K6 experimental group on pulmonary fibrosis key indexes including the hydroxyproline content and the pulmonary fibrosis area proportion are higher than those of a 22B6 experimental group.
Owner:NANKAI UNIV
Novel mouse in-situ pleural mesothelioma model and establishment method thereof
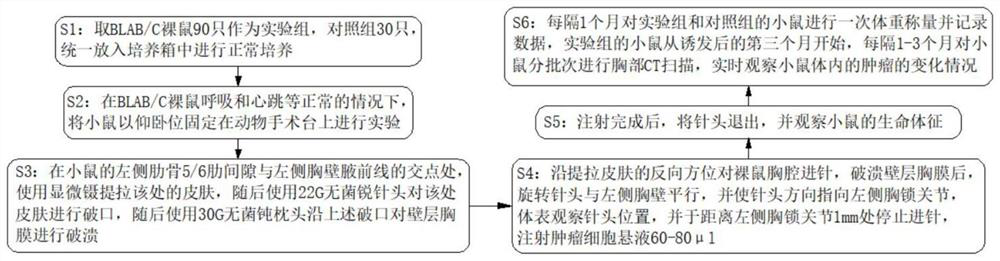
InactiveCN113599010ARestore invasive abilityRestore the effect of tumor metastasisAnimal fetteringSurgical veterinaryAnterior axillaryTumor transplantation
The invention discloses a novel mouse in-situ pleural mesothelioma model and an establishment method thereof. The establishment method comprises the following steps of 1, taking 90 BLAB / C nude mice as an experimental group and 30 control groups, and uniformly putting the experimental group and the control groups into an incubator for normal culture; 2, under the condition that BLAB / C nude mice breathe, heartbeat and the like are normal, anesthetizing the mice and fixing the mice on an animal operating table in a supine position for experiment; and 3, at the intersection point of the left rib 5 / 6 intercostal space and the left chest wall anterior axillary line of the mouse, using microscopic forceps to lift and pull the skin at the intersection point, then using a 22G sterile sharp needle head to break the skin at the intersection point, then using a 30G sterile blunt pillow to break the wall layer pleura along the broken opening. The tumor transplantation method provided by the invention is applicable to orthotopic transplantation of pleural tumors. The growth mode, the invasion ability and the tumor metastasis effect of the pleural tumor can be restored to the maximum extent, and a good mouse model is constructed for studying the pleural tumor.
Owner:FIRST AFFILIATED HOSPITAL OF KUNMING MEDICAL UNIV
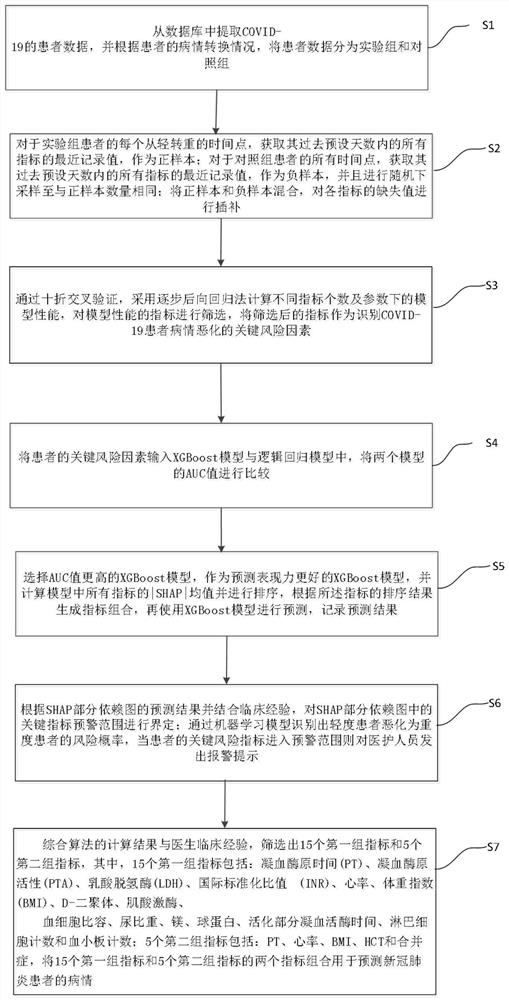
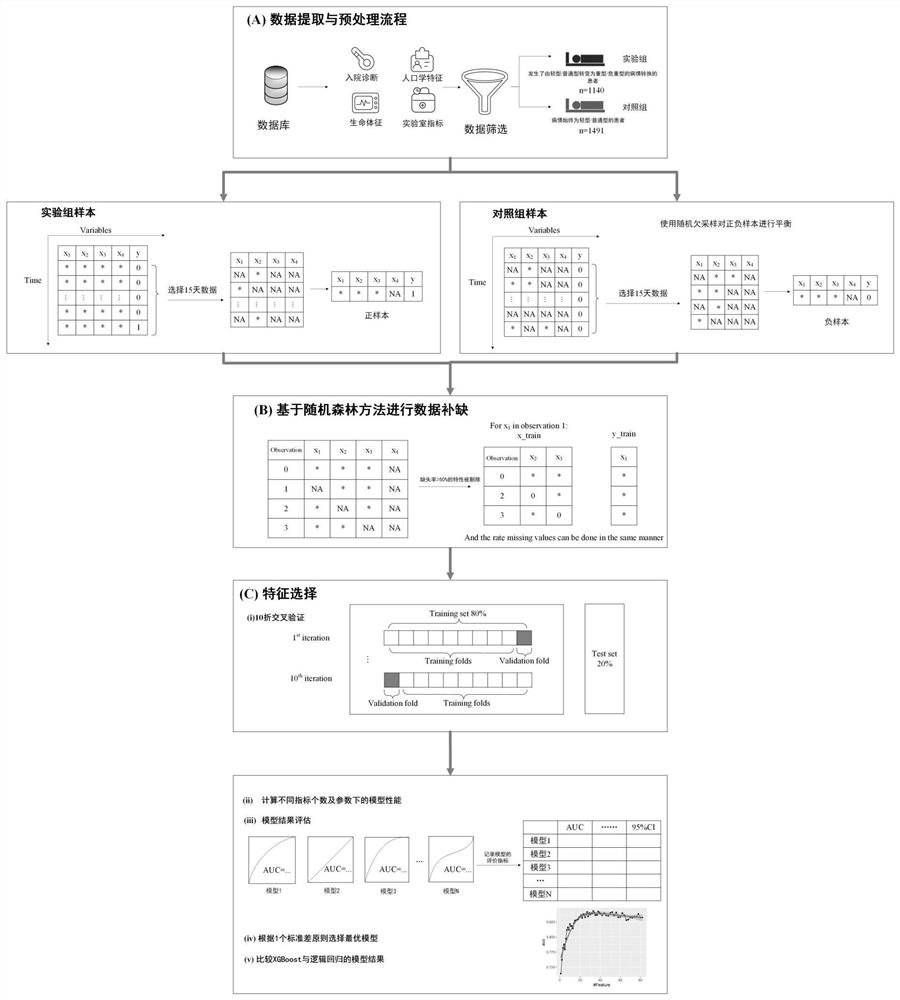
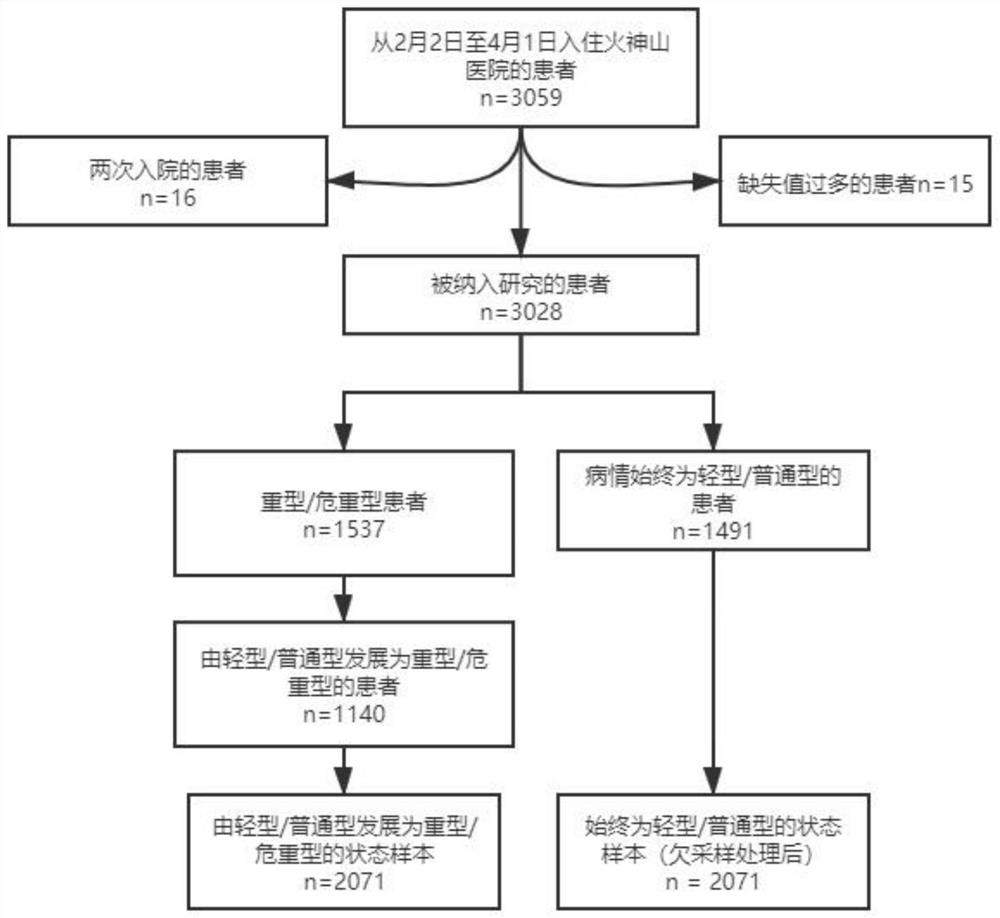
New coronal pneumonia patient outcome prediction method based on interpretable machine learning algorithm
The invention provides a new coronal pneumonia patient outcome prediction method based on an interpretable machine learning algorithm, wherein the method comprises the steps: extracting COVID-19 patient data from a database, and dividing the patient data into an experimental group and a control group according to the illness state conversion condition of a patient; interpolating the missing value of each index through random forest regression; screening the indexes of the input model, and taking the screened indexes as key risk factors for identifying the deterioration of the patient; inputting the key risk factors of the patient into the XGBoost model and the logistic regression model; selecting an XGBoost model with better prediction expressive force to generate an index combination, and performing prediction by using the XGBoost model and recording the prediction result; defining the early warning range of the key index; when the key risk index of the patient enters the early warning range, giving out an alarm prompt to medical staff. According to the invention, the calculation result of the algorithm and the clinical experience of a doctor are synthesized, and two index combinations composed of 15 first groups of indexes and 5 second groups of indexes are proposed to be used for predicting the condition of the new coronal pneumonia patient.
Owner:THE FIRST MEDICAL CENT CHINESE PLA GENERAL HOSPITAL +1
Method for detecting fibrinolytic function of blood
PendingCN112067575AEasy to operateStrong specificityMaterial analysis by optical meansDimerHemolysis
The invention relates to a method for detecting the fibrinolysis function of blood. The method comprises the following steps: a, preparing an experimental group reagent and a control group reagent; b,performing absorbance detection; and c, calculating the fibrinolysis capacity value and the fibrinolysis time value. The fibrinolysis function of blood can be directly detected; the method is relatively simple to operate; the fibrinolysis function of the blood can be quantitatively evaluated, namely the fibrinolysis capacity and the fibrinolysis time are reflected; and traditional fibrinolytic markers such as FDP and D dimers are high in non-specificity and affected by inflammation, bleeding, thrombus load and the like, and the real fibrinolytic ability can be reflected, so that the specificity is high. In addition, the method is not influenced by blood chyle, jaundice, hemolysis and other factors, and the anti-interference capability is high.
Owner:吴俊
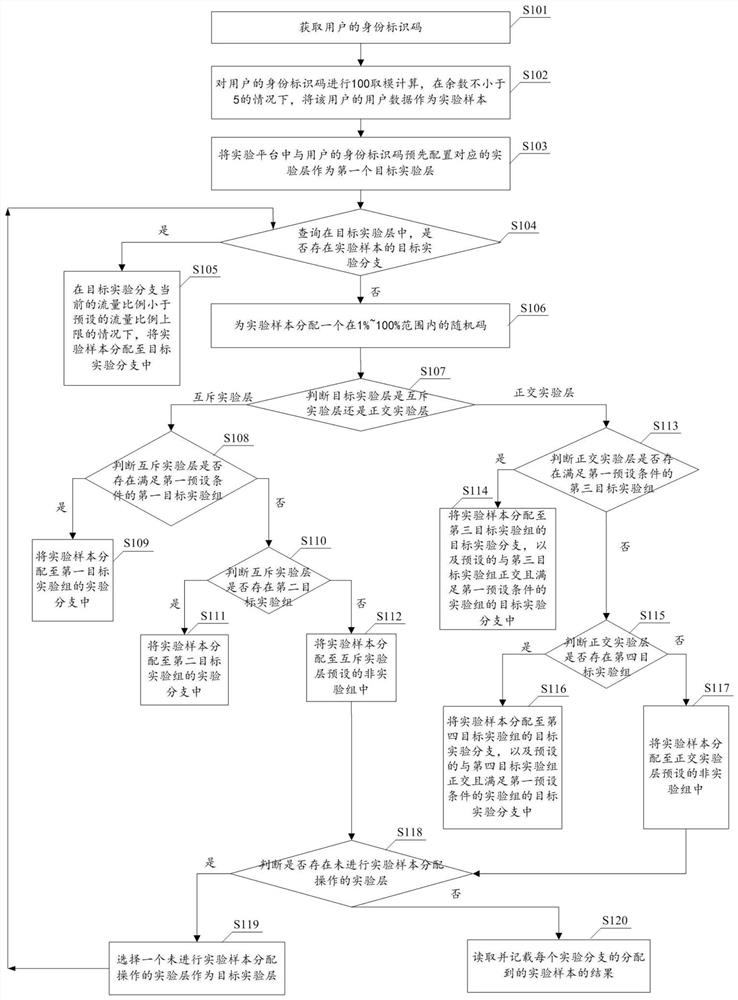
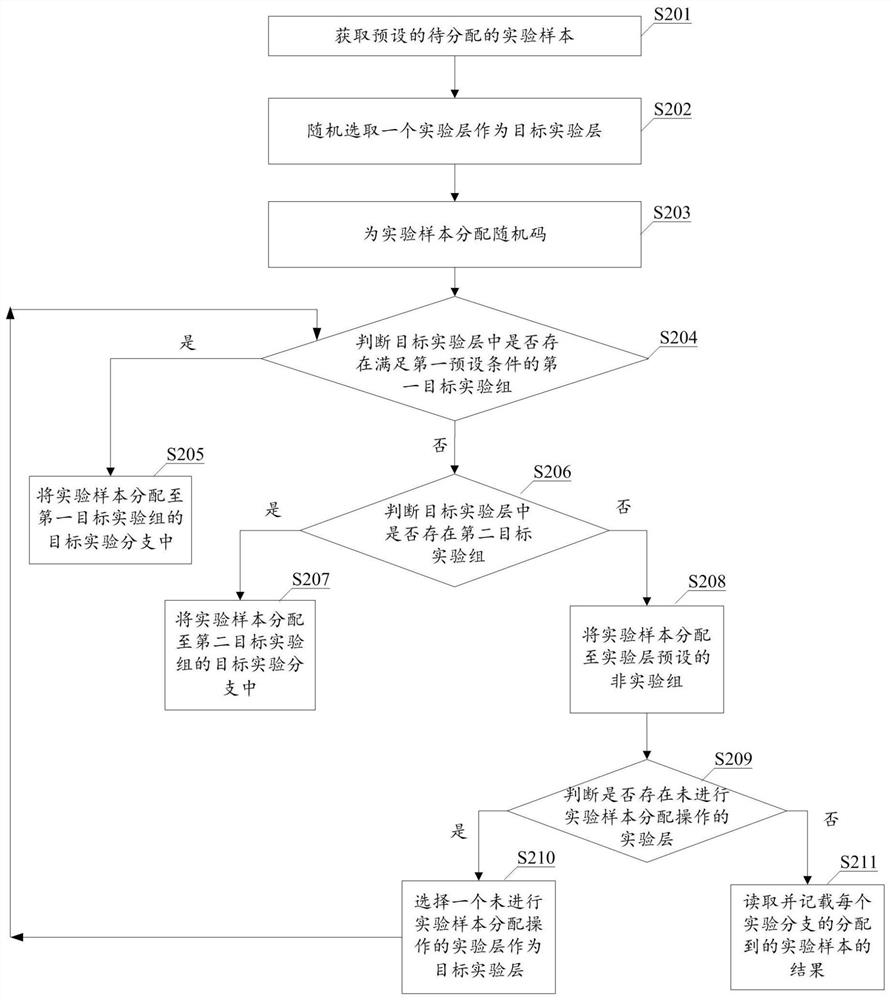
Experimental sample distribution method and device, equipment and computer readable storage medium
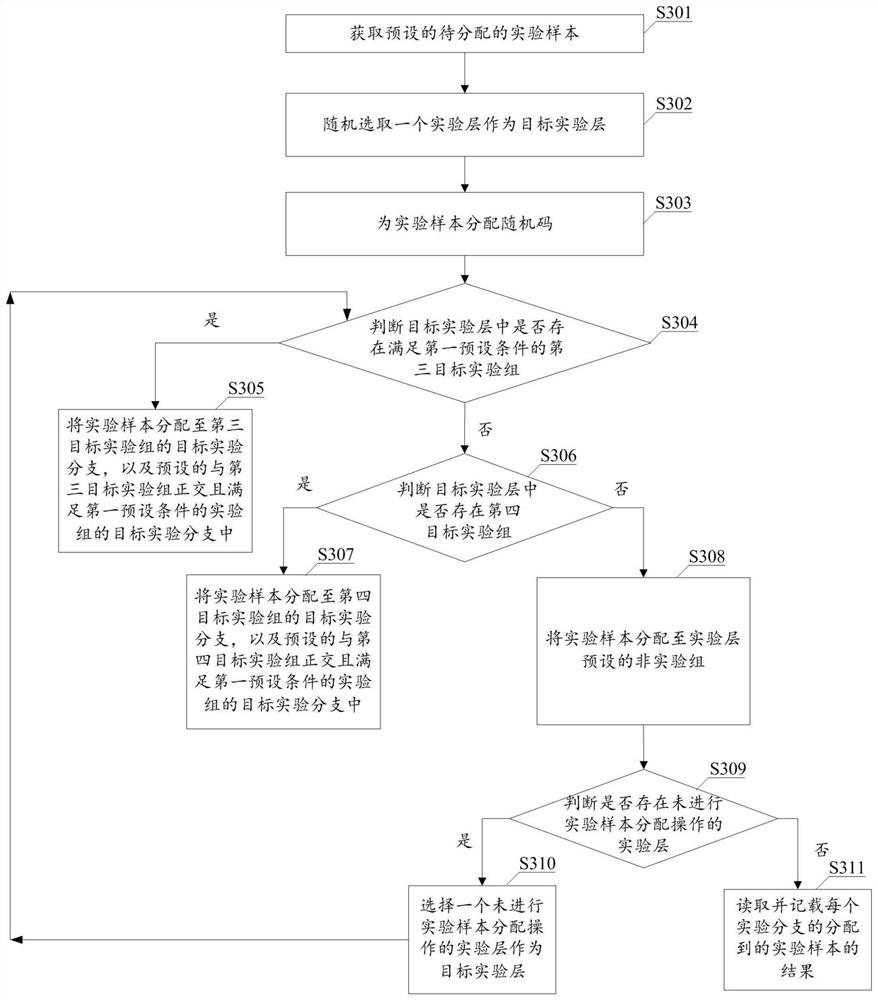
PendingCN111967798AImprove efficiencyCo-operative working arrangementsResourcesAlgorithmRandom assignment
The invention provides an experimental sample allocation method and device, equipment and a computer readable storage medium, and aims to allocate a random code to each experimental sample and allocate the experimental samples to an experimental group according to the random codes of the experimental samples and a preset allocation rule. The random codes are randomly distributed, the upper limit of the sample proportion of the experimental group is based on experiments included in the experimental group and experiments included in other experimental groups in the experimental layer where the experimental group is located, and therefore the sample proportion upper limits of different experimental groups can be different; the random code is smaller than the upper limit of the preset sample proportion of the experimental group; different experimental samples can be allocated to different experimental groups, and in addition, the situation that the number of the experimental samples allocated by a single experimental group is larger than the actually required number of the experimental groups can be prevented as the proportion of the experimental samples allocated by the experimental groups is smaller than the upper limit of the sample proportion of the experimental groups. Therefore, according to the method disclosed in the technical scheme provided by the invention, the use efficiency of the experimental sample can be improved.
Owner:度小满科技(北京)有限公司
Differential analysis method and system based on single cell samples of mixed experimental group and control group
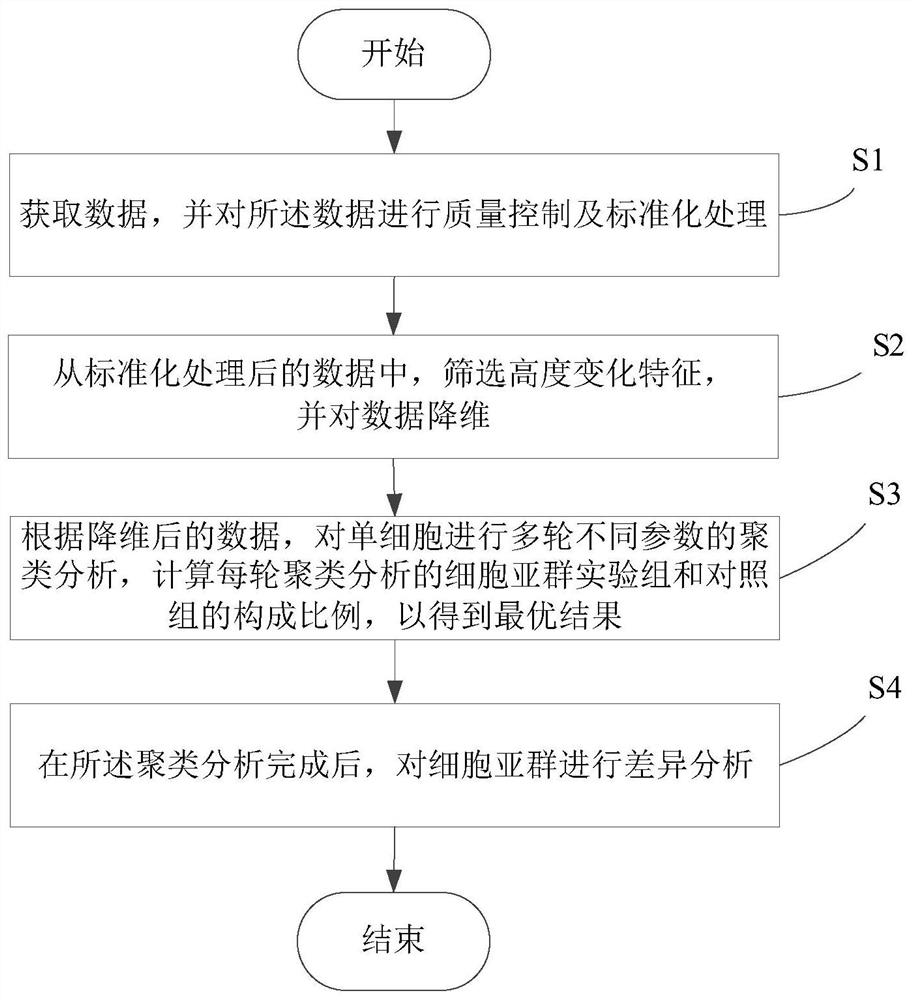
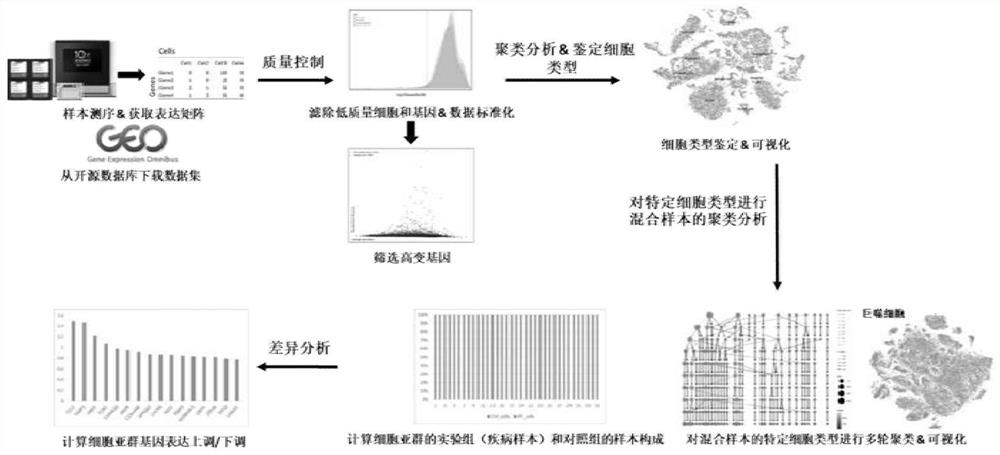
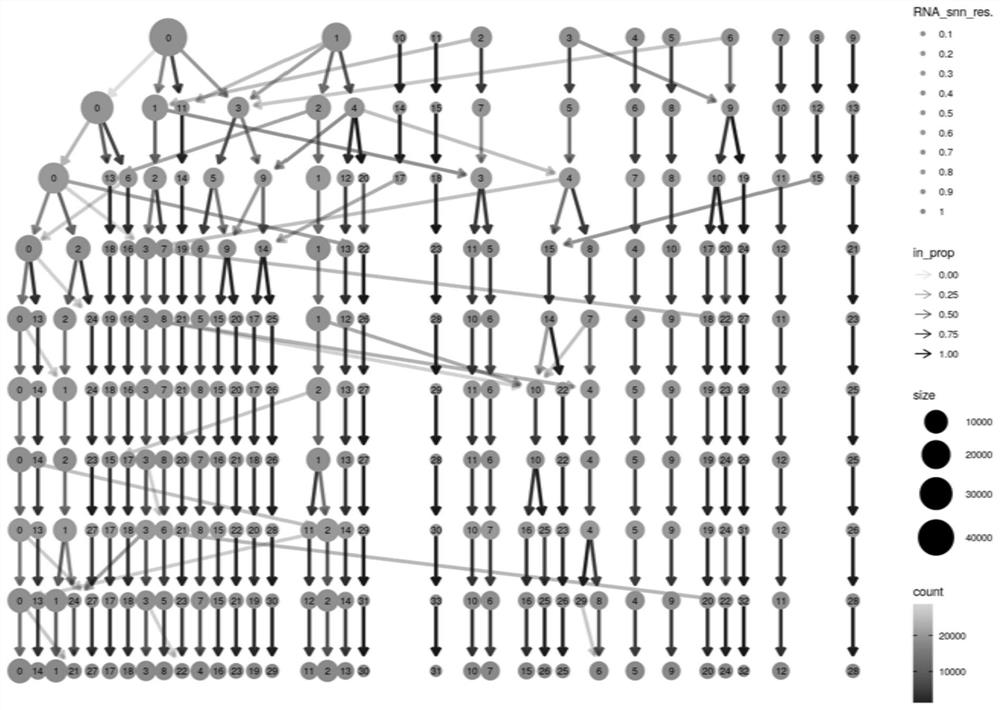
PendingCN114864003AEasy to findMedical automated diagnosisCharacter and pattern recognitionData packCell layer
The invention relates to a difference analysis method based on single cell samples of a mixed experimental group and a control group, which comprises the following steps: a, acquiring data, and carrying out quality control and standardization processing on the data, the data comprising experimental data and open source data; b, screening out hypervariant gene data from the standardized data, and carrying out dimensionality reduction on the screened data; c, performing multiple rounds of clustering analysis of different parameters on the single cells according to the data subjected to dimension reduction, and calculating the composition proportion of a cell subset experimental group and a control group of each round of clustering analysis to obtain an optimal result; and d, carrying out difference analysis on the cell subpopulation. The invention further relates to a difference analysis system based on the single cell samples of the mixed experimental group and the control group. According to the invention, the cell subgroup formed by the specific group can be better found, so that the difference analysis of the experimental group and the control group at the single cell level is realized.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
Application of p38 gamma in preparation of pancreatic cancer prognosis diagnostic reagent
ActiveCN111206098APromote invasionEasy transferMicrobiological testing/measurementDisease diagnosisTreatment and control groupsOncology
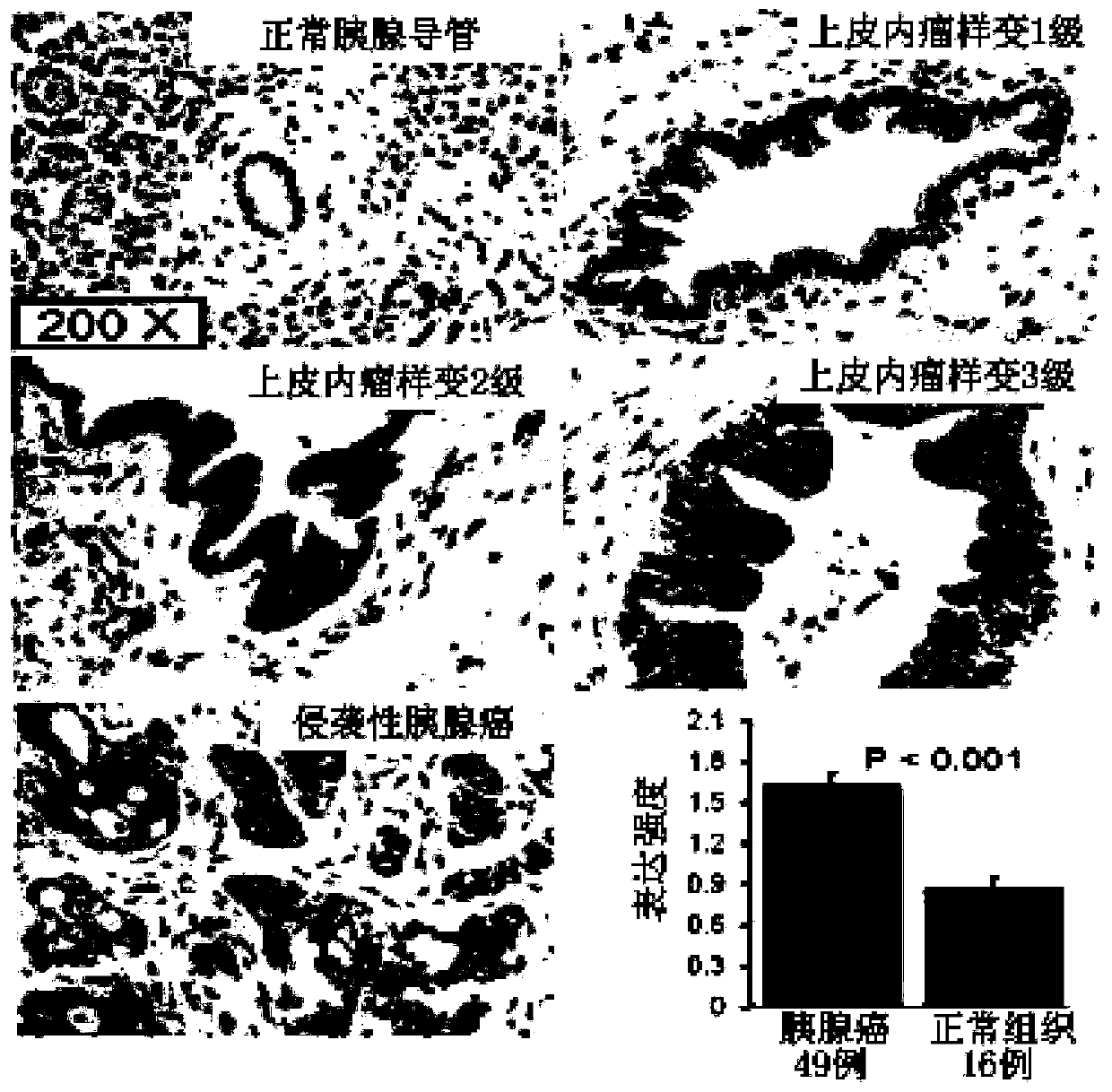
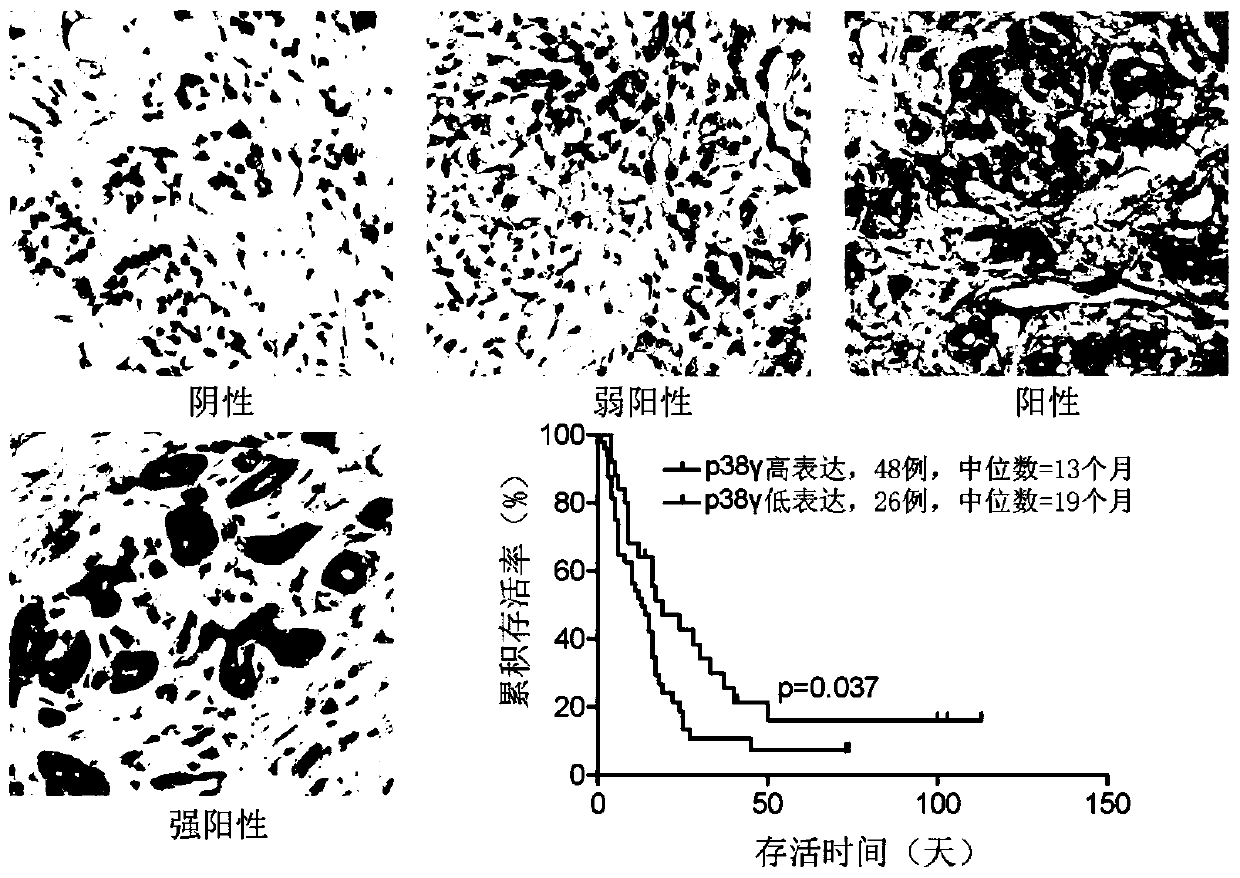
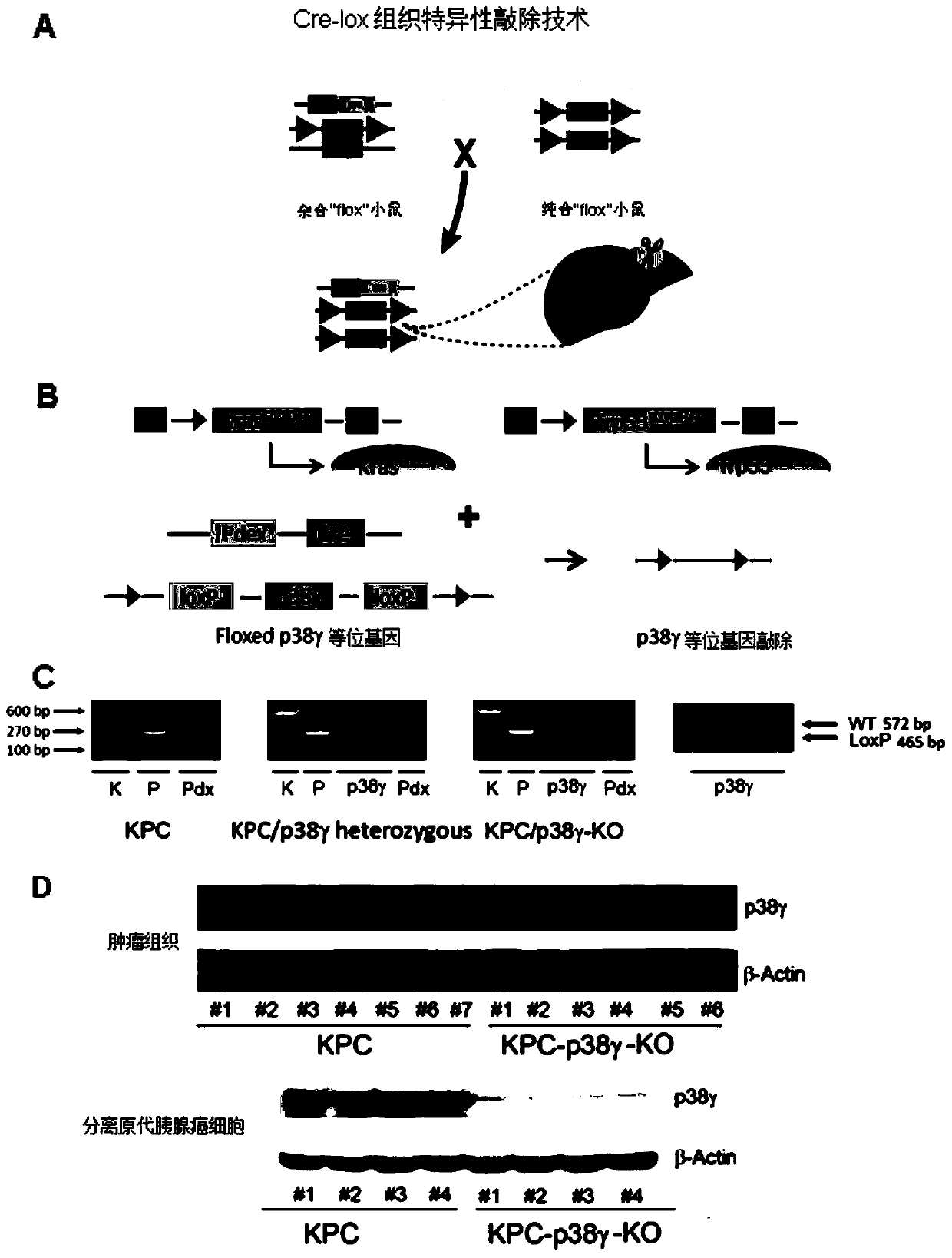
The invention discloses application of p38 gamma in preparation of a pancreatic cancer prognosis diagnostic reagent. The inventor finds that expression of p38 gamma in cancer tissues of a pancreatic cancer patient is remarkably higher than that of p38 gamma in normal tissues, the expression level of p38 gamma is gradually increased along with disease progression, and the high expression of p38 gamma is related to adverse prognosis of the patient. A pancreatic duct adenocarcinoma animal model K-RasG12D-p53R172H-Pdx-Cre (KPC) is constructed as a control group for research. A pancreatic ductal epithelial cell p38 gamma knockout pancreatic cancer animal model (KPC-p38 gamma-KO) is constructed as an experimental group by applying a Cre / LoxP technology. The p38 gamma knockout is found to be capable of remarkably reducing the size of a tumor and prolonging the lifetime of KPC mice. The median survival time of the mice in the KPC group is 160 days, most KPC-p38 gamma-KO mice still survive in the statistical time, and the median survival time is longer than 300 days. A further experimental result shows that high expression of p38 gamma can promote invasion and metastasis of a pancreatic cancer. Therefore, p38 gamma can well determine the risk and prognosis of the pancreatic cancer.
Owner:SUN YAT SEN UNIV CANCER CENT
Molecular marker related to senescence and health senescence and application of molecular marker in improving health senescence
ActiveCN111926071AAccelerated agingNervous disorderAntipyreticBiotechnologyTreatment and control groups
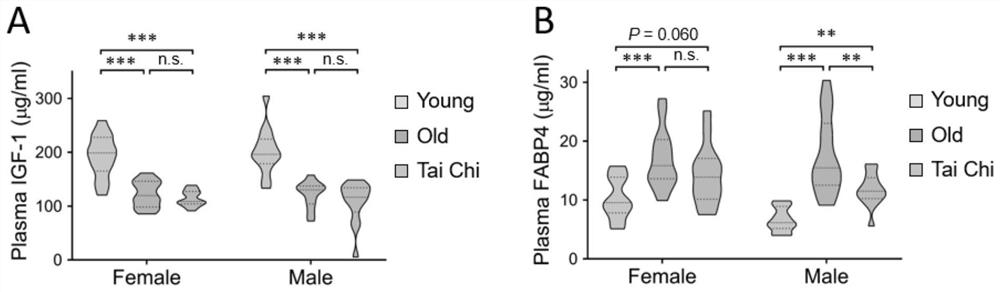
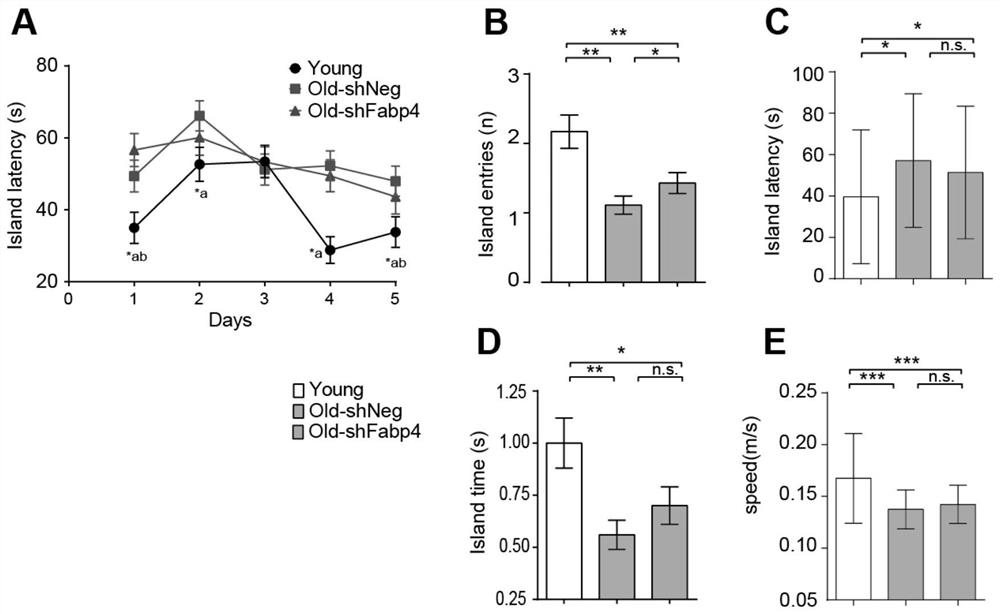
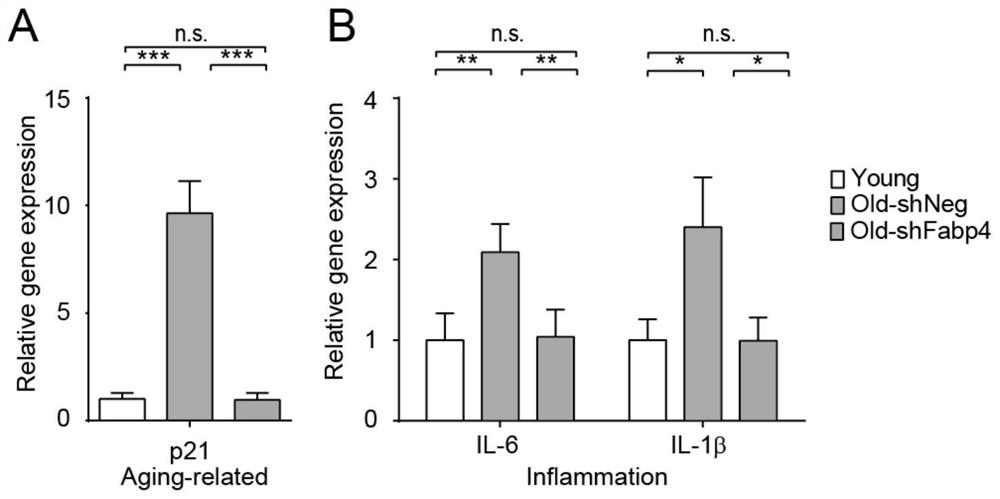
The invention belongs to the technical field of biology, and particularly relates to a molecular marker related to senescence and health senescence and application of the molecular marker in improvinghealth senescence. According to the invention, Tai Chi exercisers are used as an experimental group, protein spectra are compared with those of normal young people and old people, the FABP4 protein is screened out, the expression of the FABP4 protein in blood of old people is significantly increased compared with that of young people, but the trend of the FABP4 protein in Tai Chi exercises of oldpeople is significantly reversed. The FABP4 gene expression is knocked down or the FABP4 activity is inhibited in an old mouse, so that the cognitive function is remarkably improved, and the senescence and related inflammatory indexes are inhibited. It is prompted that the FABP4 gene has the effect of promoting senescence and can serve as a molecular target for improving healthy senescence, and anew target and a new strategy are provided for senescence delaying.
Owner:WUHAN UNIV
Camellia oil lipidosome, preparation method thereof and application of camellia oil lipidosome to preparing acute liver injury treating drugs
InactiveCN109908235AGood repeatabilityHigh activityDigestive systemPharmaceutical non-active ingredientsAlanine aminotransferaseMicroscopic exam
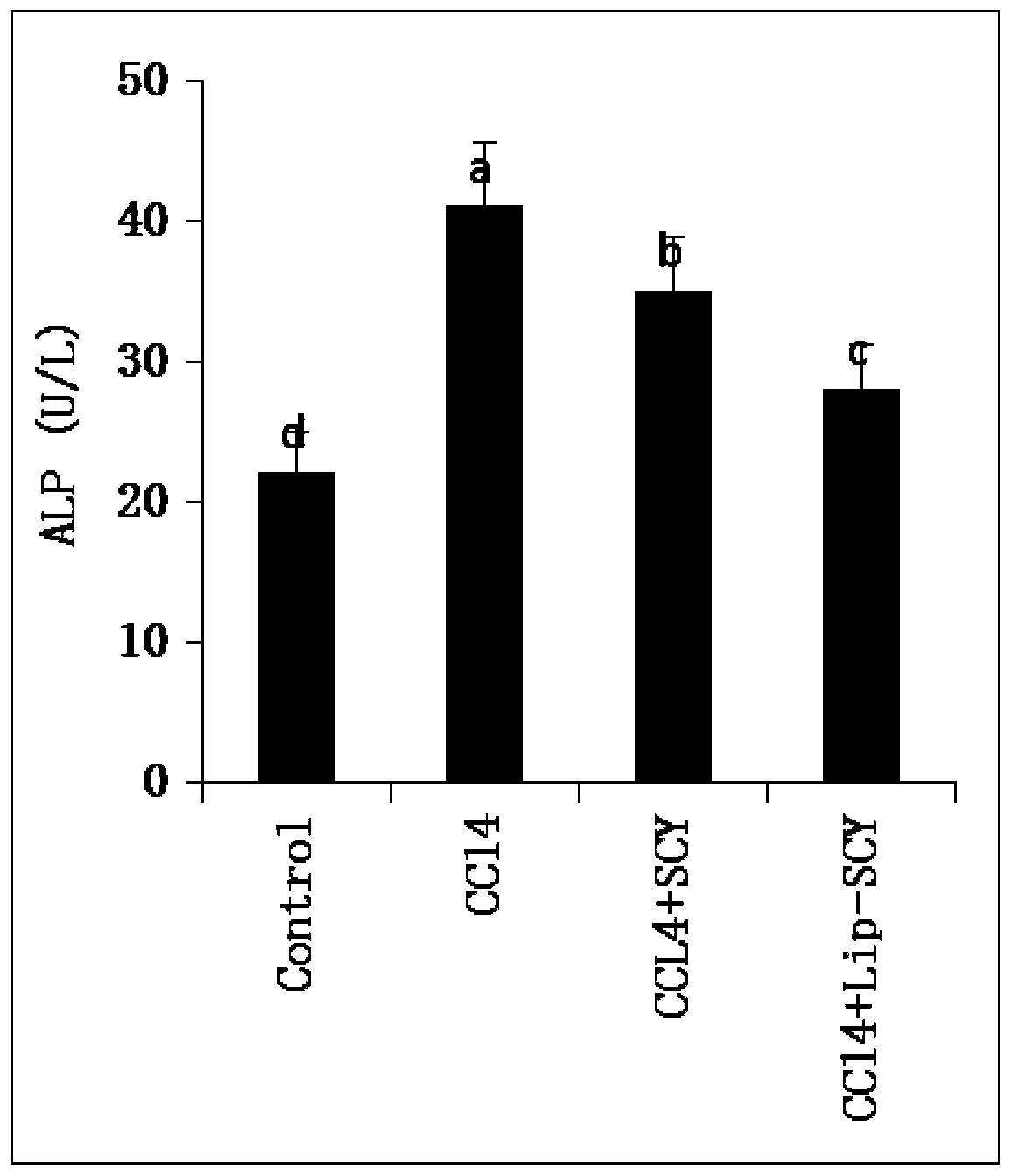
The invention provides a camellia oil lipidosome, a preparation method of the camellia oil lipidosome and application of the camellia oil lipidosome to preparing acute liver injury treating drugs andbelongs to the technical field of liver injury. The camellia oil lipidosome is prepared by suspending 1 mL of camellia oil, 0.05-1.5 g of lecithin and 0.01-0.2 g of phosphatidylserine into 5-50 mL ofdeionized water and performing ultrasonic treatment, oscillation and standing. The prepared camellia oil lipidosome is a uniform white emulsion, and under microscopic examination, is in the shape of spherical or quasi-spherical bubbles with a diameter of 1-10 mu m. Detection of the liver function indexes of activity of alkaline phosphatase (ALP) and alanine aminotransferase (ALT) in the serum of mice of an experimental group and a control group and observation of the liver tissues of the mice show that the camellia oil lipidosome can achieve significant protecting effects on acute liver injuryand is higher in efficacy than camellia oil. Therefore, the camellia oil lipidosome can be applied to preparing the acute liver injury treating drugs.
Owner:HAINAN MEDICAL COLLEGE
Lung cancer cell radiation adaptability MicroRNA expression profile detection method
PendingCN112195249AAdjust in timeImprove adaptabilityMicrobiological testing/measurementRNA extractionCancer cell
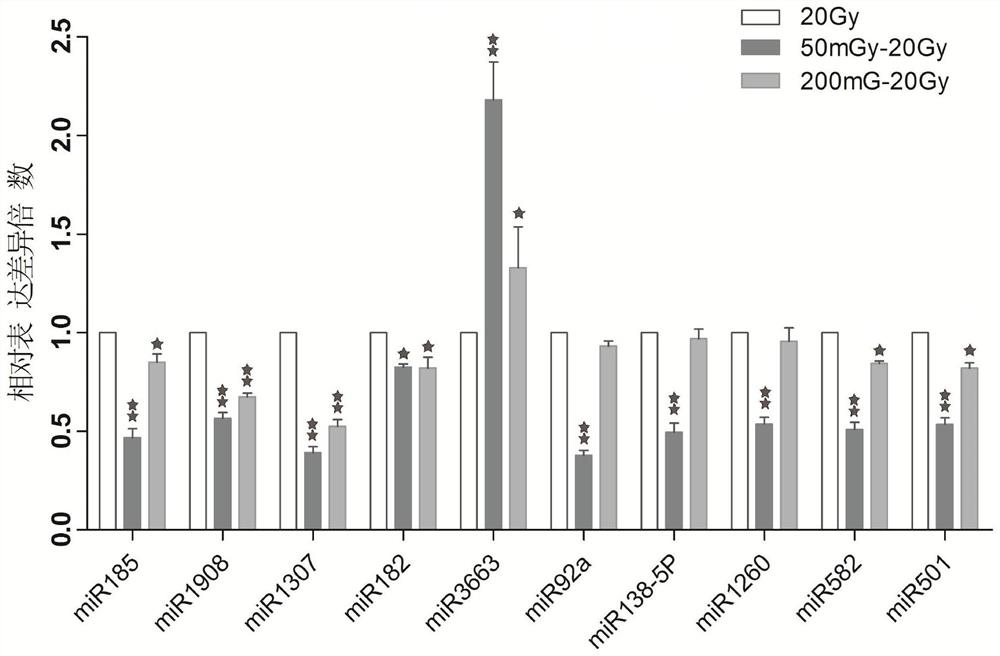
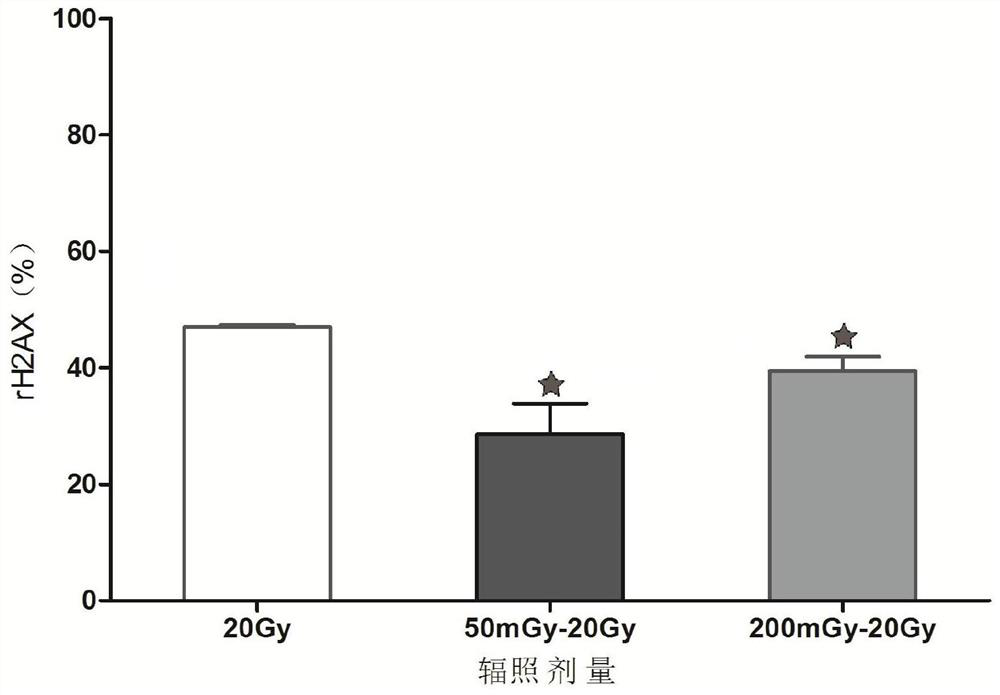
The invention provides a lung cancer cell radiation adaptability MicroRNA expression profile detection method, and belongs to the technical field of cancer cell radiation adaptability detection. The method comprises the following steps of specimen selection, dividing target lung cancer cells into an experimental group and a control group, sequentially sequentially irradiating the experimental group by adopting low-dose and high-dose rays, irradiating the control group by adopting high-dose rays until the cells are digested, and carrying out low-temperature preservation; extracting total RNA, adding an RNA extraction reagent into target lung cancer cell lines in the experimental group and the control group, cracking the target lung cancer cells, centrifuging, sucking supernate, and oscillating to obtain a water-soluble precipitate; and processing and analyzing the difference of expression profiles of the lung cancer cell MicroRNA after different doses of irradiation by using an miRNA chip. The method can be used for pre-judging the effectiveness of x-ray radiotherapy on cells before treatment and judging the adaptability change of individuals to x-ray radiation in the treatment process.
Owner:THE BEIJING PREVENTION & TREATMENT HOSPITAL OF OCCUPATIONAL DISEASE FOR CHEM IND
In-vivo lipid three-dimensional mass spectrum imaging method based on integral zebra fish model
PendingCN112098503AIntegrity guaranteedImprove integrityWithdrawing sample devicesPreparing sample for investigationChemical compoundTissue sample
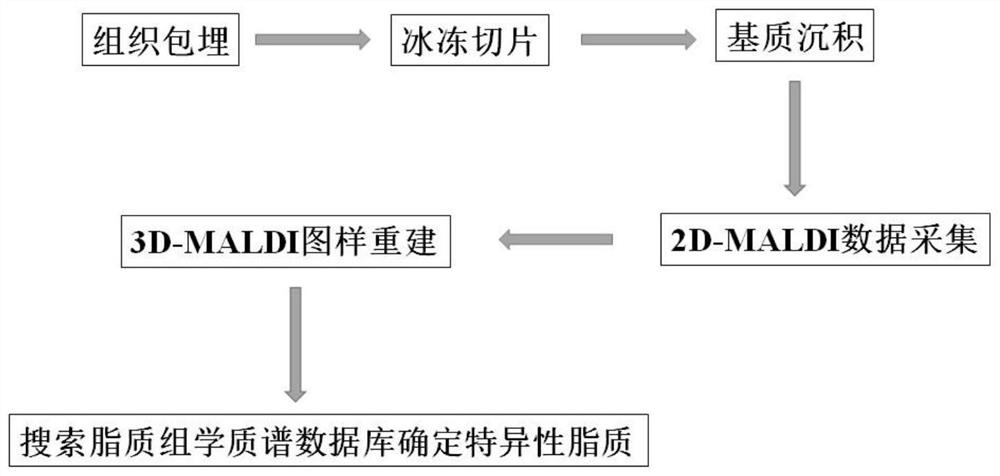
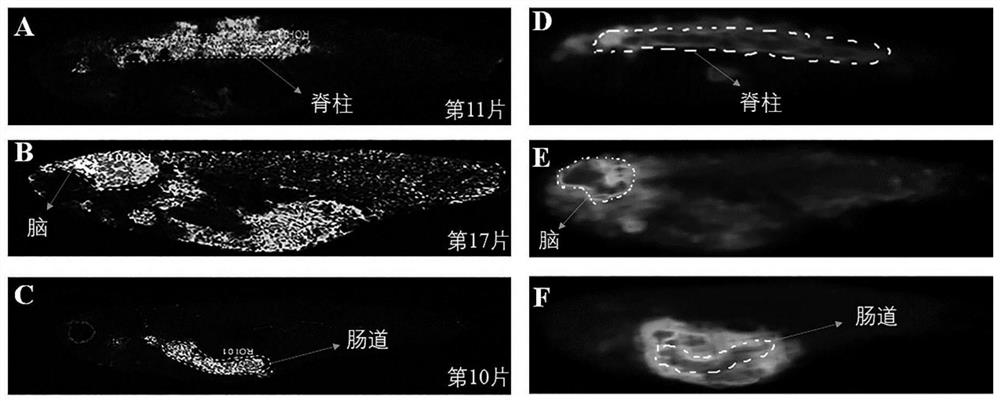
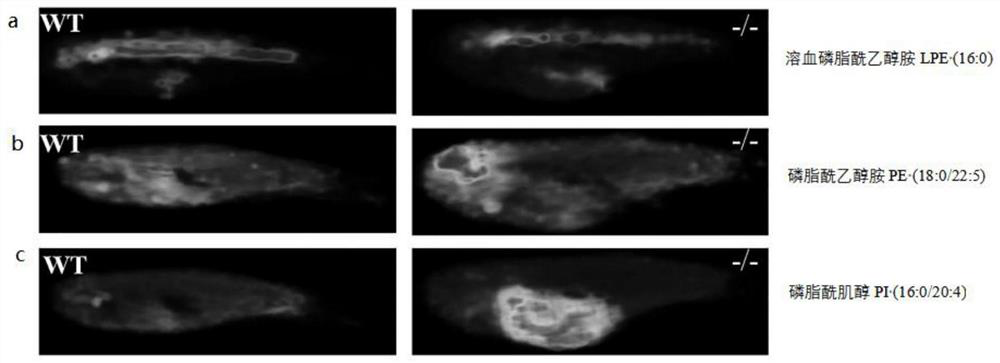
The invention belongs to the technical field of mass spectrum detection, and discloses an in-vivo lipid three-dimensional mass spectrum imaging method based on an integral zebra fish model. The invention establishes the in-vivo lipid three-dimensional mass spectrum imaging method based on the integral zebra fish model, the method is applied to the research of a zebra fish C1 type Niemann-Pick disease model, and the result shows that fifteen lipids with obvious spatial distribution difference exist in different organs of zebra fish. According to the three-dimensional MALDI mass spectrum imagingmethod established by the invention, complex sample pretreatment is not needed, various compounds in a tissue sample can be detected at the same time, and the integrity of selected tissues and a multi-dimensional visual effect can be ensured, so that the spatial distribution difference between an experimental group and a control group can be visually and comprehensively obtained; the method has the advantages of high integrity and high chemical specificity.
Owner:GUANGDONG UNIV OF TECH
A method for constructing a model based on liver metabolomics to study the enhanced immune mechanism of selenized aminopolysaccharides in black sea bream
ActiveCN110346466BImprove accuracyImprove system performanceComponent separationMass spectrometric analysisTreatment and control groupsZoology
A method for constructing a model for enhancing the immune mechanism of black sea bream by studying selenized aminopolysaccharide based on liver metabolomics, relates to the field of biomedical analysis, and includes the following steps: 1) dividing black sea bream juveniles into an experimental group and a blank group equally; 2 ) Selenized amino polysaccharide was added to the feed of the experimental group; 3) The juvenile black sea bream in the experimental group and the blank group were starved, and after anesthesia, the liver tissue was taken out and stored for future use; 4) The liver tissue samples were prepared and the quality Control samples were collected and processed using ultra-high performance liquid chromatography-time-of-flight mass spectrometry; 5) The collected metabolomic data of the liver of black sea bream were analyzed and processed to identify and screen out the information on the enhanced immune mechanism of selenized aminopolysaccharides on black sea bream. liver metabolomic biomarkers, and construct and analyze the metabolic pathways pointed to by the liver metabolomic biomarkers. The present invention has good systematicness, high accuracy, low cost and simple operation.
Owner:舟山出入境检验检疫局综合技术服务中心
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com