Application of apatinib to preparing BRAF V600E protein kinase inhibitors
A protein kinase inhibitor, apatinib technology, which is used in the application field of apatinib to prepare a BRAFV600E mutant tumor drug, and can solve problems such as no literature reports on the inhibitory effect of BRAFV600E protein kinase.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1. In vitro inhibitory screening of apatinib on various kinases
[0029] Apatinib was used to prepare a 100nM solution with DMSO, and 138 protein kinase libraries were screened based on Life's LanthaScreenbinding and ZLYTE platforms. The results showed that, in addition to the known targets VEGFR2 and KIT, apatinib can bind to BRAF V600E protein kinase, and has a good inhibitory activity on it, with an inhibition rate of 51%.
Embodiment 2
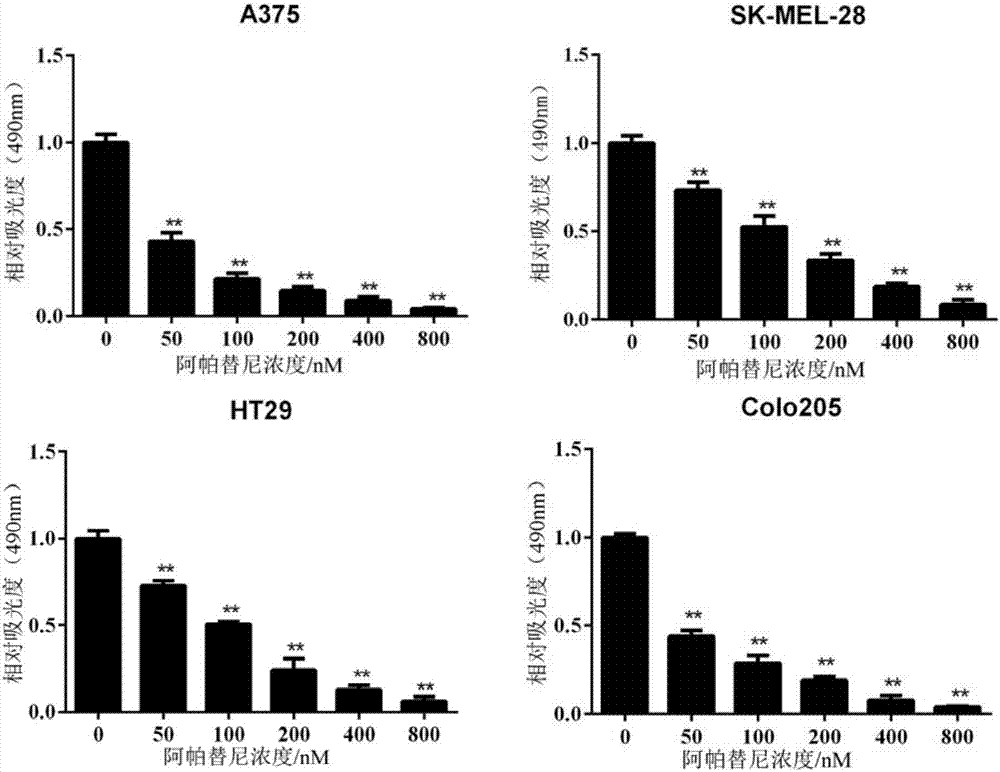
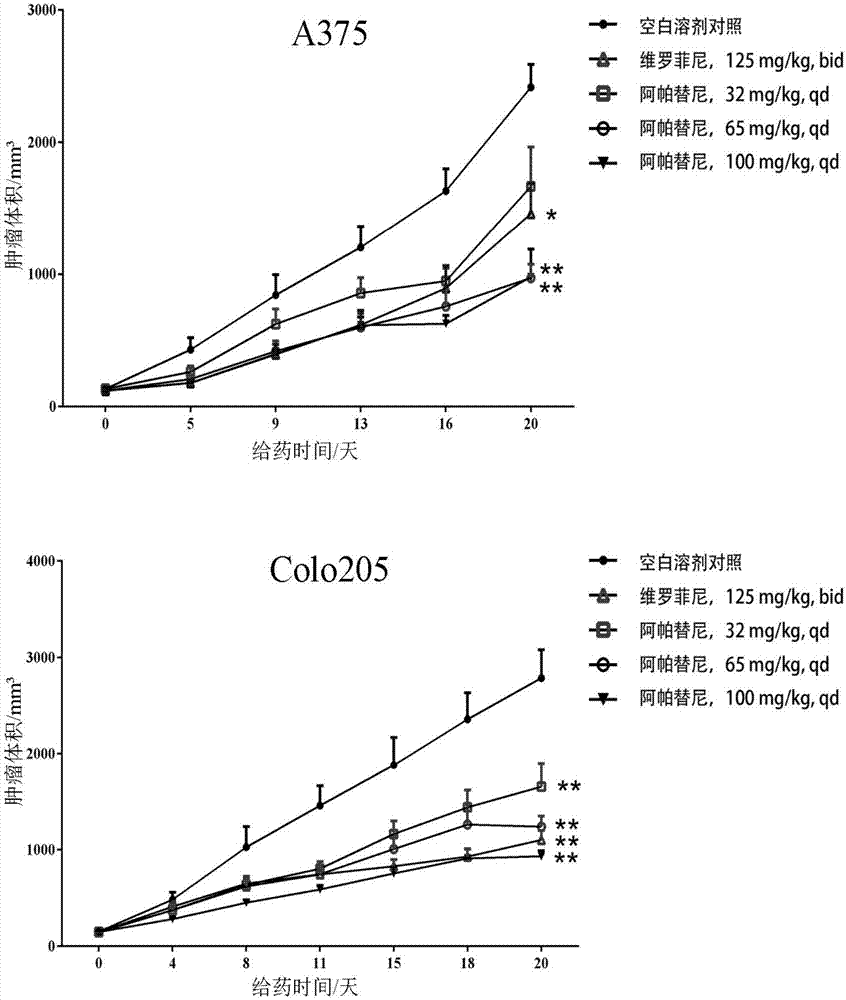
[0030] Example 2. Inhibitory Effect of Apatinib on the Growth of Human Melanoma and Colorectal Cancer Cell Lines in Vitro
[0031] Human melanoma cells (A375, SK-MEL-28) and colorectal cancer cells (Colo205, HT29) in the logarithmic growth phase were taken, and the cell suspension was prepared by trypsinization method, and the cell pellet was collected by centrifugation. RPMI1640 complete medium (the final concentrations of apatinib were 0, 50, 100, 200, 400, 800 nM) to adjust the cell density to 1 × 10 5 cells / mL, inoculate cells into 96-well cell culture plate, add 100 μL cell suspension to each well, shake the culture plate to make it evenly distributed, 37 and 5% CO 2 The cells were cultured under these conditions, and the cell viability was detected by CCK-8 method 48 hours after inoculation. Before detection, add 10 μL of CCK-8 solution to each well of cells, shake gently to make it evenly distributed, and store at 37 and 5% CO 2 Continue culturing for 4 hours, detect ...
Embodiment 3
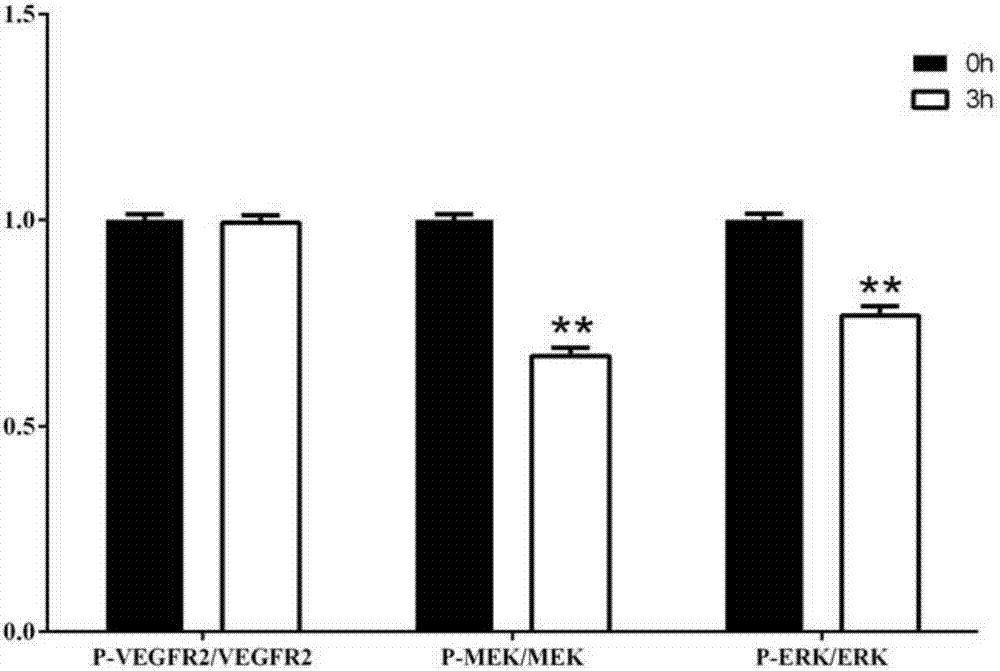
[0033] Example 3. Inhibitory Effect of Apatinib on MAPK Signaling Pathway
[0034] Human melanoma A375 cells in the logarithmic growth phase were taken, and the cell suspension was prepared by trypsinization method, and the cell pellet was collected by centrifugation, and the cell density was adjusted to 1×10 5 cells / mL, inoculate cells into 6-well cell culture plate, add 2mL cell suspension to each well, gently shake the culture plate to make the distribution even, 37 and 5% CO 2 Cultivate under conditions, and remove the medium 3 hours after inoculation, add 1mL dPBS to the culture plate to wash the cells twice, remove the dPBS, add 0.5mL cell lysate to each well, extract the total cell protein according to the kit instructions, and quantify it by BCA method Total cell protein concentration. Quantified total cell protein, 10 μL per group for vertical electrophoresis, SDS-polyacrylamide gel separation gel concentration is 10%, after 90 minutes of electrophoresis at 110V vol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com