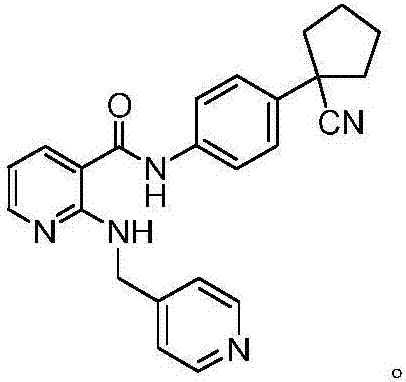
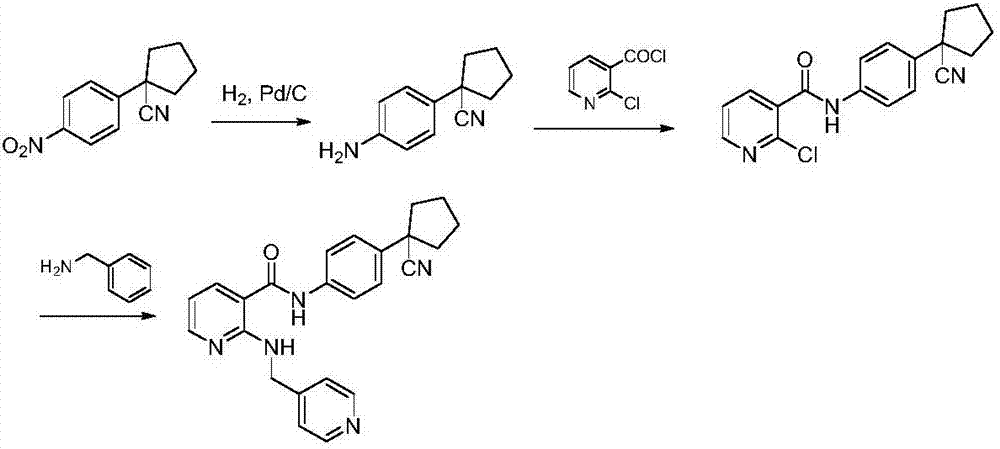
Synthesis method of apatinib for treating stomach cancer
A synthesis method and apatinib technology, applied in the field of drug synthesis, can solve the problems of harsh reaction conditions, difficult purification of products and yields, etc., and achieve the effects of few reaction steps, simple reaction and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
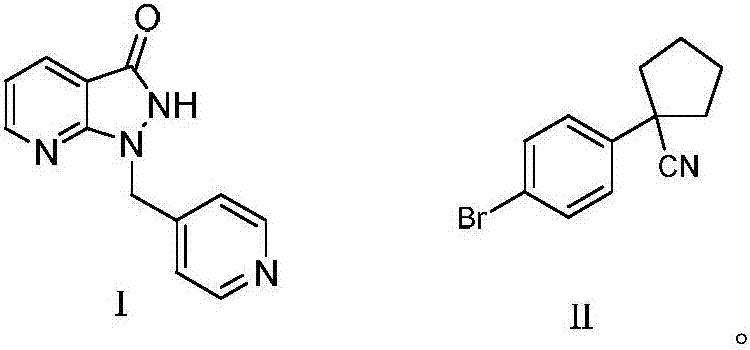
[0028] Synthesis of compounds shown in formula I
[0029] 37.7g (220mmol) of methyl 2-chloro-3-pyridinecarboxylate and 127.2g (1.2mol) of sodium carbonate were mixed in 240ml mixed solvent at room temperature, and said mixed solvent was acetonitrile and A mixed solution of water, then 23.8g (200mmol) of 4-diazomethyl-pyridine was added to the system to carry out a contact reaction for 3 hours, and the reaction was monitored, and the reaction liquid was added with water, extracted with dichloromethane, and dried over anhydrous sodium sulfate , concentrated under reduced pressure, recrystallized from petroleum ether, and dried to obtain 39.6 g of the compound represented by formula I, with a yield of 87.6% and a HPLC purity of 99.31%. MS(ESI):m / z[M+H] + 227.10.
[0030] 1 HNMR (400MHz, CDCl 3 ): δ10.17(s,1H),8.26-8.19(d,2H),8.15-8.09(d,1H),7.79-7.71(d,1H),7.48-7.44(d,2H),6.76-6.70 (m, 1H), 4.57-5.49 (d, 2H).
Embodiment 2
[0032] Synthesis of compounds shown in formula I
[0033] 20.6g (120mmol) of methyl 2-chloro-3-pyridinecarboxylate was mixed with 55.2g (400mmol) of potassium carbonate in 160ml mixed solvent at room temperature, and the mixed solvent was acetonitrile and water with a volume ratio of 8:1 Then 11.9g (100mmol) of 4-diazomethyl-pyridine was added to the system for contact reaction for 3.5 hours, and the reaction was monitored. Water was added to the reaction solution, extracted with dichloromethane, and dried over anhydrous sodium sulfate. Concentrate under reduced pressure, recrystallize from petroleum ether, and dry to obtain 20 g of the compound represented by formula I, with a yield of 88.3% and a purity of 99.22% by HPLC.
Embodiment 3
[0035] Synthesis of compounds shown in formula I
[0036] 18.9g (110mmol) of methyl 2-chloro-3-pyridinecarboxylate and 41.4g (300mmol) of potassium carbonate were mixed in 180ml mixed solvent at room temperature, and the mixed solvent was acetonitrile and water with a volume ratio of 10:1 4-diazomethyl-pyridine 11.9g (100mmol) was then added to the system for contact reaction for 3 hours, monitored until the reaction was complete, adding water to the reaction solution, extracting with dichloromethane, drying over anhydrous sodium sulfate, Concentrate under reduced pressure, recrystallize from petroleum ether, and dry to obtain 19.6 g of the compound represented by formula I, with a yield of 86.8% and an HPLC purity of 99.40%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com