Method for determining apatinib drug concentration in human plasma
A technology of apatinib and drug concentration, which is applied in the field of determination of apatinib drug concentration in human plasma, can solve the problems of consumption of organic solvents, long analysis time, large lower limit of quantification, etc., and achieves good reproducibility and sensitivity The effect of high, short analysis time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
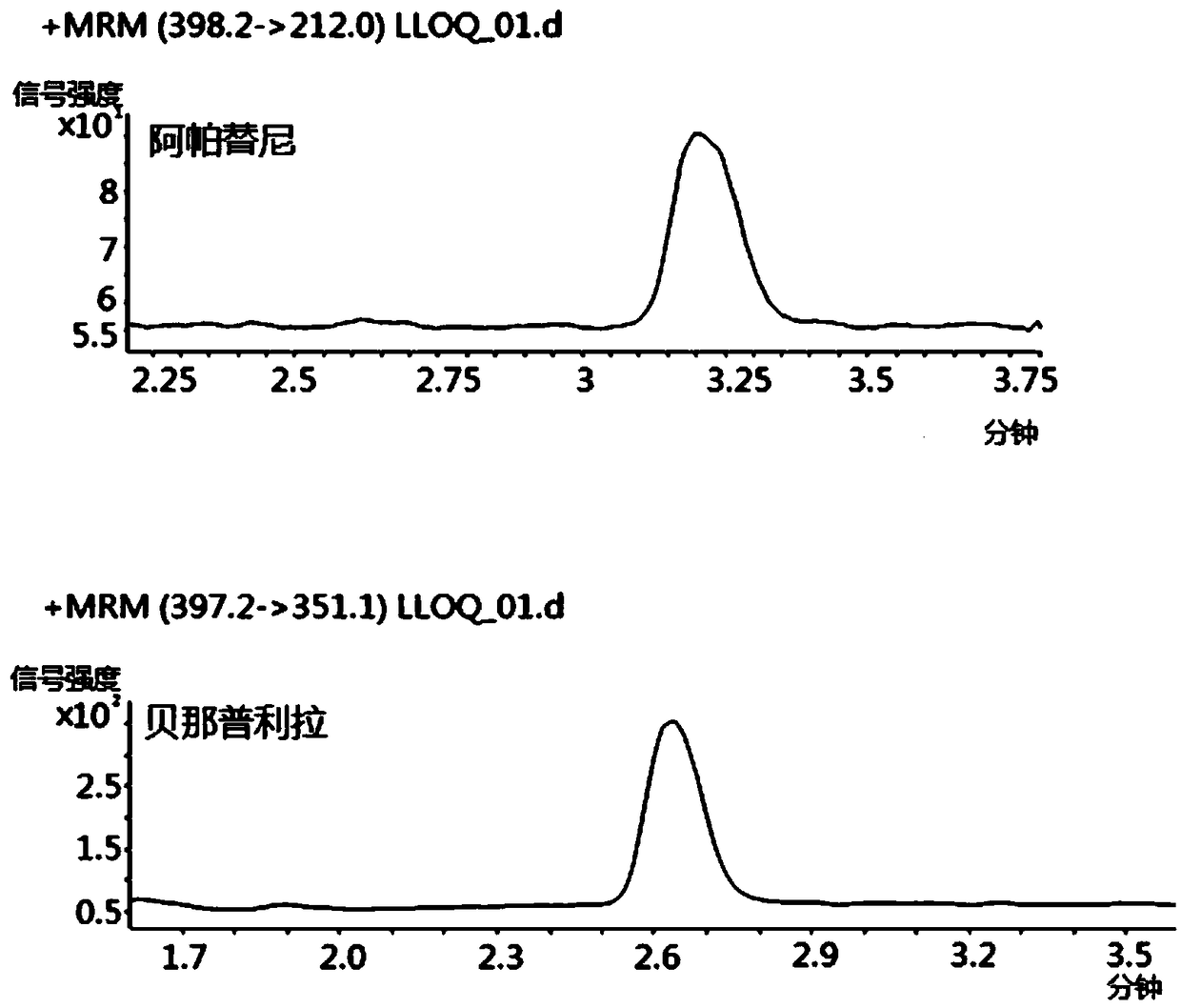
[0034] A method for measuring the drug concentration of apatinib in human plasma, which is detected by high performance liquid chromatography tandem mass spectrometry, comprising the following steps:
[0035] (1) Preparation of standard working solution
[0036] Accurately weigh 1.02 mg of apatinib standard substance and place it in a 1.5mL centrifuge tube, accurately add 1.02mL of pure methanol solution to dissolve, and obtain standard stock solution A, that is, apatinib solution with a concentration of 1mg / mL. Stock solution A was diluted with pure methanol solution, diluted into apatinib solutions with concentrations of 0.04, 0.1, 0.2, 0.5, 1.0, 4.0, 10.0, 20.0, 40.0ug / mL, and stored at -80°C;
[0037] (2) Preparation of internal standard working solution
[0038] Accurately weigh 1.0 mg of benazeprilat standard substance and place it in a 1.5 mL centrifuge tube, accurately add 1.0 mL of pure methanol solution to dissolve to obtain standard stock solution B, dilute standar...
Embodiment 2
[0046] (1) Preparation of standard working solution
[0047] Accurately weigh 1.02 mg of apatinib standard substance and place it in a 1.5mL centrifuge tube, accurately add 1.02mL of pure methanol solution for dissolution to obtain standard stock solution A, that is, apatinib solution with a concentration of 1mg / mL, and standard Stock solution A was diluted with pure methanol solution, diluted into apatinib solutions with concentrations of 0.04, 0.1, 0.2, 0.5, 1.0, 4.0, 10.0, 20.0, 40.0ug / mL, and stored at -80°C;
[0048] (2) Preparation of internal standard working solution
[0049] Accurately weigh 1.0 mg of benazeprilat standard substance and place it in a 1.5 mL centrifuge tube, accurately add 1.0 mL of pure methanol solution to dissolve to obtain standard stock solution B, dilute standard stock solution B with pure methanol to obtain a concentration of 5ug / mL internal standard working solution, and stored at -80°C;
[0050] (3) Detection of centrifugation of blood
[0...
Embodiment 3
[0057] (1) Preparation of standard working solution
[0058] Accurately weigh 1.02 mg of apatinib standard substance and place it in a 1.5mL centrifuge tube, accurately add 1.02mL of pure methanol solution for dissolution to obtain standard stock solution A, that is, apatinib solution with a concentration of 1mg / mL, and standard Stock solution A was diluted with pure methanol solution, diluted into apatinib solutions with concentrations of 0.04, 0.1, 0.2, 0.5, 1.0, 4.0, 10.0, 20.0, 40.0ug / mL, and stored at -80°C;
[0059] (2) Preparation of internal standard working solution
[0060] Accurately weigh 1.0 mg of benazeprilat standard substance and place it in a 1.5 mL centrifuge tube, accurately add 1.0 mL of pure methanol solution to dissolve to obtain standard stock solution B, dilute standard stock solution B with pure methanol to obtain a concentration of 5ug / mL internal standard working solution, and stored at -80°C;
[0061] (3) Detection of centrifugation of blood
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com