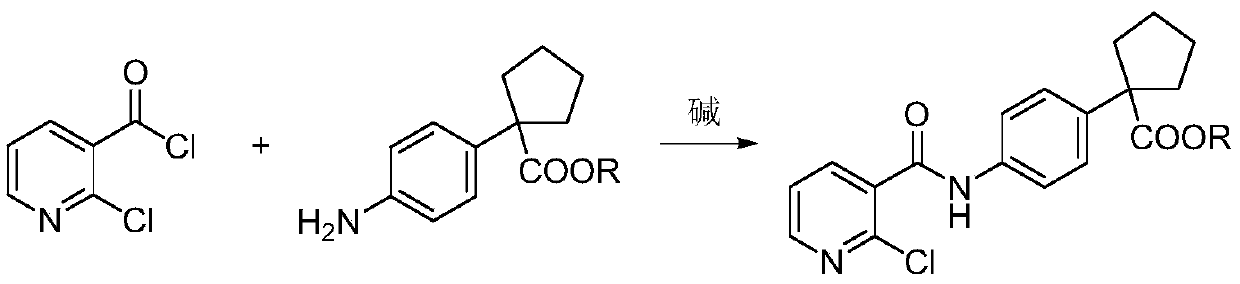
Preparation method for apatinib
A technology of apatinib and phenyl, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of difficult elimination of impurities, difficult separation and purification, and high cost, and achieve the effects of less impurities, lower costs, and simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
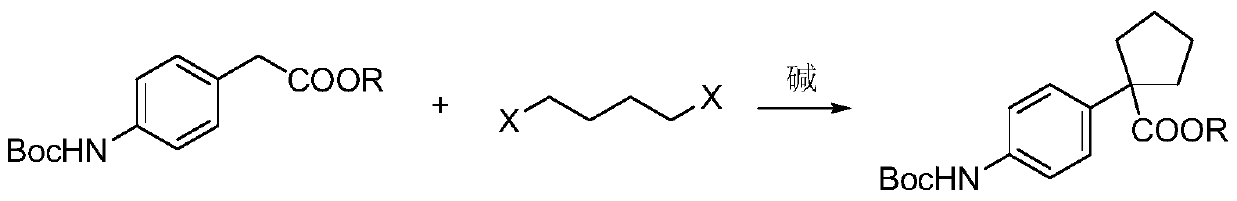
[0053] A) Preparation of methyl 1-[4-(tert-butoxycarbonylamino)phenyl]cyclopentanecarboxylate:
[0054] Add 60% sodium hydride (5.7g, 141.3mmol), chloroform (300mL) and methyl 4-(tert-butoxycarbonylamino)phenylacetate (25.0g, 94.2mmol) to the reaction flask, stir for 10min, add dropwise 1,4 -Dibromobutane (24.4g, 113.1mmol) in chloroform (20mL) solution, the reaction mixture was stirred and reacted at 60°C for 12h, after the reaction was completed, the reaction solution was lowered to 5°C, dilute hydrochloric acid was added dropwise to adjust the pH=7, and the reaction solution reduced Remove the organic solvent by rotary evaporation, add ethyl acetate for extraction, dry over magnesium sulfate, concentrate to dryness by rotary evaporation, recrystallize from isopropanol, and dry to obtain 1-[4-(tert-butoxycarbonylamino)phenyl]cyclopentane Methyl formate, off-white to white solid (26.8g), yield 89.0%;
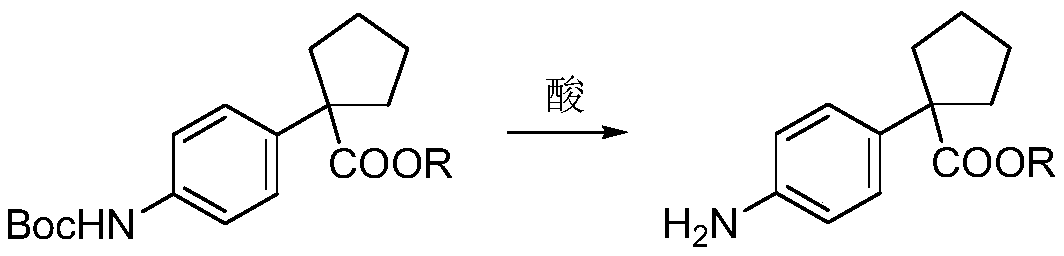
[0055] B) Preparation of methyl 1-(4-aminophenyl)cyclopentanecarboxylate:...
Embodiment 2
[0066] A) Preparation of ethyl 1-[4-(tert-butoxycarbonylamino)phenyl]cyclopentanecarboxylate:
[0067] Potassium hydride (49.5g, 1.23mol), toluene (1400mL) and ethyl 4-(tert-butoxycarbonylamino)phenylacetate (123.0g, 0.44mol) were added to the reaction flask, stirred for 10min, and 1,4-bis Bromobutane (190.1g, 0.88mol) in toluene (200mL) solution, the reaction mixture was stirred and reacted at 80°C for 10h, after the reaction was completed, the reaction solution was lowered to 5°C, dilute hydrochloric acid was added dropwise to adjust the pH=7, the reaction solution was decompressed and spun Remove the organic solvent by evaporation, extract with ethyl acetate, dry over magnesium sulfate, concentrate to dryness by rotary evaporation, recrystallize from isopropanol, and dry to obtain ethyl 1-[4-(tert-butoxycarbonylamino)phenyl]cyclopentanecarboxylate Ester, off-white to white solid (128.5g), yield 87.5%;
[0068] B) Preparation of ethyl 1-(4-aminophenyl)cyclopentanecarboxylat...
Embodiment 3
[0079] A) Preparation of methyl 1-[4-(tert-butoxycarbonylamino)phenyl]cyclopentanecarboxylate:
[0080] Potassium tert-butoxide (296.1g, 2.64mol), N,N-dimethylformamide (5000mL) and methyl 4-(tert-butoxycarbonylamino)phenylacetate (200.0g, 0.75mol) were added to the reaction flask, Stir for 10 min, add dropwise a solution of 1,4-dichlorobutane (239.4g, 1.88mol) in N,N-dimethylformamide (300mL), and raise the reaction mixture to 100°C and stir for 6h. After the reaction is complete, the reaction solution Decrease to 5°C, add dilute hydrochloric acid dropwise to adjust the pH to 7, remove the organic solvent by rotary evaporation under reduced pressure, add ethyl acetate for extraction, dry over magnesium sulfate, concentrate to dryness by rotary evaporation, recrystallize from isopropanol, and dry to obtain 1 -[4-(tert-butoxycarbonylamino)phenyl]cyclopentanecarboxylic acid methyl ester, off-white to white solid (219.1g), yield 91.0%;
[0081] B) preparation of 1-(4-aminophenyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com