Preparation method and application of apatinib-coated chitosan sodium alginate microsphere
A technology of sodium alginate and chitosan, which is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc. It can solve the problems of affecting the recovery effect, high cost of apatinib, and patient economics. Burden and other issues, to achieve the effect of reducing economic burden, improving absorption and utilization, and reducing oral intake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
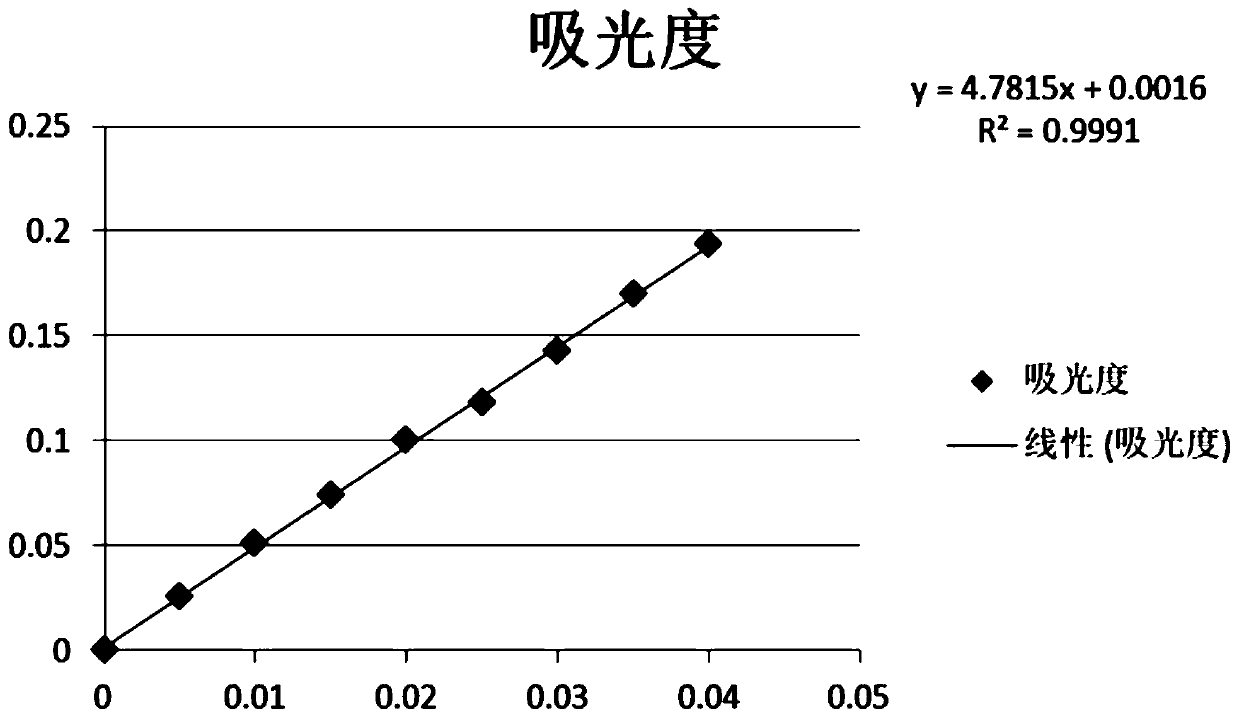
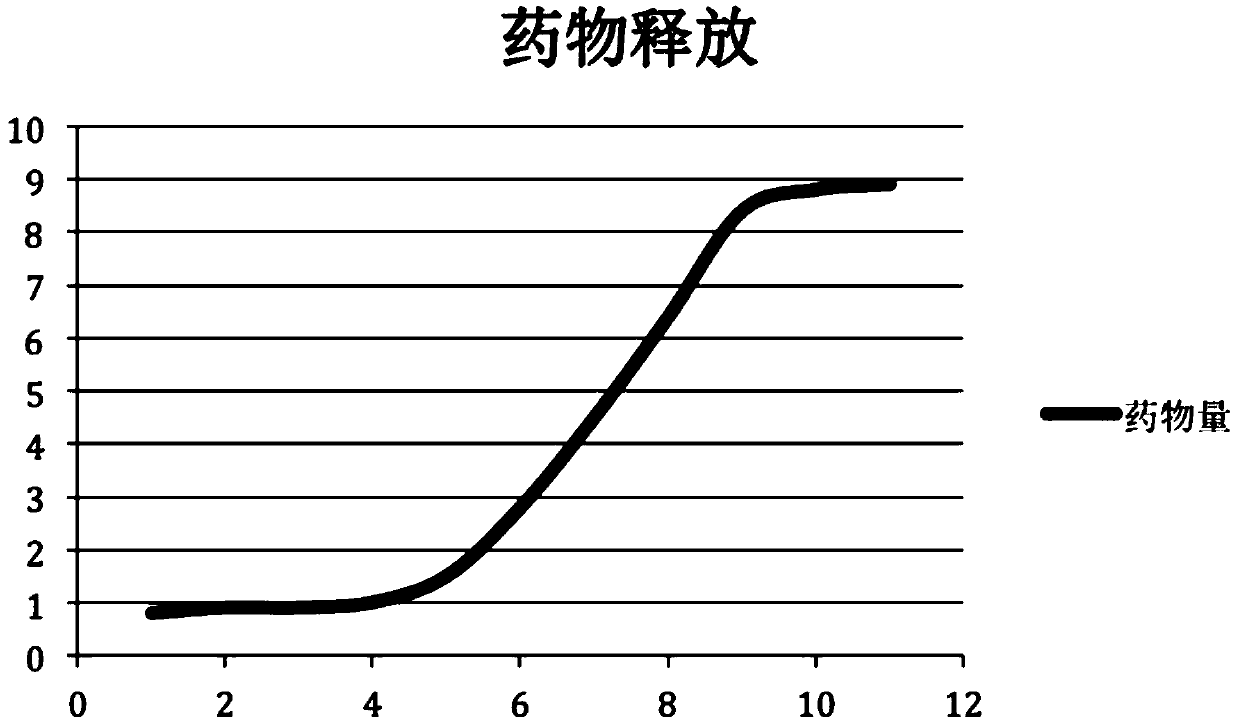
Image
Examples
Embodiment Construction
[0020] The present invention will be further described below in conjunction with the accompanying drawings and specific embodiments.
[0021] Prepare chitosan sodium alginate microspheres loaded with Apatinib according to the following method, comprising the following steps:
[0022] Step 1: prepare an alginate solution with a mass concentration of 0.0315%, and a chitosan solution with a mass concentration of 1% acetic acid of 0.07 wt%; magnetically stir the two solutions overnight for standby;
[0023] Step 2: Prepare an apatinib solution with a concentration of 10 mg / mL, dissolve 10 mg of apatinib in 1ml of methanol solution; mix with 23.5 ml of the alginate solution prepared in step 1, and disperse evenly with ultrasound to obtain a mixture solution;
[0024] Step 3: Dispense 1.5 ml of 0.2 wt% CaCl using a microsyringe pump 2 The solution is injected into the mixed solution obtained in step 2 at a speed of 0.1 ml / min, and magnetically stirred at the same time;
[0025] S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com