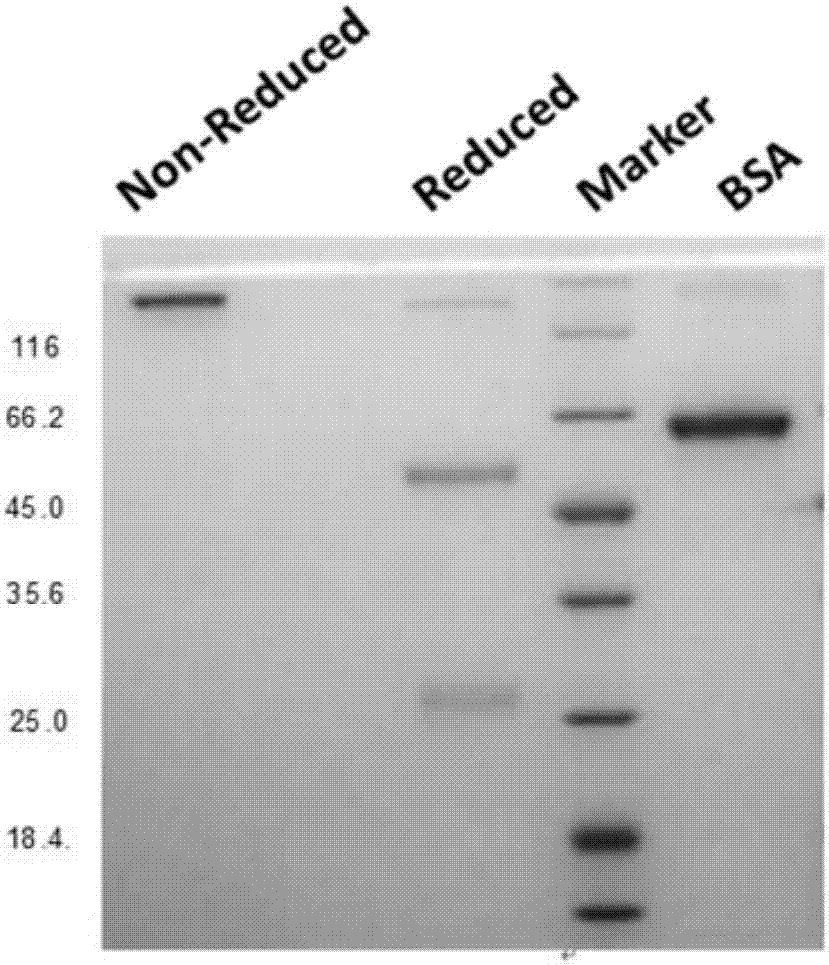
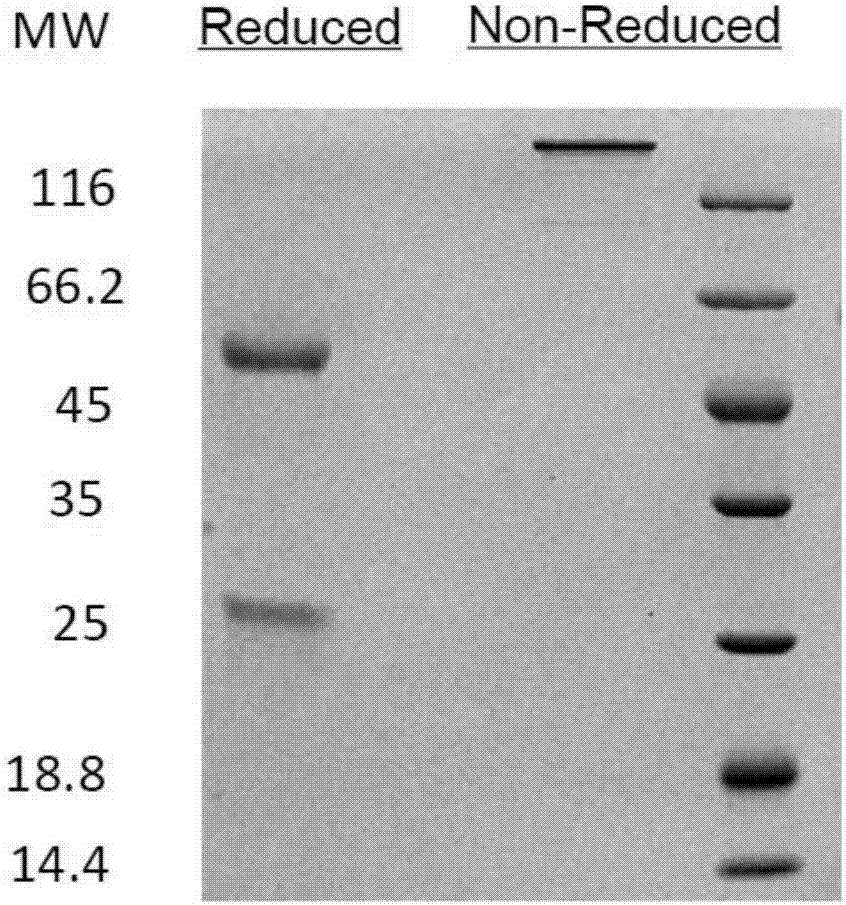
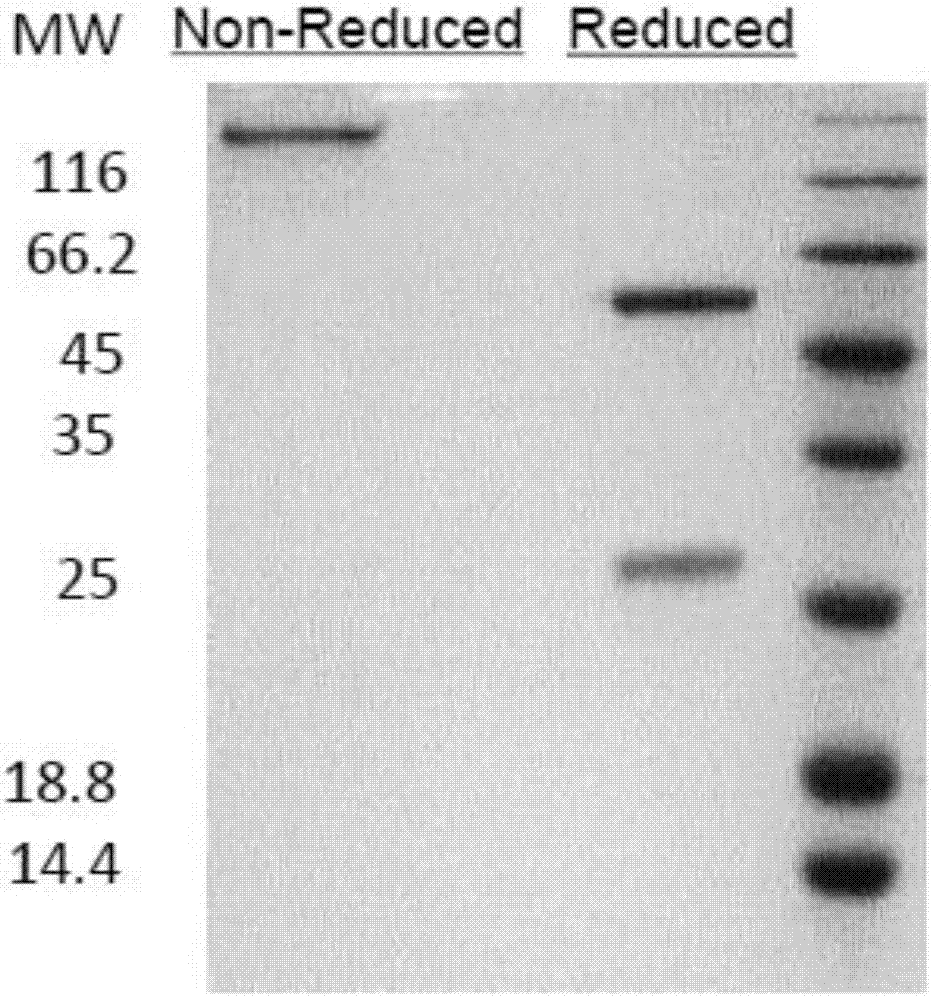
Anti-CTLA4-anti-PD-1 bifunctional antibody, pharmaceutical composition and use thereof
A PD-1, bispecific antibody technology, applied in the field of tumor therapy and molecular immunology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0310] Example 1: Preparation of Anti-CTLA4 Antibody 4G10
[0311] 1. Preparation of hybridoma cell line LT002
[0312] The antigen CTLA4-mFc used to prepare the anti-CTLA4 antibody is a fusion protein of human CTLA4 (GenbankID: NP_005205.2) extracellular region and mouse IgG1Fc. Splenocytes from immunized BALB / C mice (purchased from Guangdong Medical Experimental Animal Center) were fused with mouse myeloma cells to form hybridoma cells, referring to established methods (for example, Stewart, S.J., "Monoclonal Antibody Production", in Basic Methods in Antibody Production and Characterization, Eds. G.C. Howard and D.R. Bethell, Boca Raton: CRC Press, 2000).
[0313] The fusion protein CTLA4-mFc was digested with TEV protease, and the CTLA4 protein was obtained by column purification. Using CTLA4 protein as an antigen to coat the microtiter plate, performing indirect ELISA screening, and obtaining hybridoma cells secreting new antibodies specifically combined with CTLA4. T...
Embodiment 2
[0317] Example 2: Sequence Analysis of Anti-CTLA4 Antibody 4G10
[0318] Sequence Analysis of Antibody 4G10
[0319] The mRNA was extracted from the LT002 cell line cultured in Example 1 according to the method of Cultured Cell Bacteria Total RNA Extraction Kit (Tiangen, Cat. No. DP430).
[0320] According to Invitrogen III First-Strand Synthesis System for RT-PCR Kit Instructions Synthesize cDNA and perform PCR amplification.
[0321] The PCR amplified product was directly cloned by TA, and the specific operation was carried out according to the instructions of the pEASY-T1 Cloning Kit (TransgenCT101) kit.
[0322] The products of TA clones were directly sequenced, and the sequencing results were as follows:
[0323] Nucleic acid sequence of heavy chain variable region: (372bp)
[0324] CAGGTCAAGCTGCAGGAGTCTGGACCTGAGCTGGTGAAGCCTGGAGCTTCAATGAAGATATCCTGCAAGGCTTCTGGTTACTCATTCACTGGCTACACCATGAACTGGGTGAAGCAGAGCCATGGAAAGAACCTTGAATGGATTGGACTTATTAATCCTTACAATAATATTACTAACTACAAC...
Embodiment 3
[0332] Example 3: Design and preparation of anti-CTLA4 humanized antibodies 4G10H1L1, 4G10H3L3 and 4G10H4L3
[0333] 1. Design of light chain and heavy chain sequences of anti-CTLA4 humanized antibodies 4G10H1L1, 4G10H3L3 and 4G10H4L3
[0334] According to the three-dimensional crystal structure of CTLA4 protein (Nat.Struct.Biol.(1997) 4p.527) and the sequence of antibody 4G10 obtained in Example 2, the antibody model was simulated by computer, and mutations were designed according to the model to obtain antibodies 44G10H1L1, 4G10H3L3 and 4G10H4L3 The variable region sequence (antibody constant region sequence, from NCBI database, the heavy chain constant region is Iggamma-1chain C region, ACCESSION: P01857, the light chain constant region is Ig kappa chain Cregion, ACCESSION: P01834).
[0335] The designed variable region sequence is as follows:
[0336] (1) Heavy chain and light chain sequences of humanized monoclonal antibody 4G10H1L1
[0337] Nucleic acid sequence of h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com