Injection preparation of anti-IL-17RA monoclonal antibody
A technology for monoclonal antibodies and injection preparations, which can be applied in the direction of antibodies, anti-inflammatory agents, and medical preparations with non-active ingredients, which can solve the problems of limited dosing volume and impact.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-15
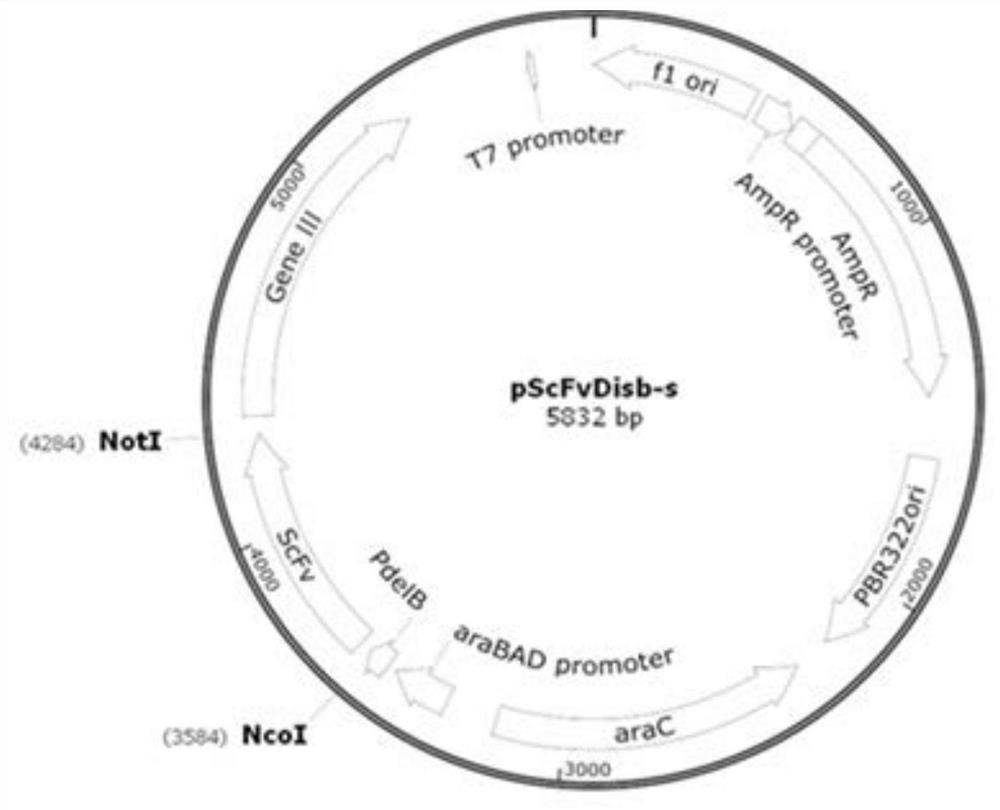
[0050] Examples 1-15 of the present invention all provide an injection preparation of an anti-IL-17RA monoclonal antibody, the preparation includes a pharmacodynamic molecule and a buffer solution, and the pharmacodynamic molecule is an anti-IL-17RA monoclonal antibody with a protein content of 120 mg / ml , wherein, in the preparation provided by the present invention, the anti-IL-17RA monoclonal antibody is screened from a fully synthesized phage library using phage library display technology, and the phage antibody library display technology is a kind of coding exogenous antibody firstly established by Smith. The gene of the source protein or polypeptide is inserted into the phage coat protein gene, and the foreign protein or polypeptide is fused with the phage coat protein and expressed on the surface of the phage. The phage antibody library uses the above principles to express antibodies with different specificities or their functional fragments (Fab, Fv, ScFv) on the surfac...
Embodiment 16-19
[0104] Embodiments 16-19 of the present invention respectively provide an injection preparation of an anti-IL-17RA monoclonal antibody, the preparation includes a pharmacodynamic molecule and a buffer solution, and the pharmacodynamic molecule is an anti-IL-17RA monoclonal antibody with a protein content of 100 mg / ml , the amino acid sequence of the anti-IL-17RA monoclonal antibody is the same as in Examples 1-15.
[0105] The monoclonal antibody also includes a heavy chain constant region and a light chain constant region. The heavy chain constant region is one of human IgG1, IgG2, IgG3, and IgG4, and the light chain constant region is human CK.
[0106] Preferably, the heavy chain constant region is human IgG1. The components and contents of the buffer solutions in the injection preparations provided in Examples 16-19 are shown in Table 2.
[0107] Each component content table in the buffer solution in table 2 embodiment 16-19
[0108]
[0109]
Embodiment 20-22
[0111] Embodiments 20-22 of the present invention respectively provide an injection preparation of an anti-IL-17RA monoclonal antibody, the preparation includes a pharmacodynamic molecule and a buffer solution, and the pharmacodynamic molecule is an anti-IL-17RA monoclonal antibody with a protein content of 150 mg / ml , the amino acid sequence of the anti-IL-17RA monoclonal antibody is the same as in Examples 1-15.
[0112] The monoclonal antibody also includes a heavy chain constant region and a light chain constant region. The heavy chain constant region is one of human IgG1, IgG2, IgG3, and IgG4, and the light chain constant region is human CK.
[0113] The components and contents of the buffer solutions in the injection preparations provided in Examples 20-22 are shown in Table 3.
[0114] Each component content table in the buffer solution in the embodiment 20-22 of table 3
[0115]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com