Sclerostin and the inhibition of wnt signaling and bone formation
A compound, bone injury technology, applied in the field of sclerostin and protein sclerostin, can solve problems such as bone mass increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
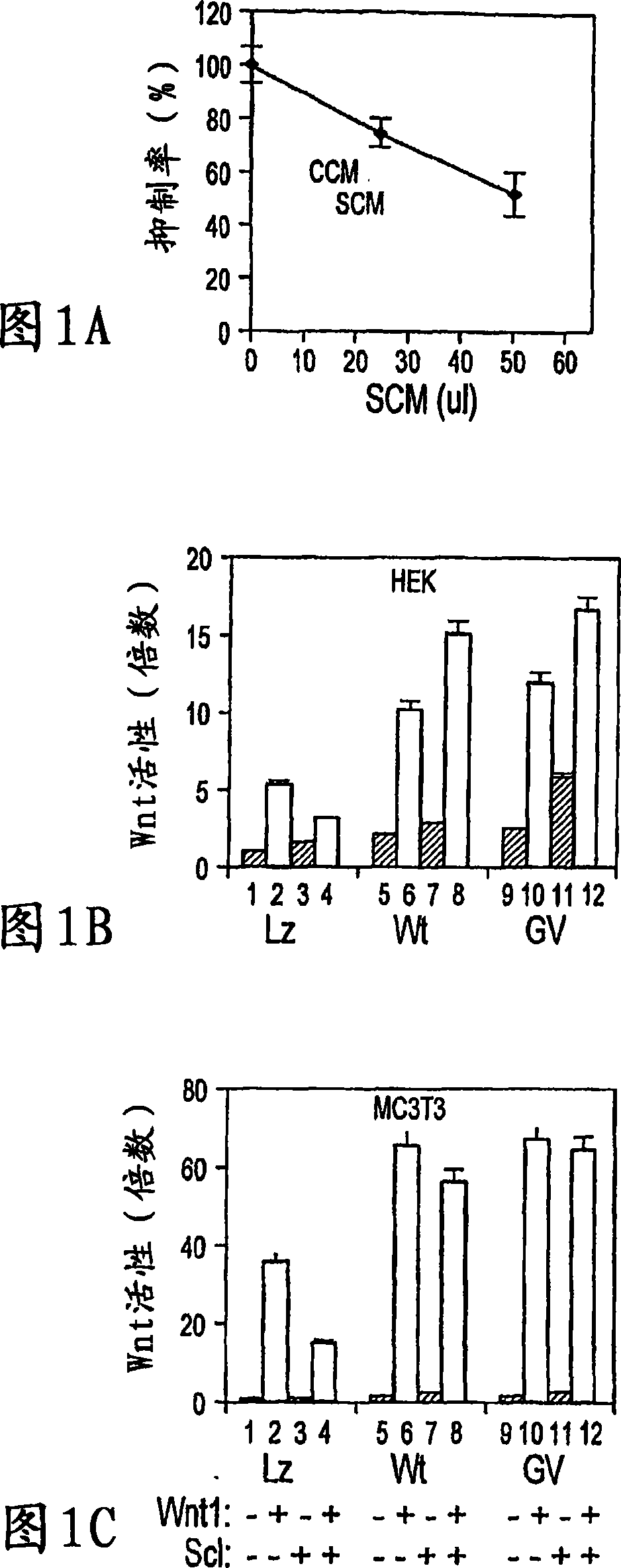
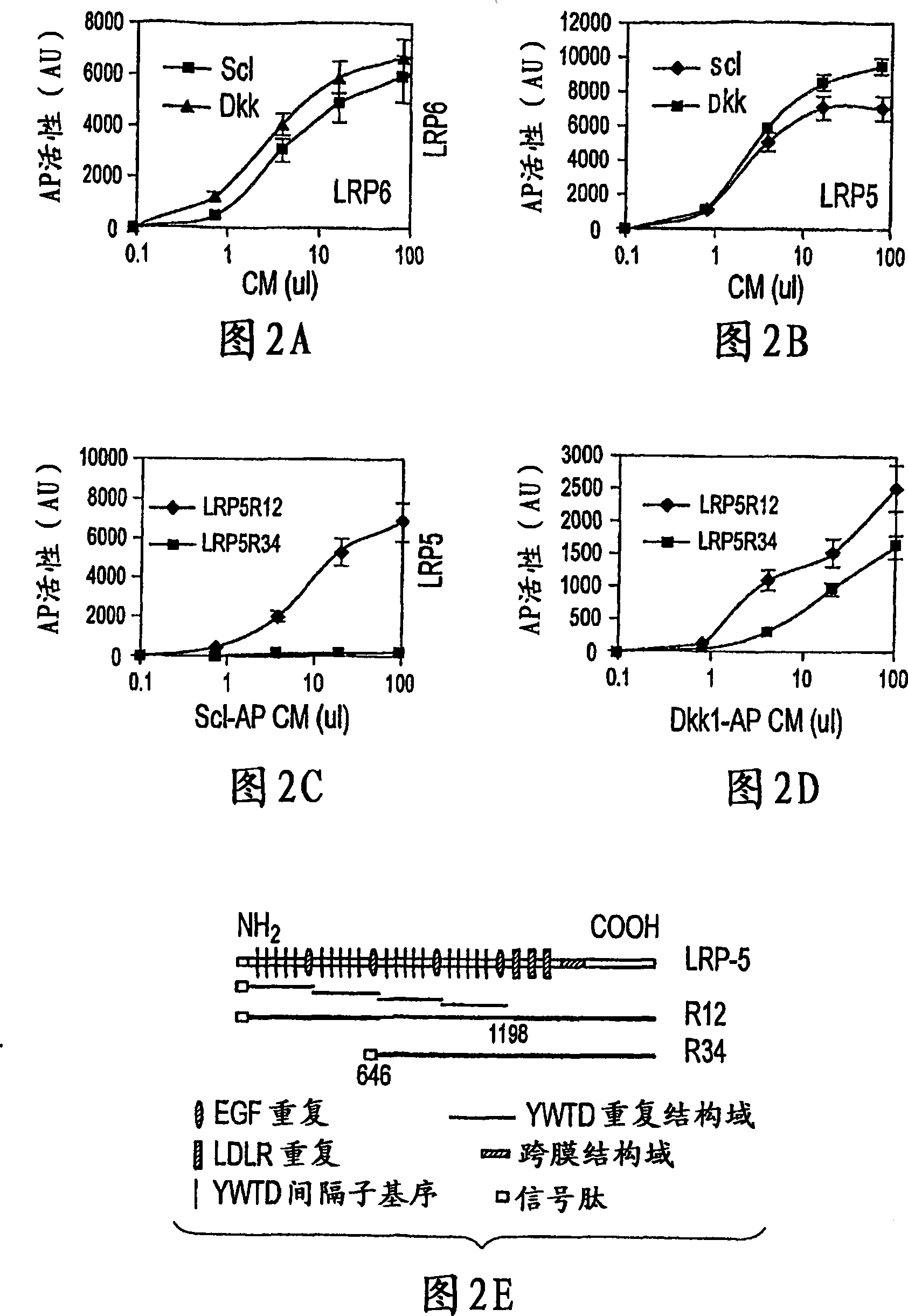
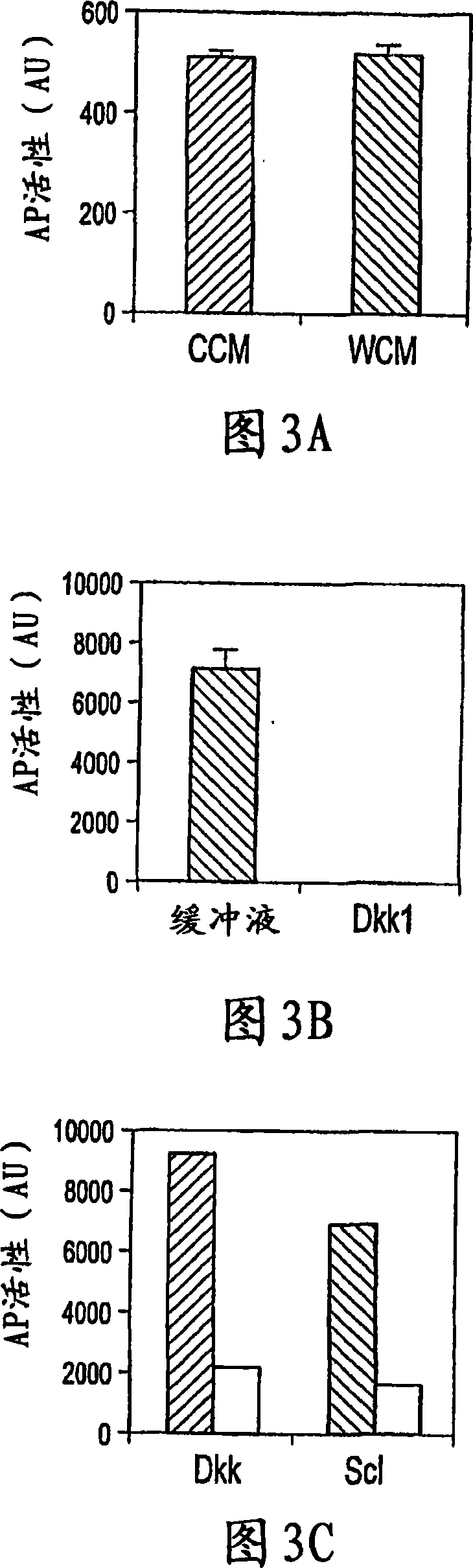
[0014] Because WISE and sclerostin share homology, experiments were performed to determine whether sclerostin could have an effect on canonical Wnt signaling. The effect of conditioned medium (CM) containing mouse sclerostin on Wnt3a-induced activation of canonical Wnt signaling was determined in human embryonic kidney (HEK) cells using a LEF-1-based reporter assay. CM containing sclerostin showed significant inhibition of Wnt3a activity in a dose-dependent manner ( Figure 1A ). Higher doses were not tested as the control CM started to show significant inhibition at 50 microliters. To further confirm this effect of sclerostin, sclerostin and other canonical Wnt (Wnt1) were co-expressed in HEK cells, and sclerostin showed up to 60% inhibition of the activity of co-expressed Wnt-1 ( Figure 1B , column 2&4). Interestingly, coexpression of LRP5 abolished the antagonistic effect of sclerostin on Wnt signaling, and a slight stimulation of Wnt1 signaling by sclerostin was also ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com