Kit for detecting hyaluronic acid and detection method and application of kit
A technology of hyaluronic acid and kits, applied in biological testing, measuring devices, material inspection products, etc., can solve problems such as radioactive pollution, long detection cycle, and unsatisfactory repeatability of ELISA methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
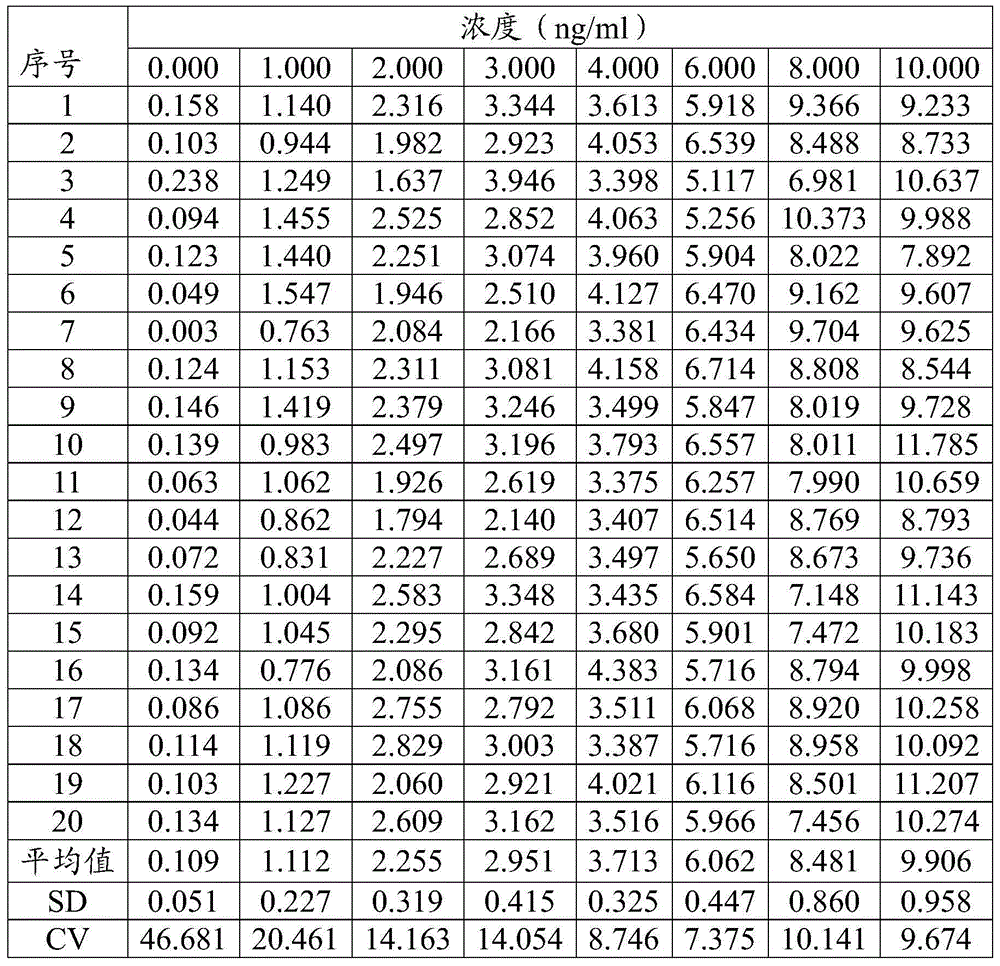
[0063] A test kit for detecting hyaluronic acid, comprising the following components:
[0064] 1) Magnetic microsphere system: magnetic microsphere solution coated with hyaluronic acid antigen (HA antigen).
[0065] Among them, the working concentration of the magnetic microspheres: 0.5 mg / ml, and the working concentration of the HA antigen: 2 μg / ml.
[0066] 2) Marker system: ABEI solution labeled with anti-hyaluronan-binding protein monoclonal antibody (HABP monoclonal antibody).
[0067] Wherein: the working concentration of anti-hyaluronan-binding protein monoclonal antibody: 25 μg / ml, and the working concentration of ABEI: 100 ng / ml.
[0068] 3) Free hyaluronic acid binding protein solution.
[0069] Wherein: the working concentration of hyaluronic acid binding protein: 600ng / ml.
[0070] 4) Calibrator solution: a low-point calibrator solution with a hyaluronic acid concentration of 35.566 ng / ml and a high-point calibrator solution with a concentration of 1124.68 ng / ml...
Embodiment 2
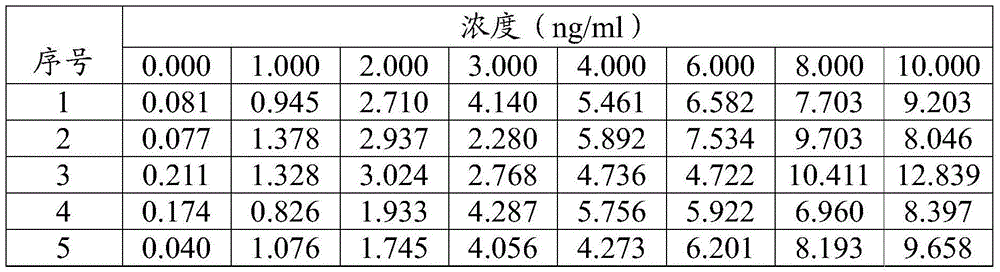
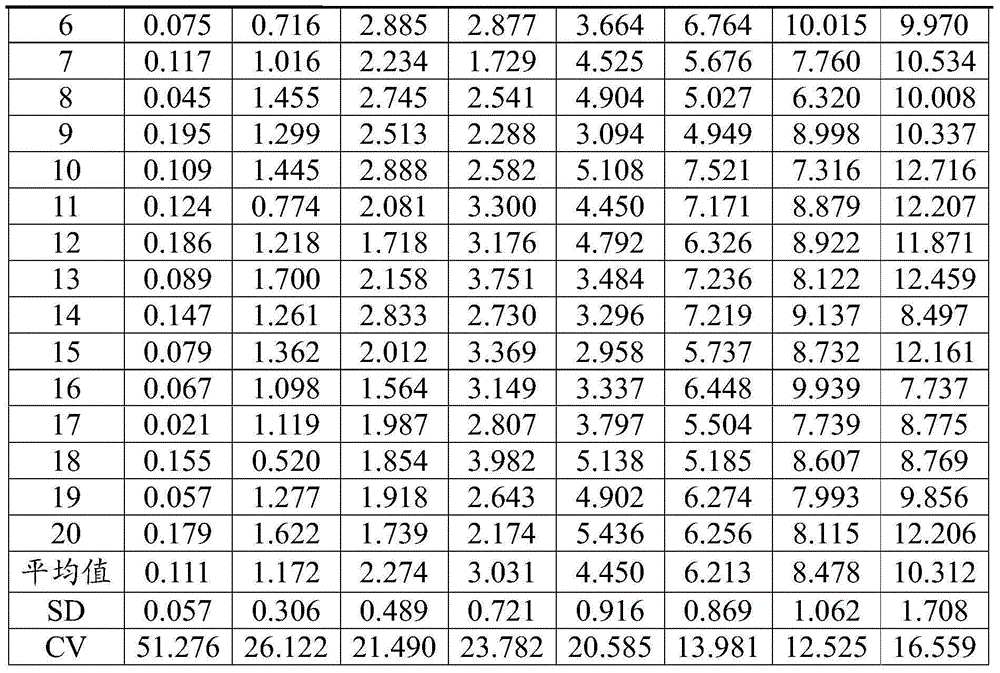
[0096] A test kit for detecting hyaluronic acid, which is the same as the test kit in Example 1.
[0097] The method for detecting hyaluronic acid (one-time cleaning method) of the present embodiment comprises the following steps:
[0098] 1) Reaction: Add 40 μl of the sample to be tested (serum), high and low concentration calibrator into the cuvette respectively, then add 20 μl of magnetic microsphere solution coated with HA antigen, and add 100 μl of free hyaluronic acid binding protein solution at the same time And 100 μl of HABP monoclonal antibody-labeled ABEI solution, incubate at 37°C for 15 minutes, so that the HA in the sample to be tested competes with the HA antigen attached to the magnetic microspheres for binding to the hyaluronic acid binding protein.
[0099] 2) Detection: Apply an external magnetic field to precipitate the above reaction product, remove the supernatant, wash with buffer, and then add chemiluminescent exciters (NaOH and H 2 o 2 ), detect the ...
Embodiment 3
[0101] A test kit for detecting hyaluronic acid, which is basically the same as the test kit in Example 1, except that:
[0102] 1) Magnetic microsphere system:
[0103] A solution of magnetic microspheres coated with streptavidin (SA).
[0104] Among them, the working concentration of the magnetic microspheres: 0.5 mg / ml, and the working concentration of the SA antigen: 1 μg / ml.
[0105] Biotin-labeled hyaluronic acid antigen solution.
[0106] Among them, the working concentration of Biotin: 200ng / ml, the working concentration of HA antigen: 2000ng / ml.
[0107] The preparation method of the kit for detecting hyaluronic acid in this example refers to the preparation method in Example 1, except for the following steps, the rest are the same as the method in Example 1.
[0108] 1. Preparation of magnetic microsphere system.
[0109] 1. Preparation of magnetic microsphere solution coated with streptavidin (SA).
[0110] The preparation method of the magnetic microsphere sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com