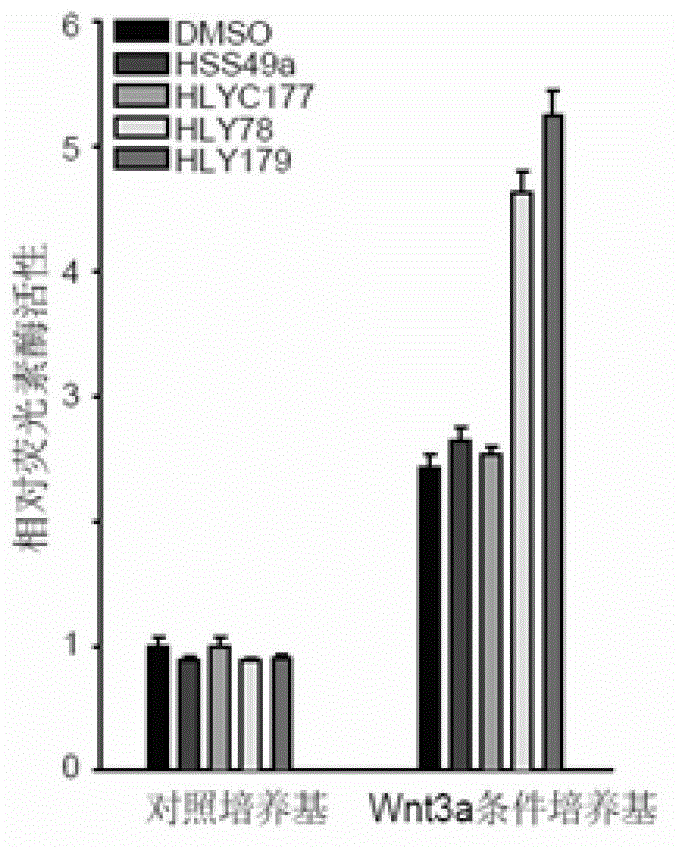
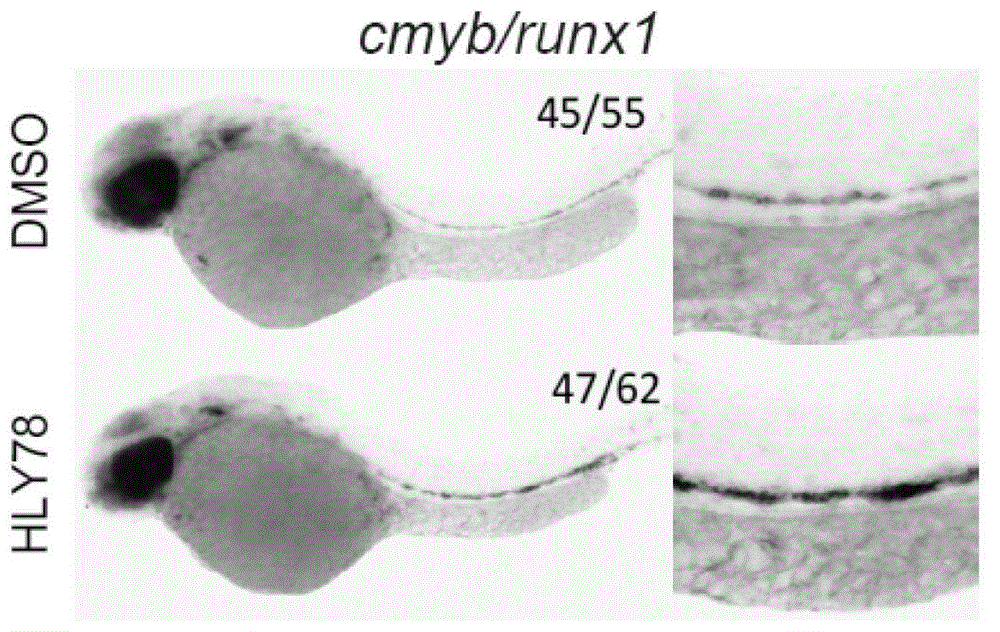
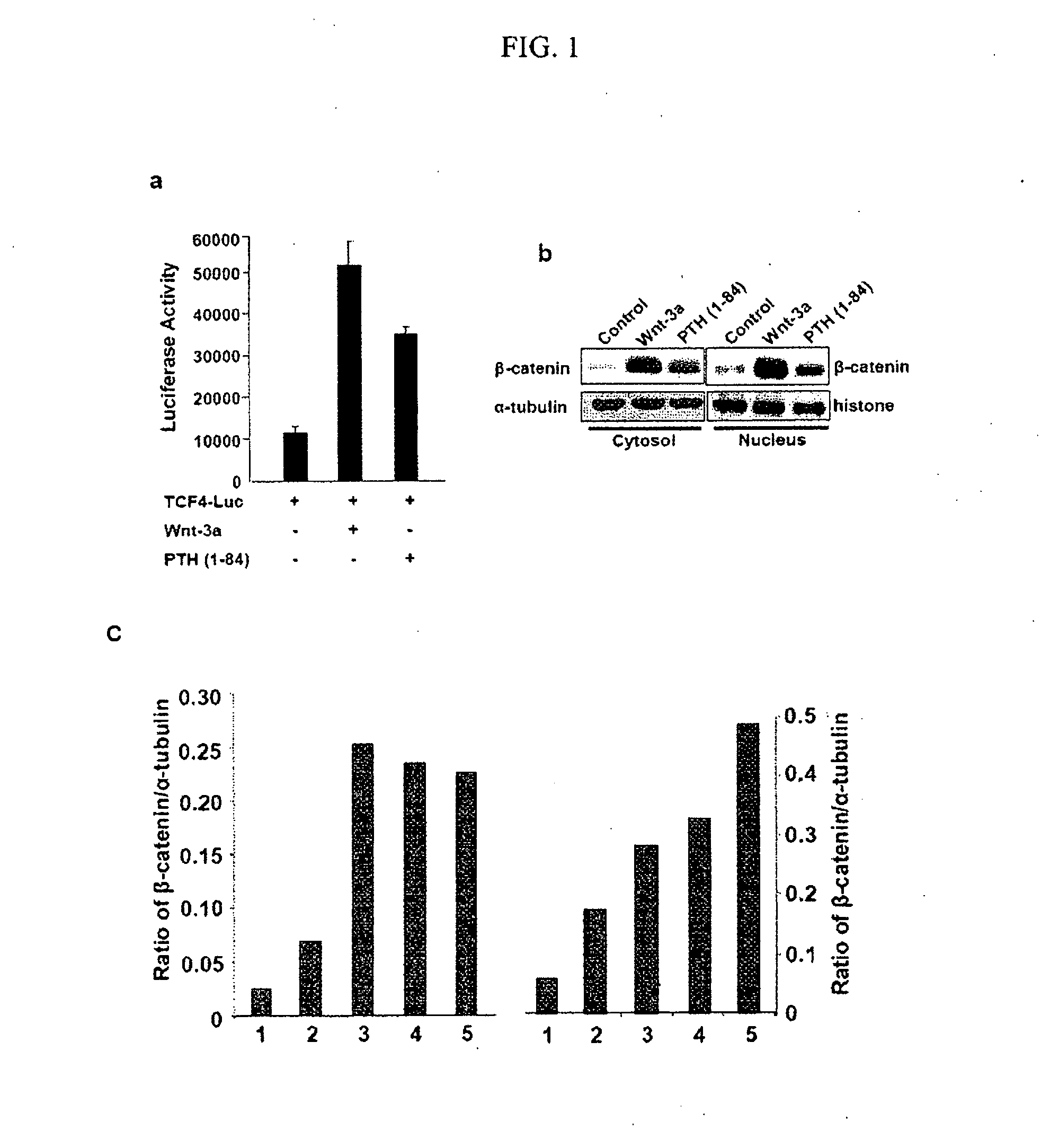
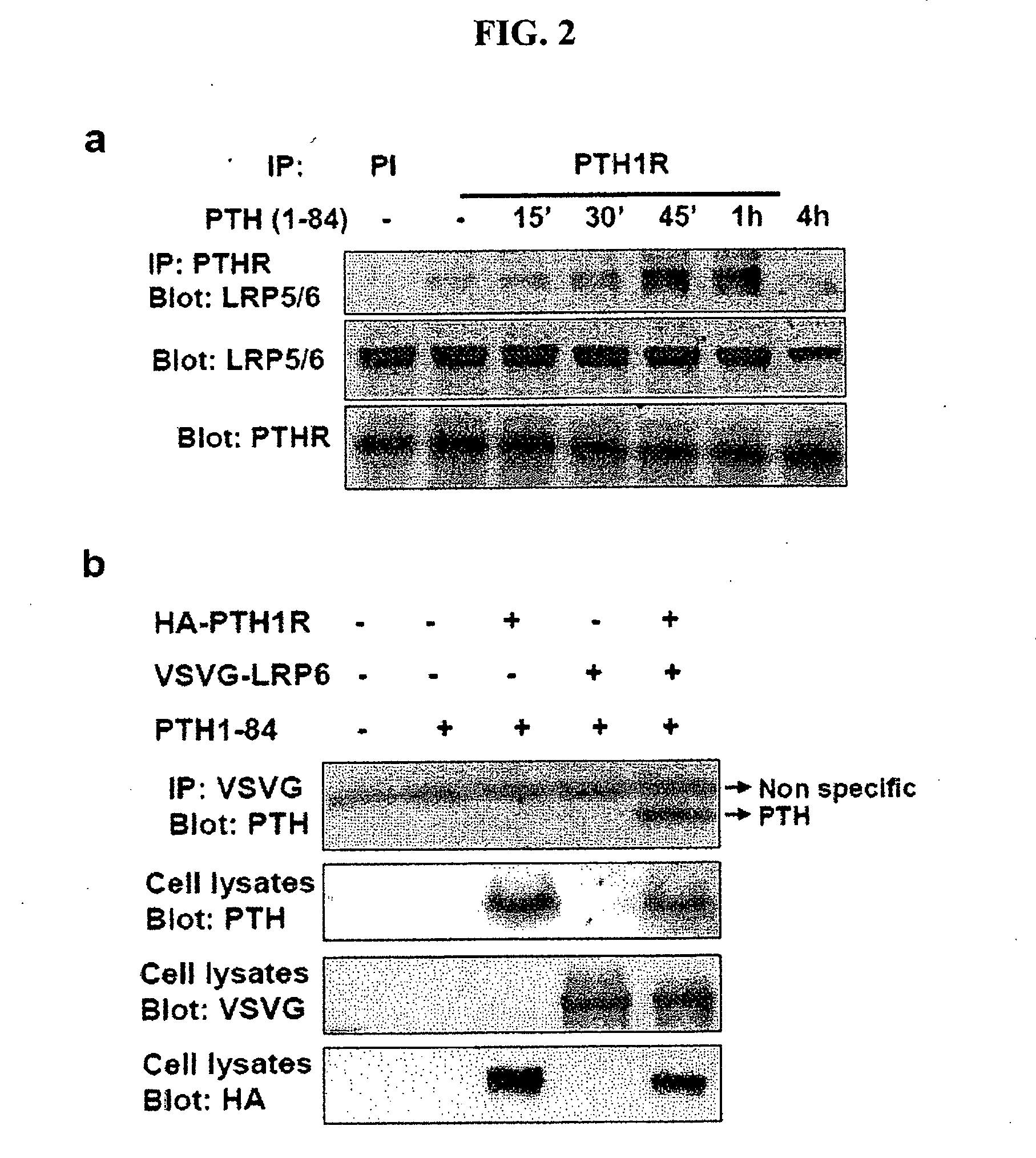
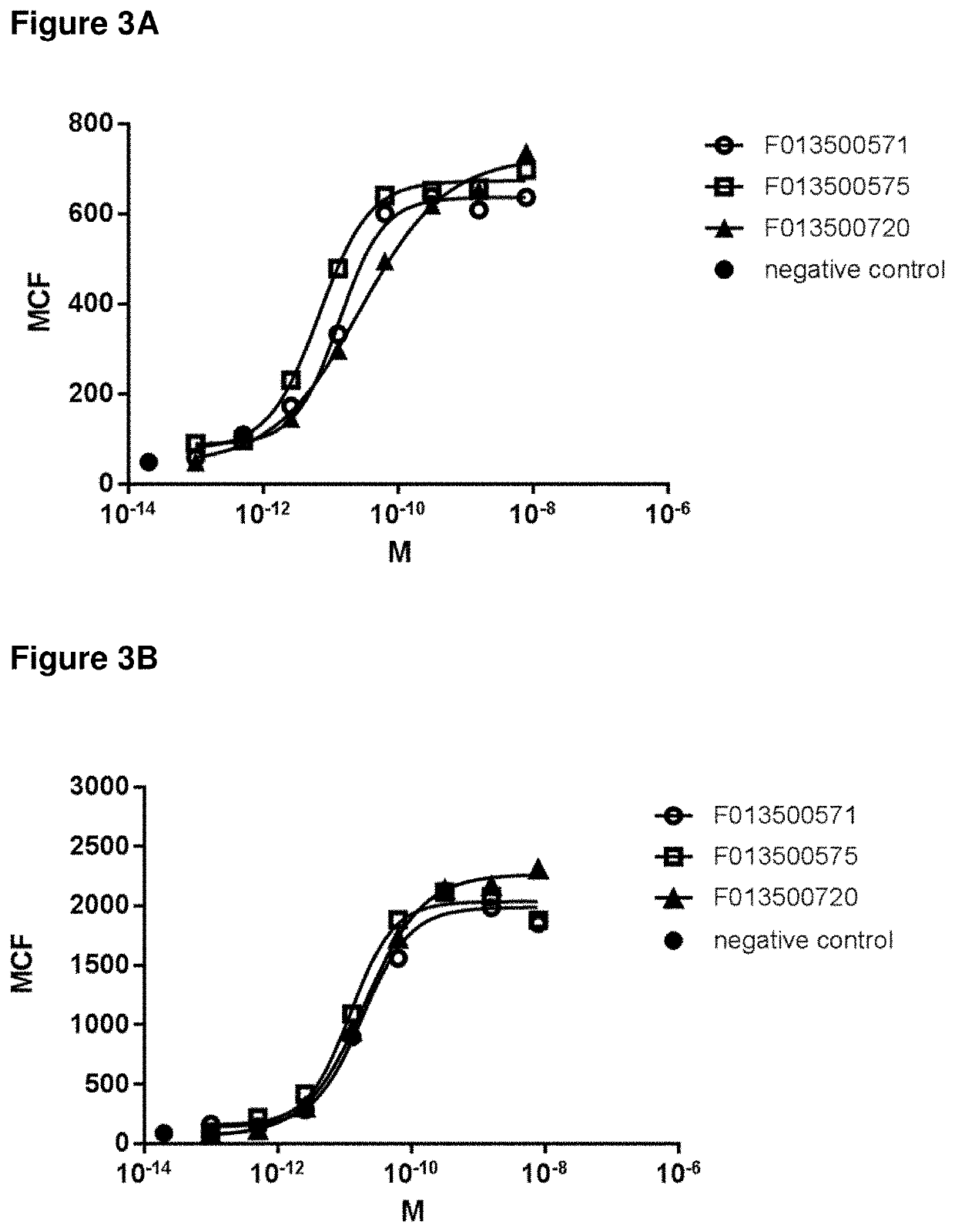
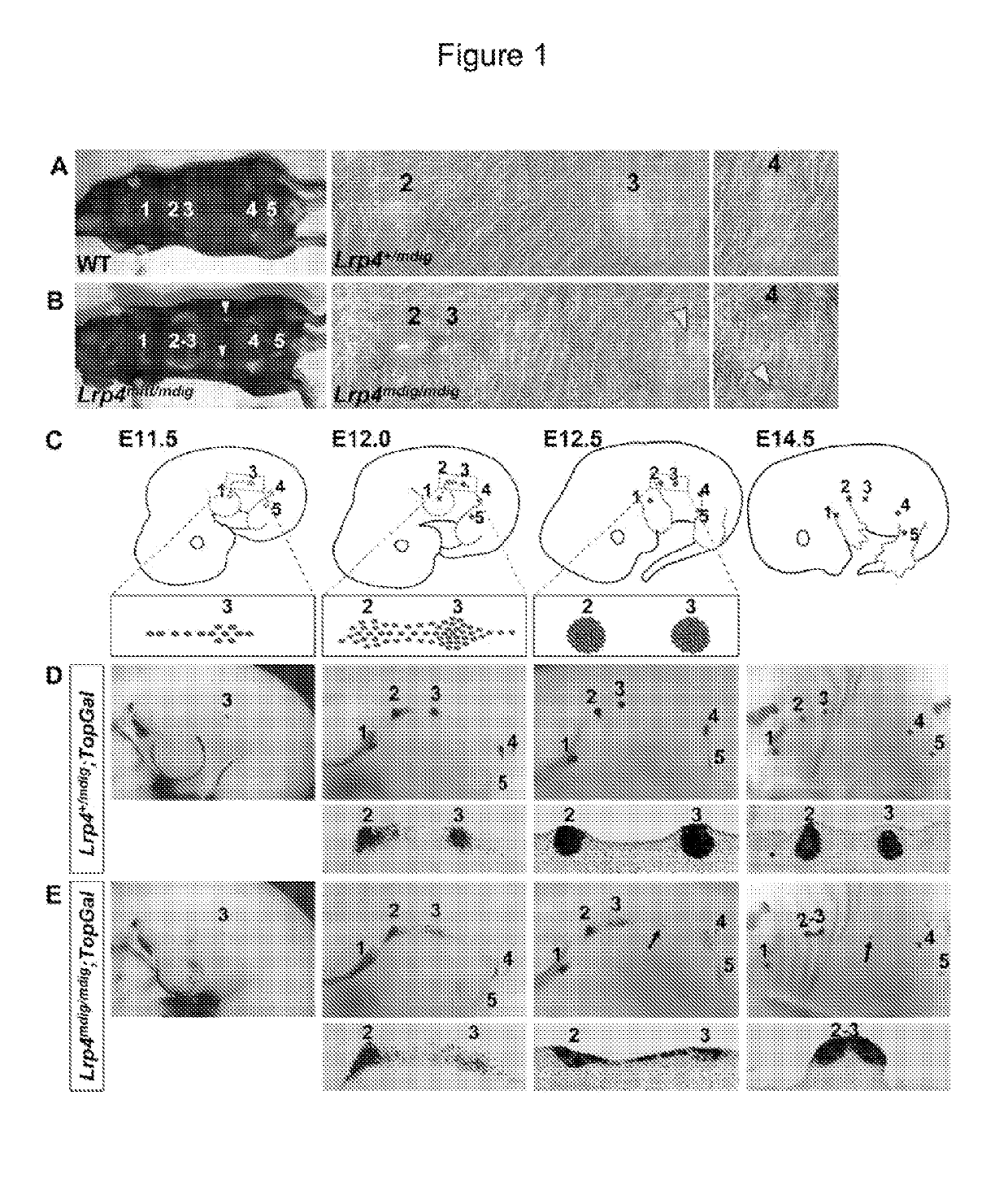
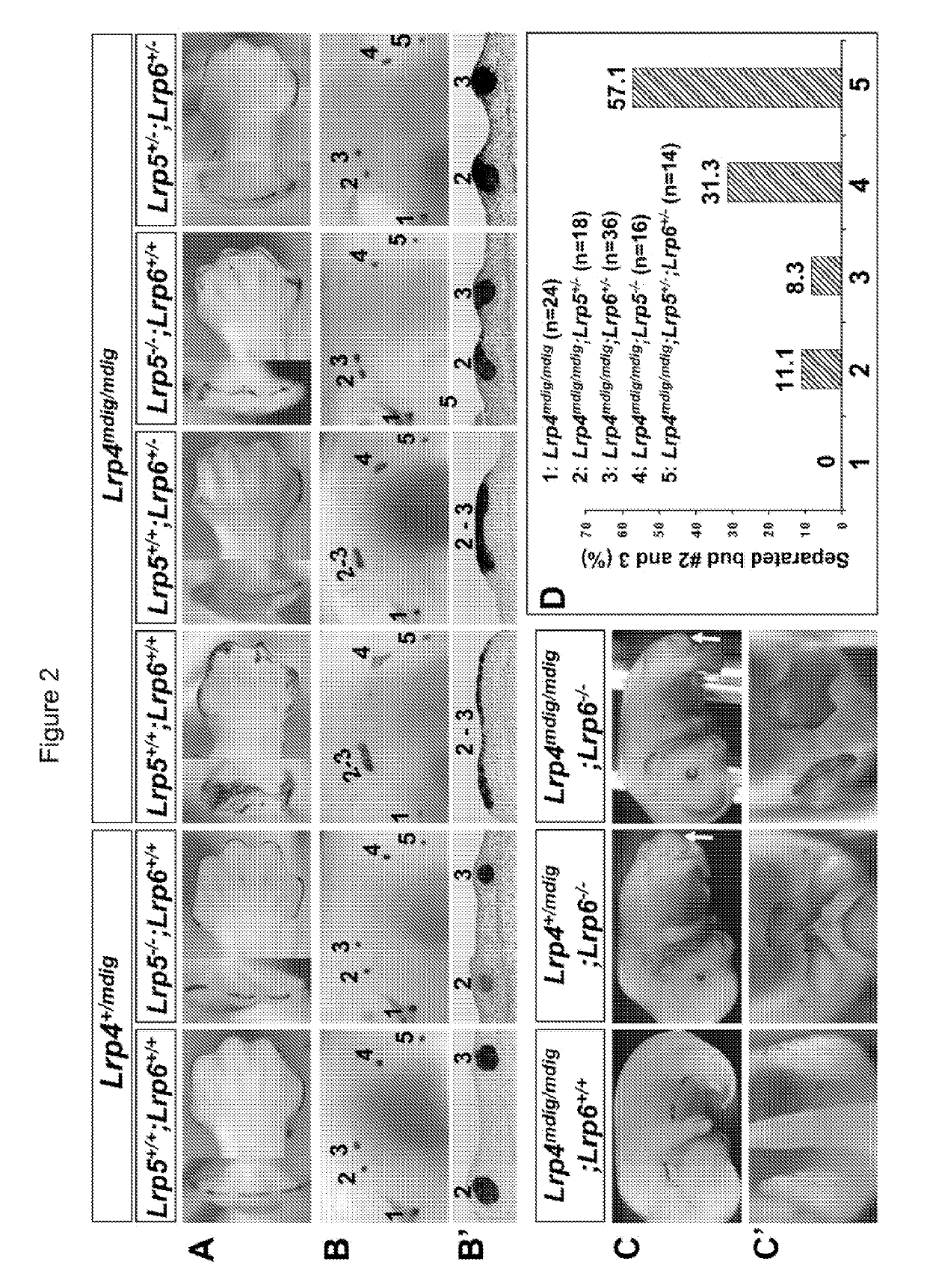
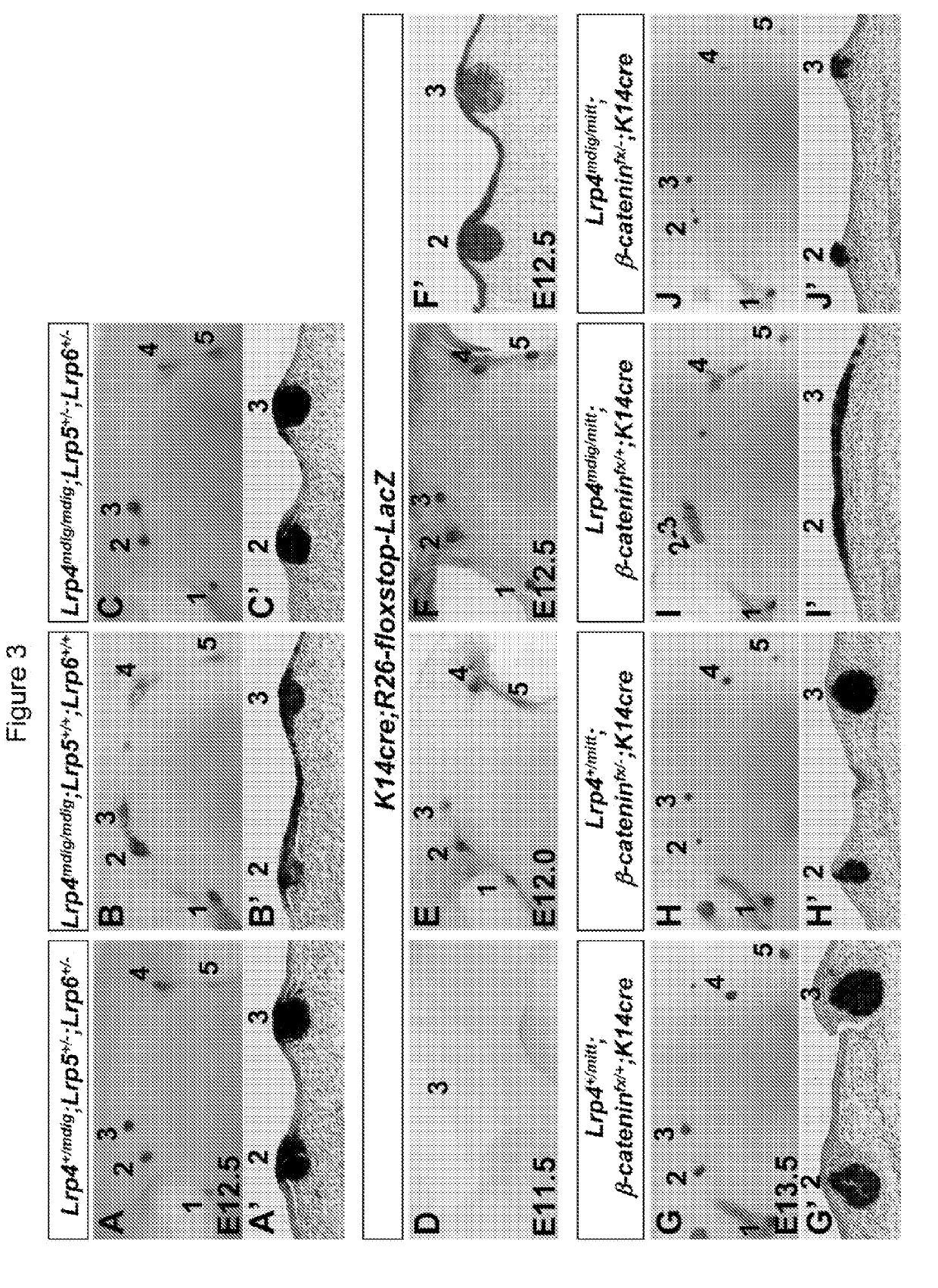
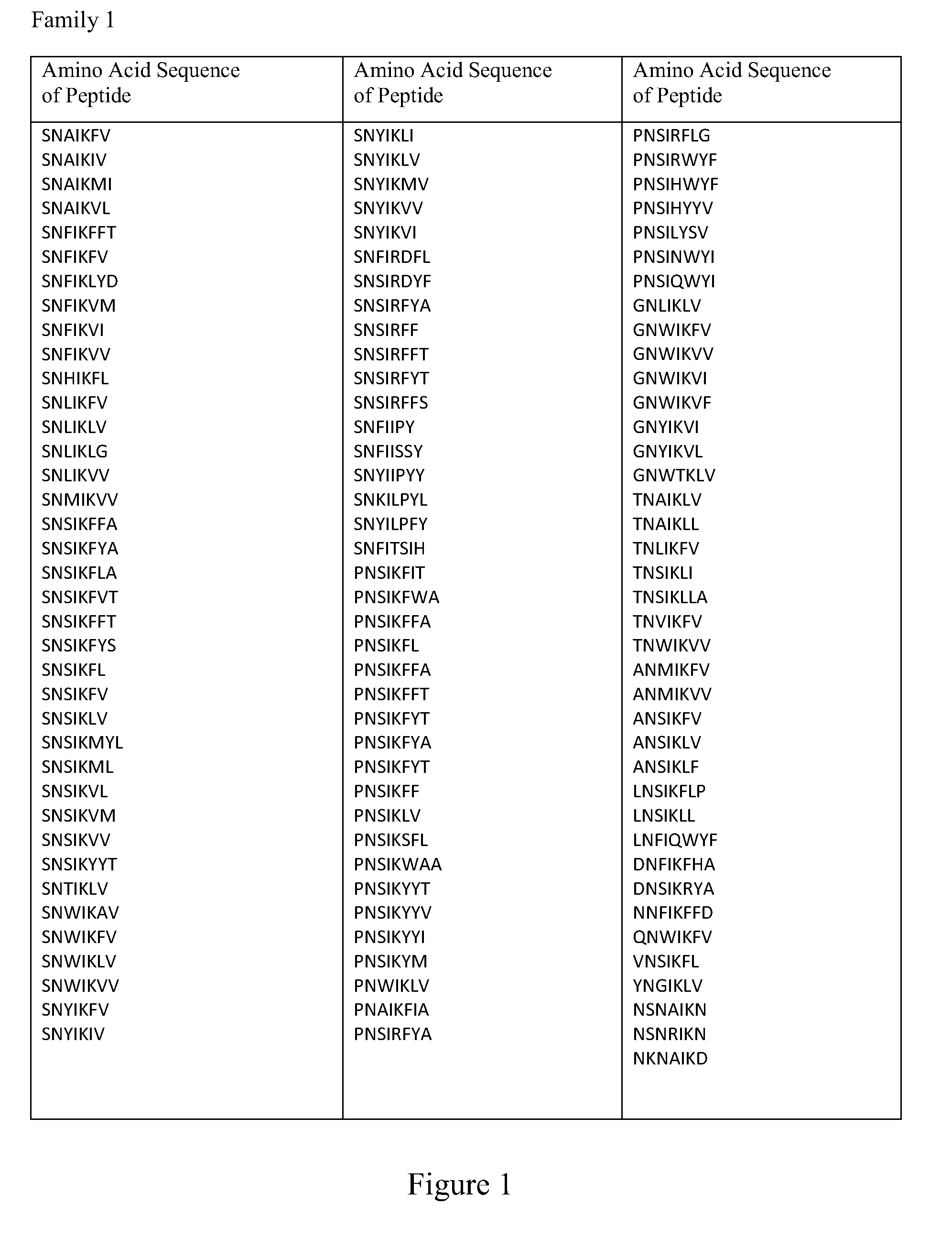Patents
Literature
37 results about "LRP5" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
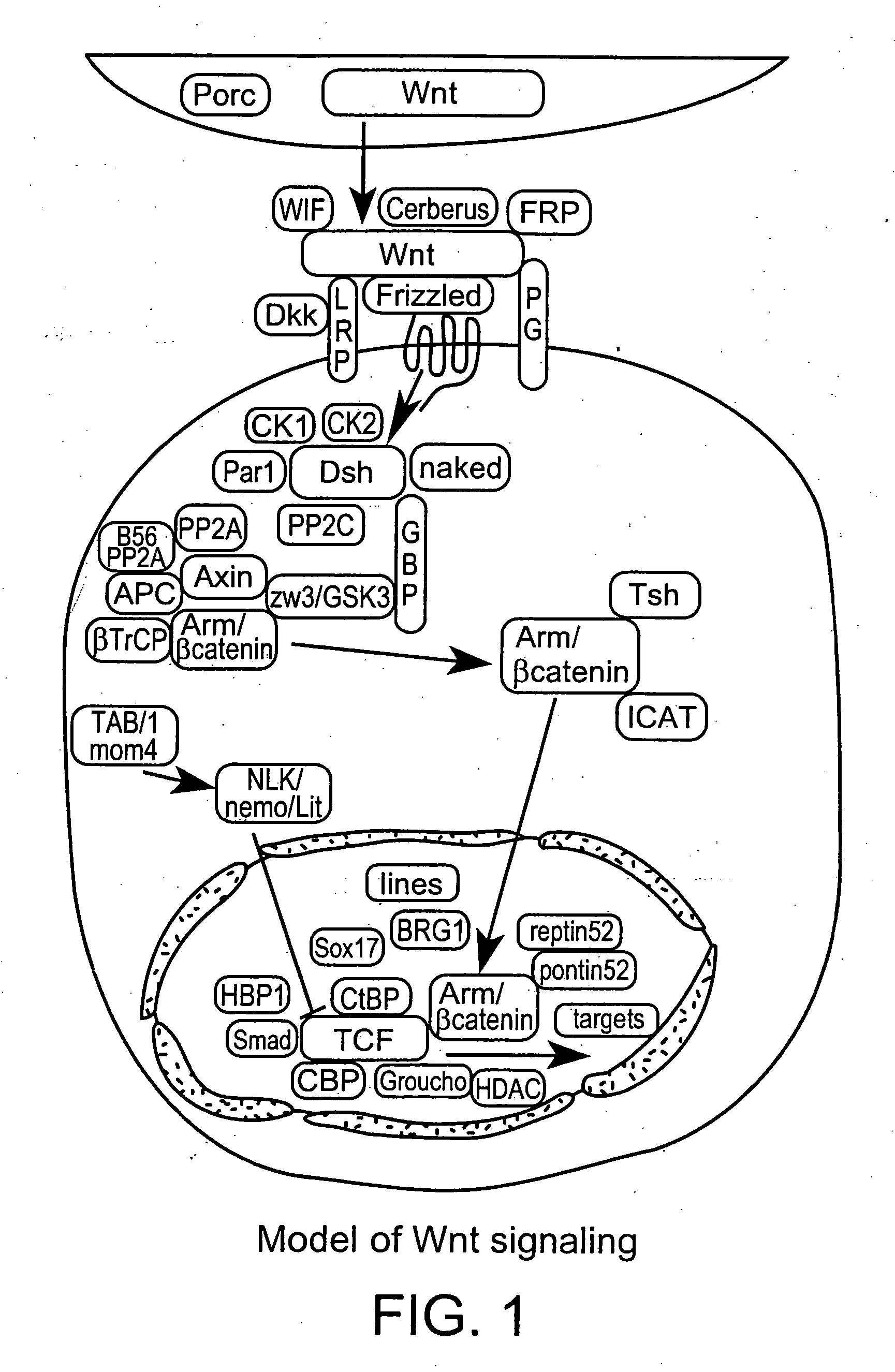
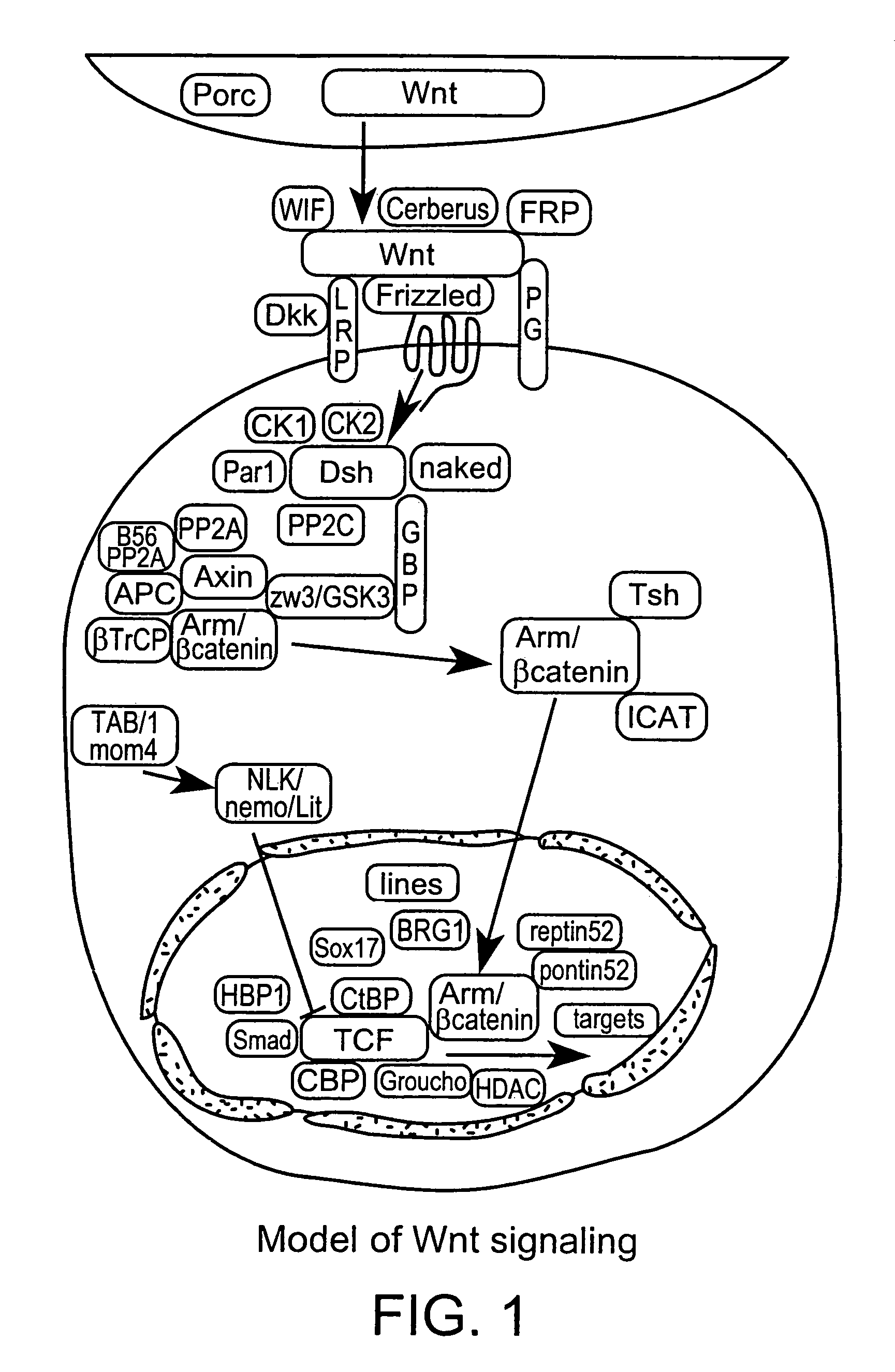
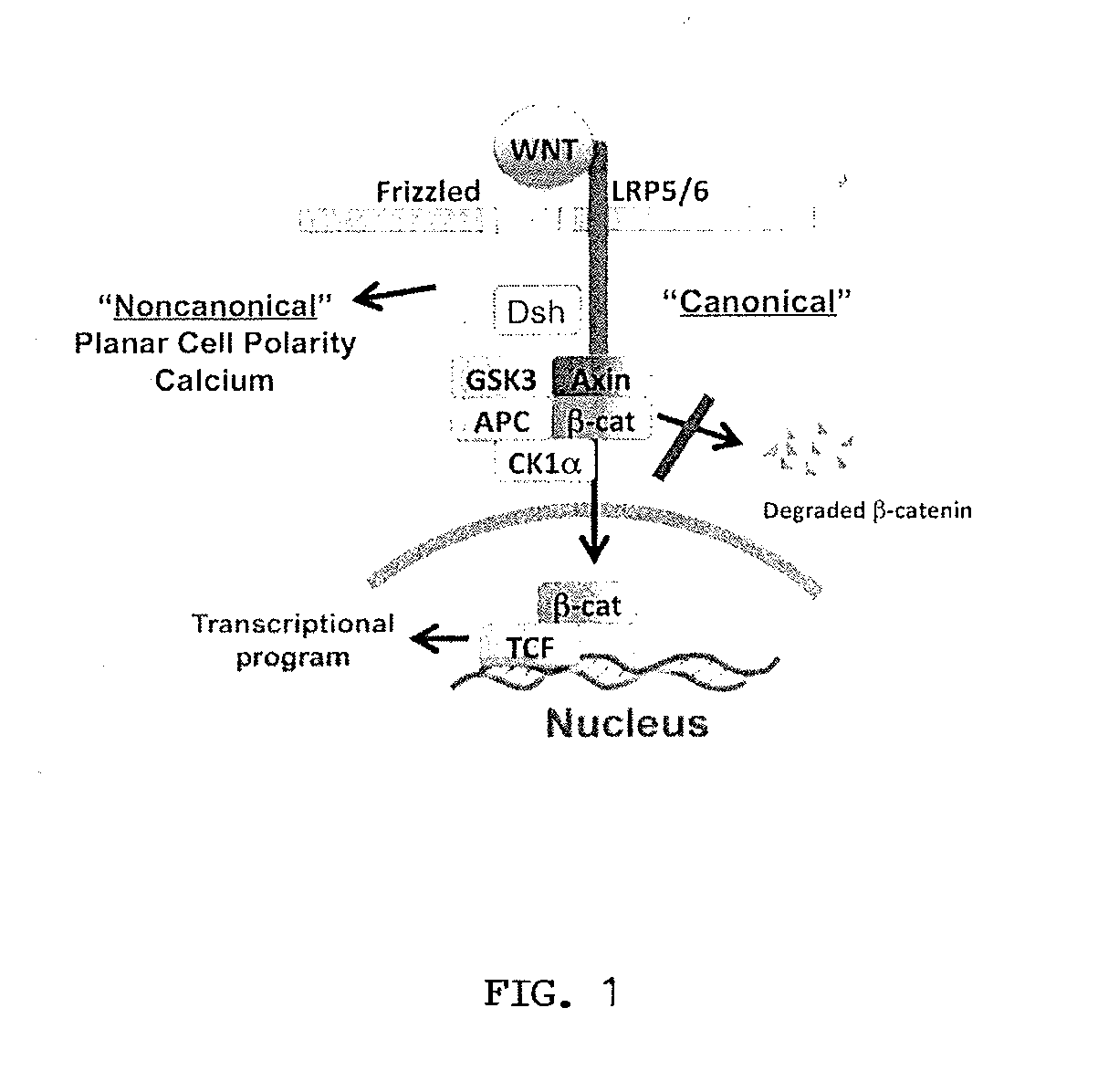
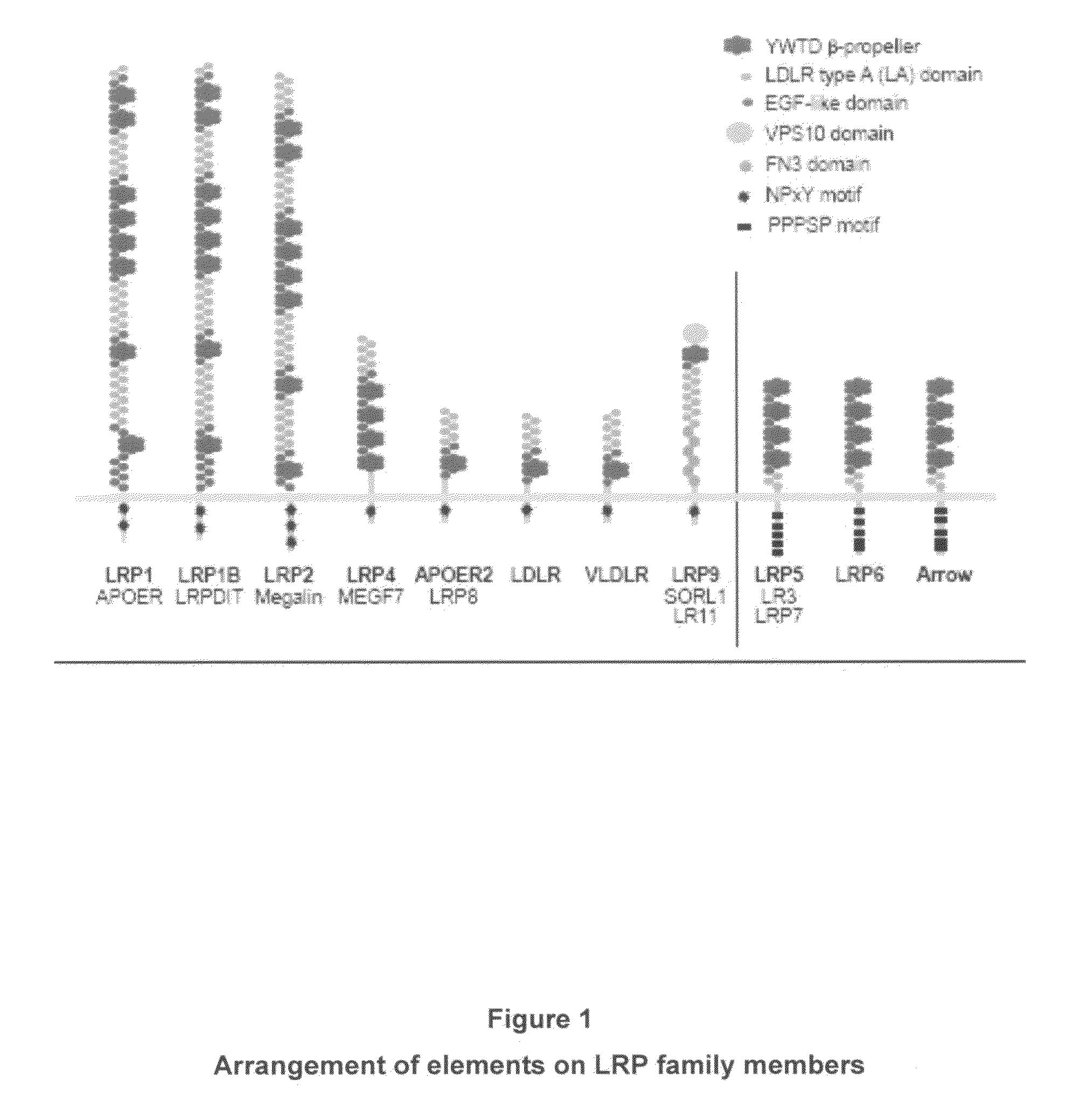
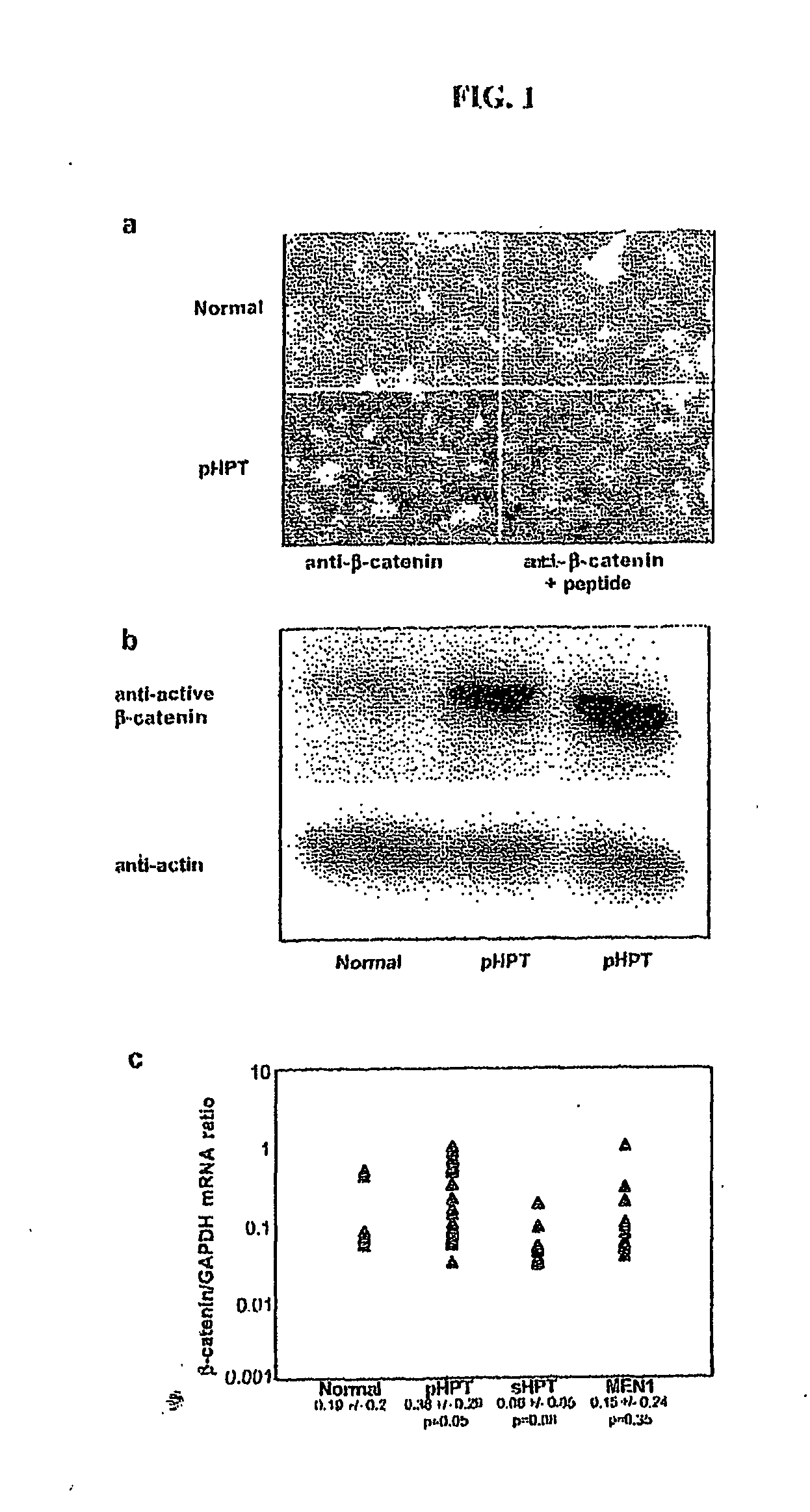
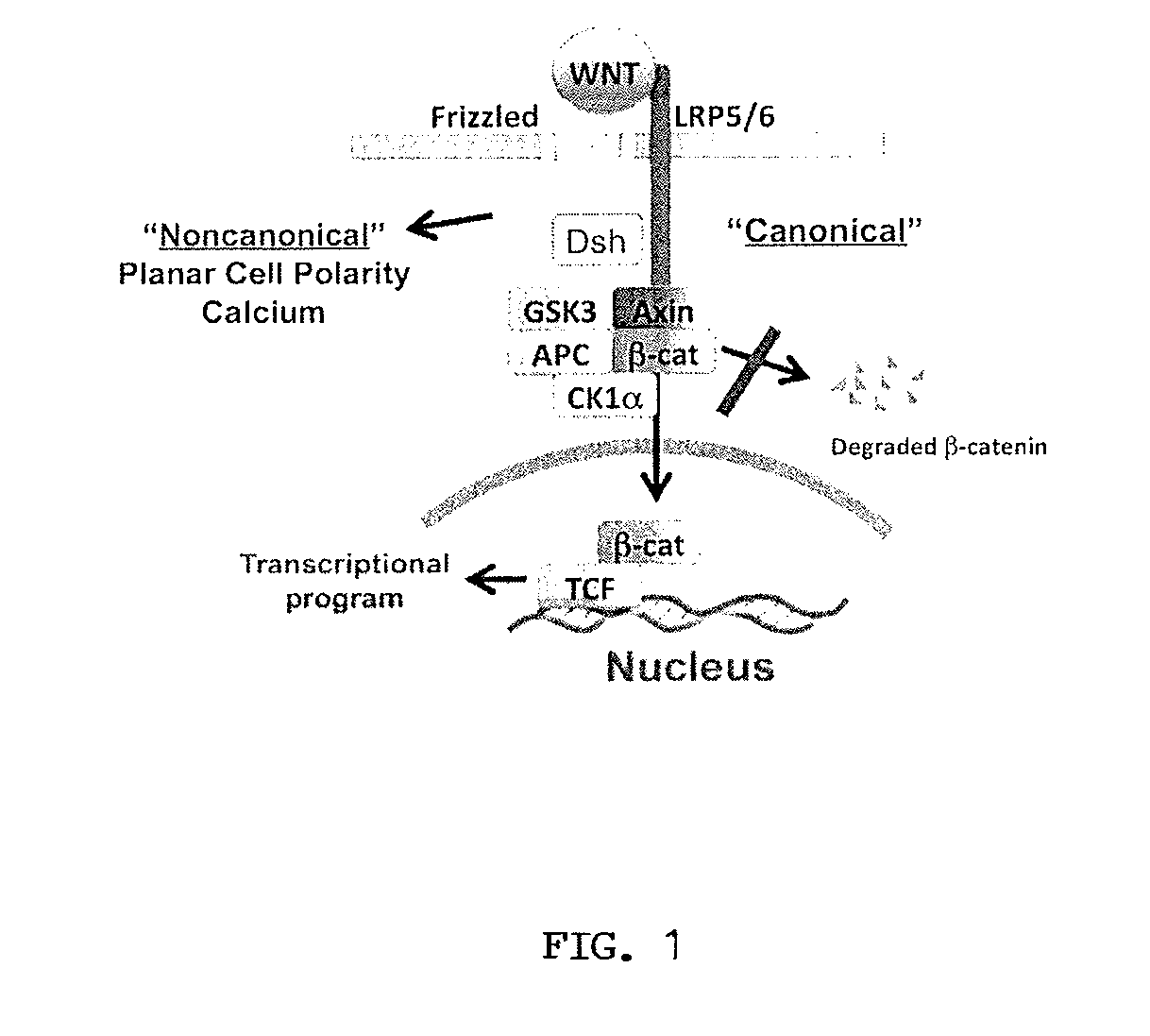
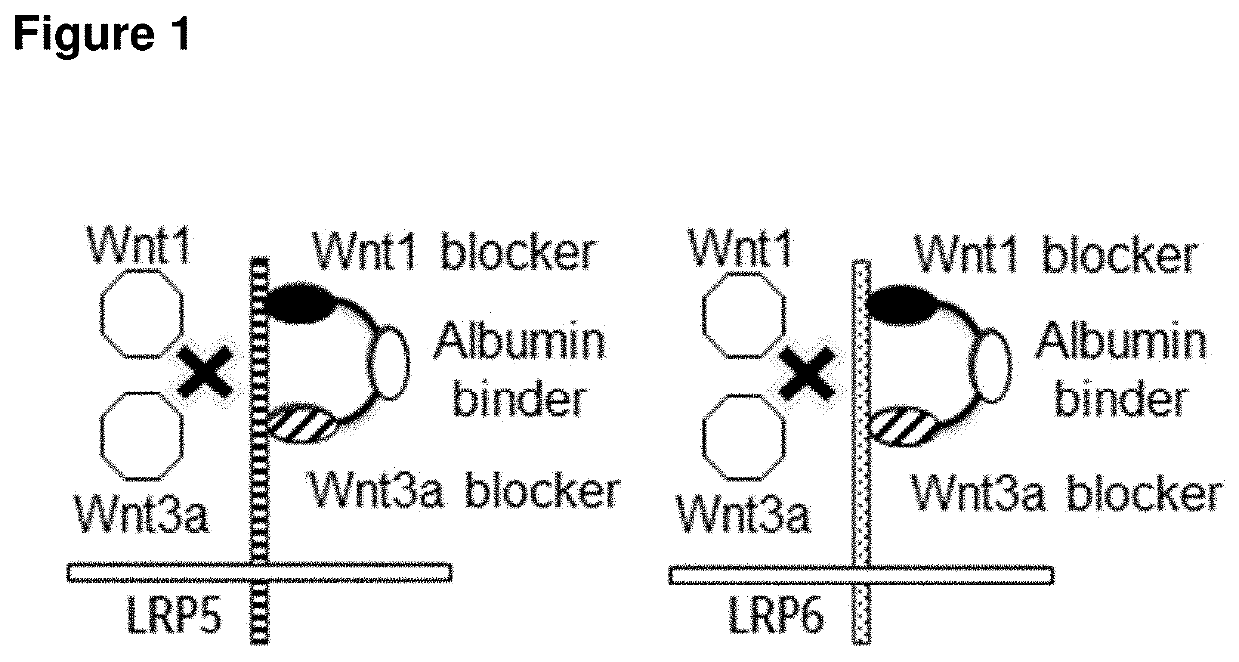
Low-density lipoprotein receptor-related protein 5 is a protein that in humans is encoded by the LRP5 gene. LRP5 is a key component of the LRP5/LRP6/Frizzled co-receptor group that is involved in canonical Wnt pathway. Mutations in LRP5 can lead to considerable changes in bone mass. A loss-of-function mutation causes osteoporosis-pseudoglioma (decrease in bone mass), while a gain-of-function mutation causes drastic increases in bone mass.
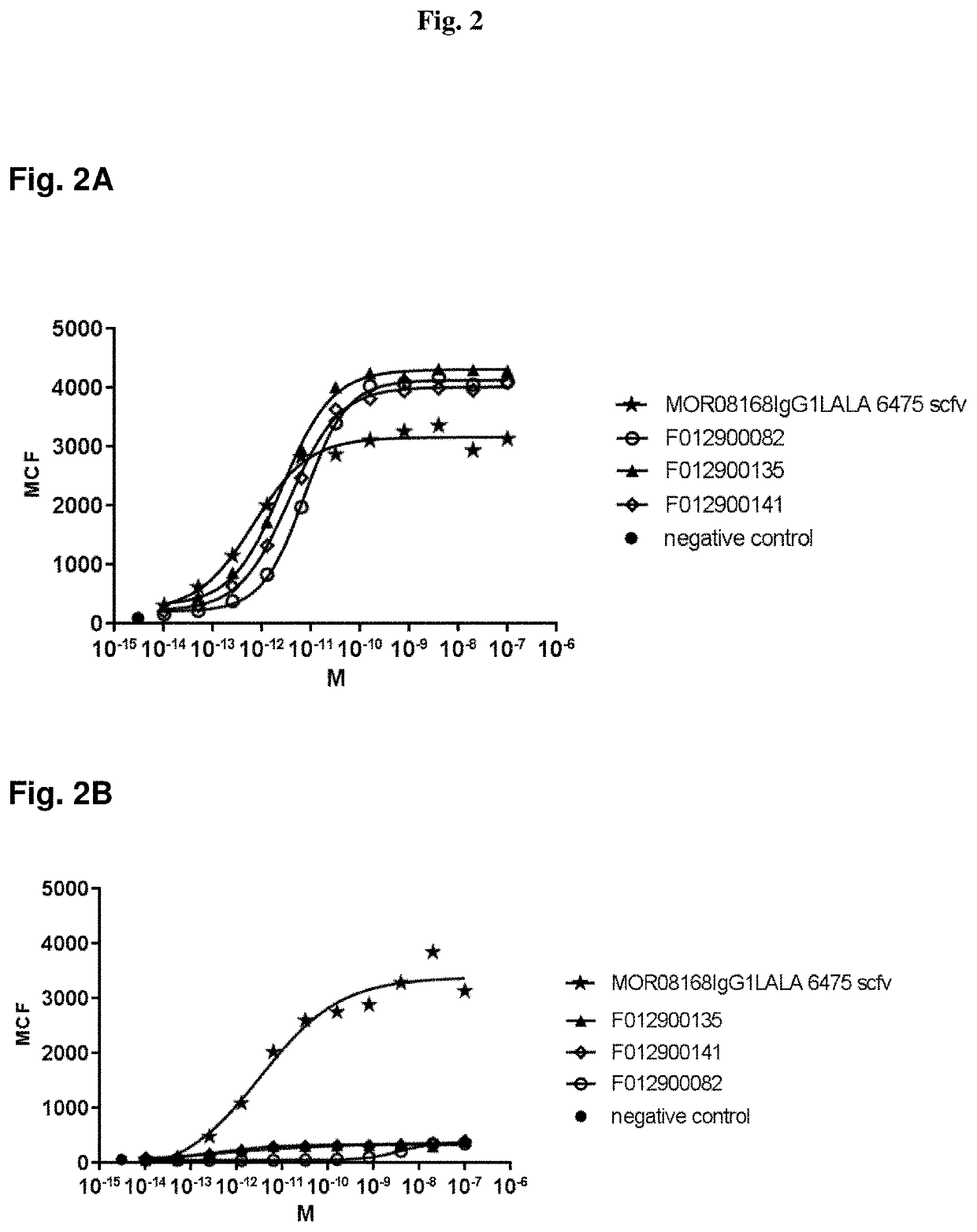
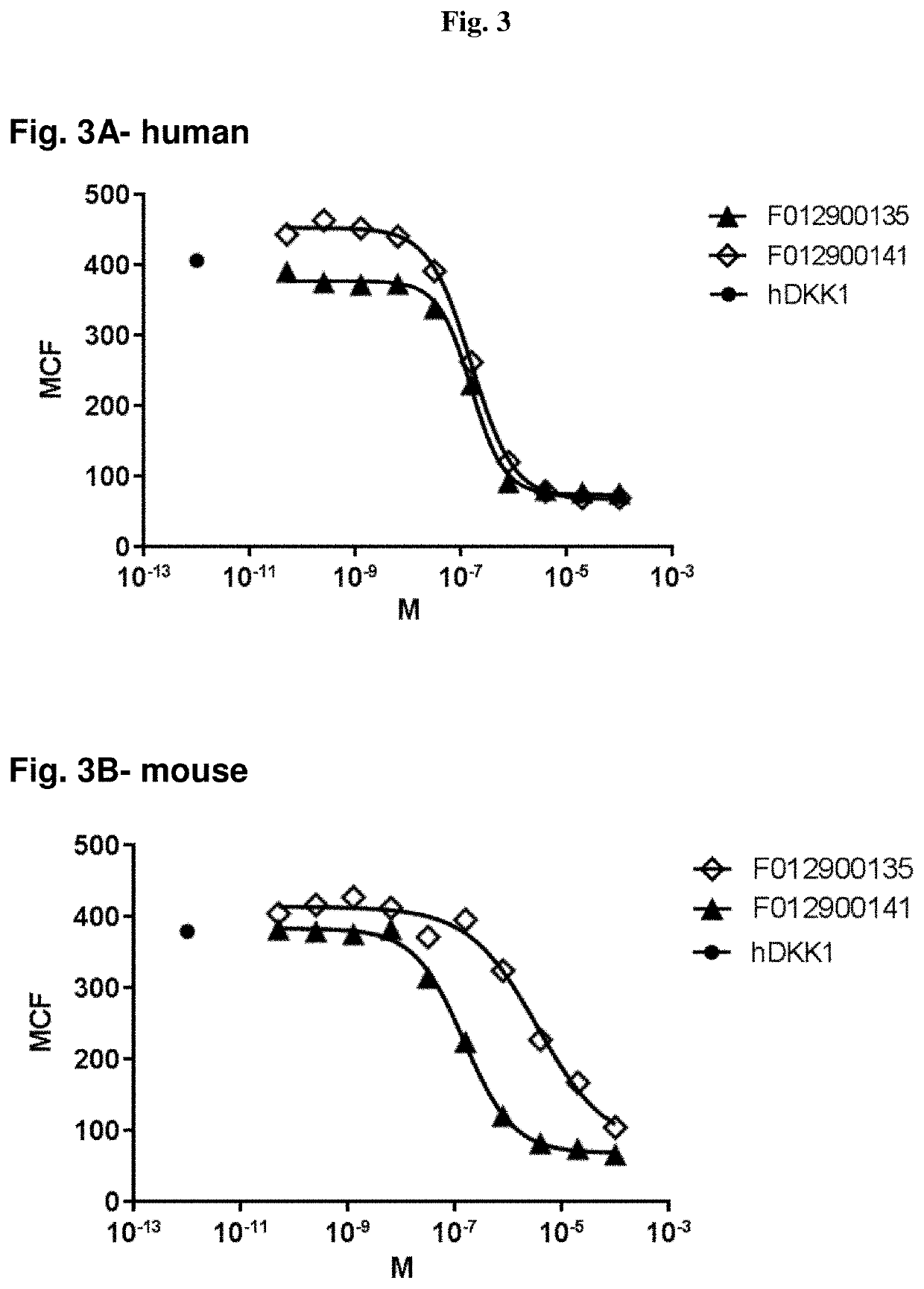
Reagents and method for modulating Dkk-mediated interactions
The present invention provides reagents, compounds, compositions, and methods relating to novel interactions of the extracellular domain of LRP5, HBM (a variant of LRP5), and / or LRP6 with Dkk, including Dkk-1. The various nucleic acids, polypeptides, antibodies, assay methods, diagnostic methods, and methods of treatment of the present invention are related to and impact on Dkk, LRP5, LRP6, HBM, and Wnt signaling. Dkk, LRP5, LRP6, HBM, and Wnt are implicated in bone and lipid cellular signaling. Thus, the present invention provides reagents and methods for modulating lipid levels and / or bone mass and is useful in the treatment and diagnosis of abnormal lipid levels and bone mass disorders, such as osteoporosis.
Owner:GENOME THERAPEUTICS
Antibodies specific for DKK-1
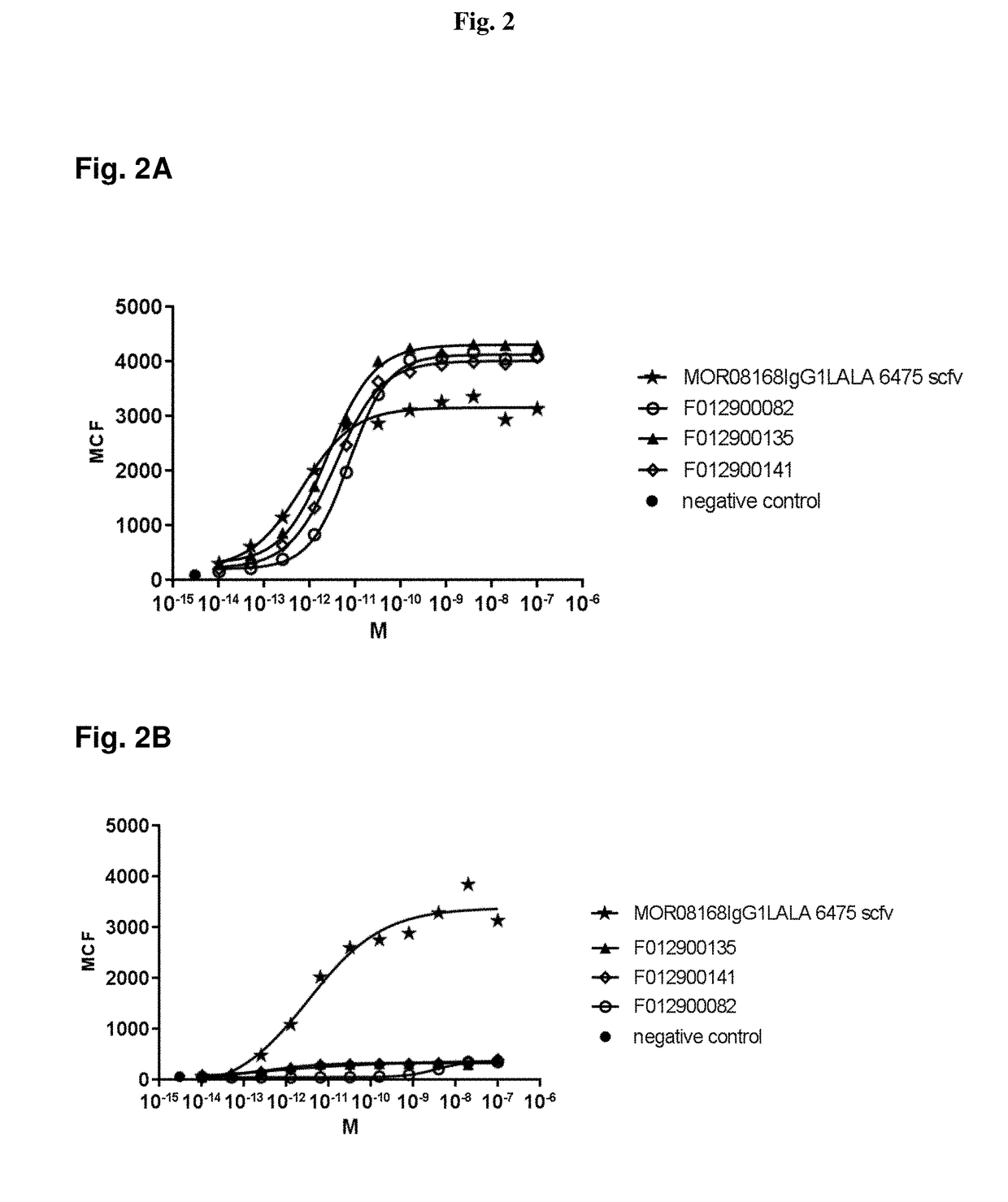
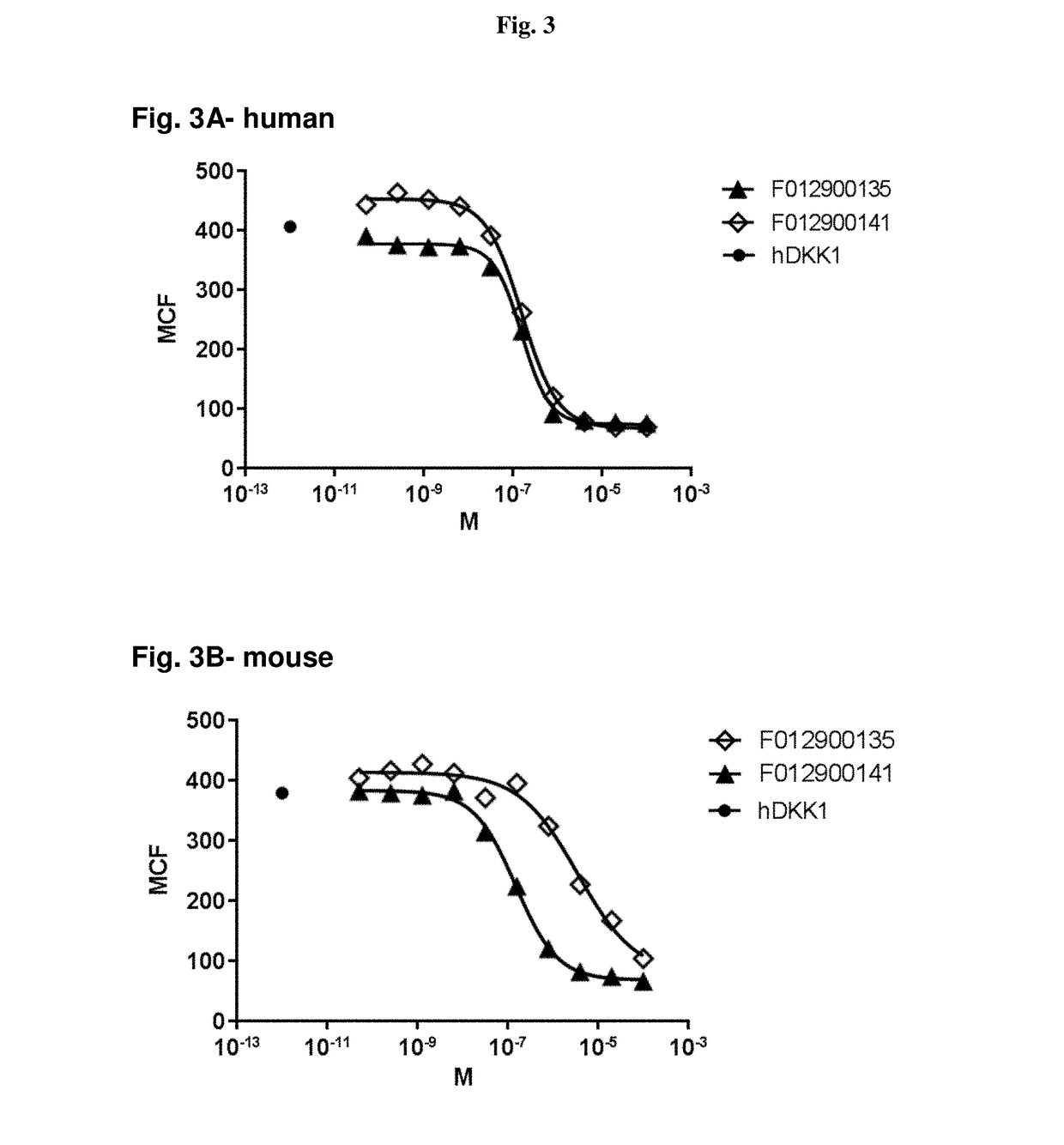
Antibodies specific for Dkk-1, an inhibitor of the osteoanabolic Wnt / LRP5 signaling pathway, are described. The antibodies, which inhibit binding of Dkk-1 to LRP5, are useful in compositions for stimulating bone growth, in particular, compositions for treating bone disorders which result in a loss in bone, for example, osteoporosis.
Owner:MEDIMMUNE LTD
Reagents and method for modulating Dkk-mediated interactions
The present invention provides reagents, compounds, compositions, and methods relating to novel interactions of the extracellular domain of LRP5, HBM (a variant of LRP5), and / or LRP6 with Dkk, including Dkk-1. The various nucleic acids, polypeptides, antibodies, assay methods, diagnostic methods, and methods of treatment of the present invention are related to and impact on Dkk, LRP5, LRP6, HBM, and Wnt signaling. Dkk, LRP5, LRP6, HBM, and Wnt are implicated in bone and lipid cellular signaling. Thus, the present invention provides reagents and methods for modulating lipid levels and / or bone mass and is useful in the treatment and diagnosis of abnormal lipid levels and bone mass disorders, such as osteoporosis.
Owner:GENOME THERAPEUTICS
Antibodies That Inhibit WNT Signaling And Methods Of Using The Same
InactiveUS20120276089A1Extend your lifeIncrease physical strengthImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseMonoclonal antibody
The present invention is directed to monoclonal antibodies and fragments thereof directed to LRP5 / 6 that find use in the prevention and treatment of cardiac remodeling and cancer. Also disclosed are methods for using such monoclonal antibodies in the prevention and treatment of such diseases.
Owner:VANDERBILT UNIV
Methods for treating inflammation
The present invention relates to the field of therapeutic methods, compositions and uses thereof, that affect, directly or indirectly, the behavior of LRP receptors. These compositions and methods result in the treatment of inflammatory, immunological and metabolic conditions. More particularly, the methods and compositions of the invention are directed to the identification of small molecules, drugs and / or pharmacological agents that affect the Wnt pathway by affecting normal complex formation among various signaling receptors, the LRP5 and LRP6 receptor, and related ligands.
Owner:ENZO BIOCHEM
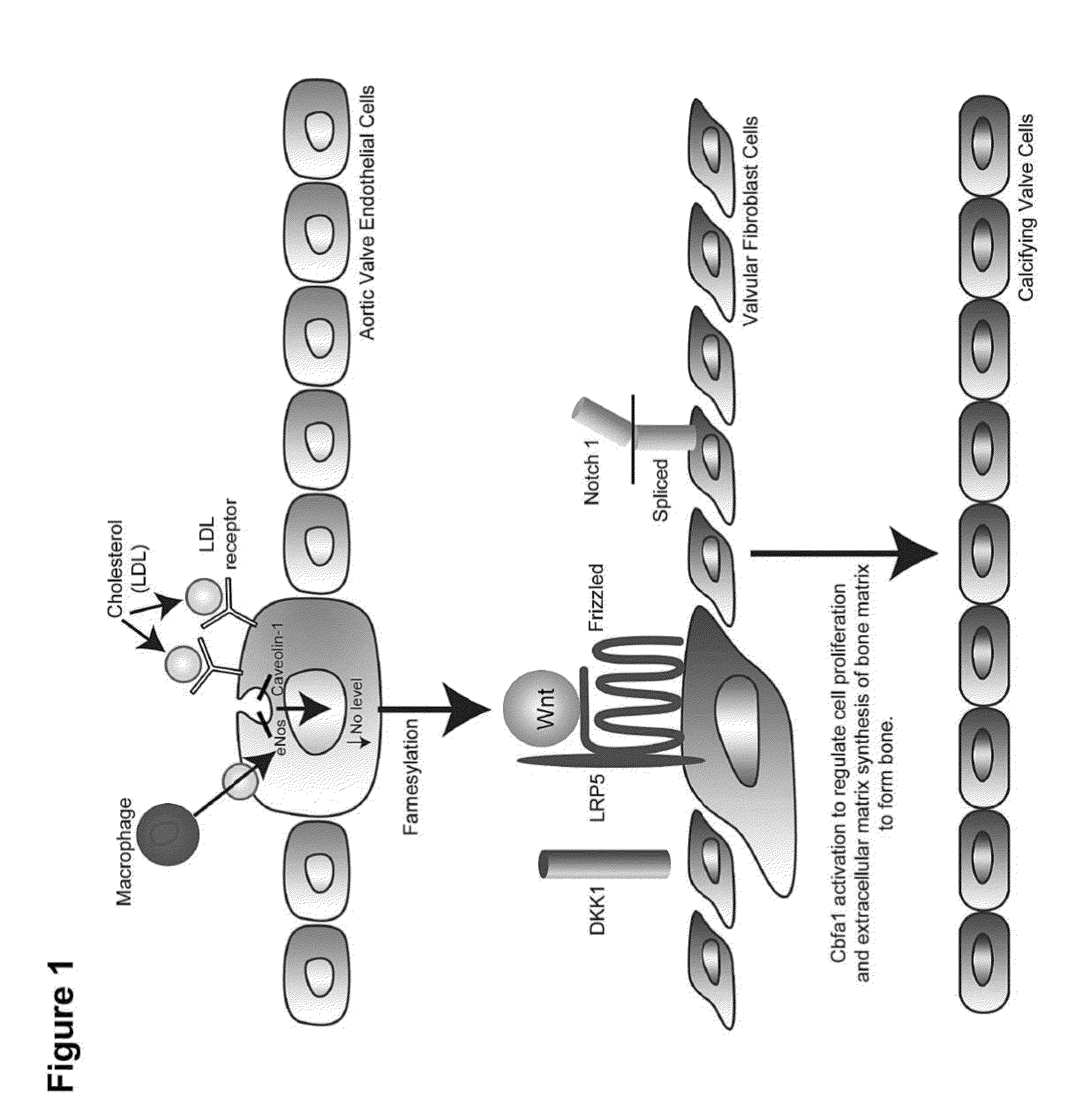
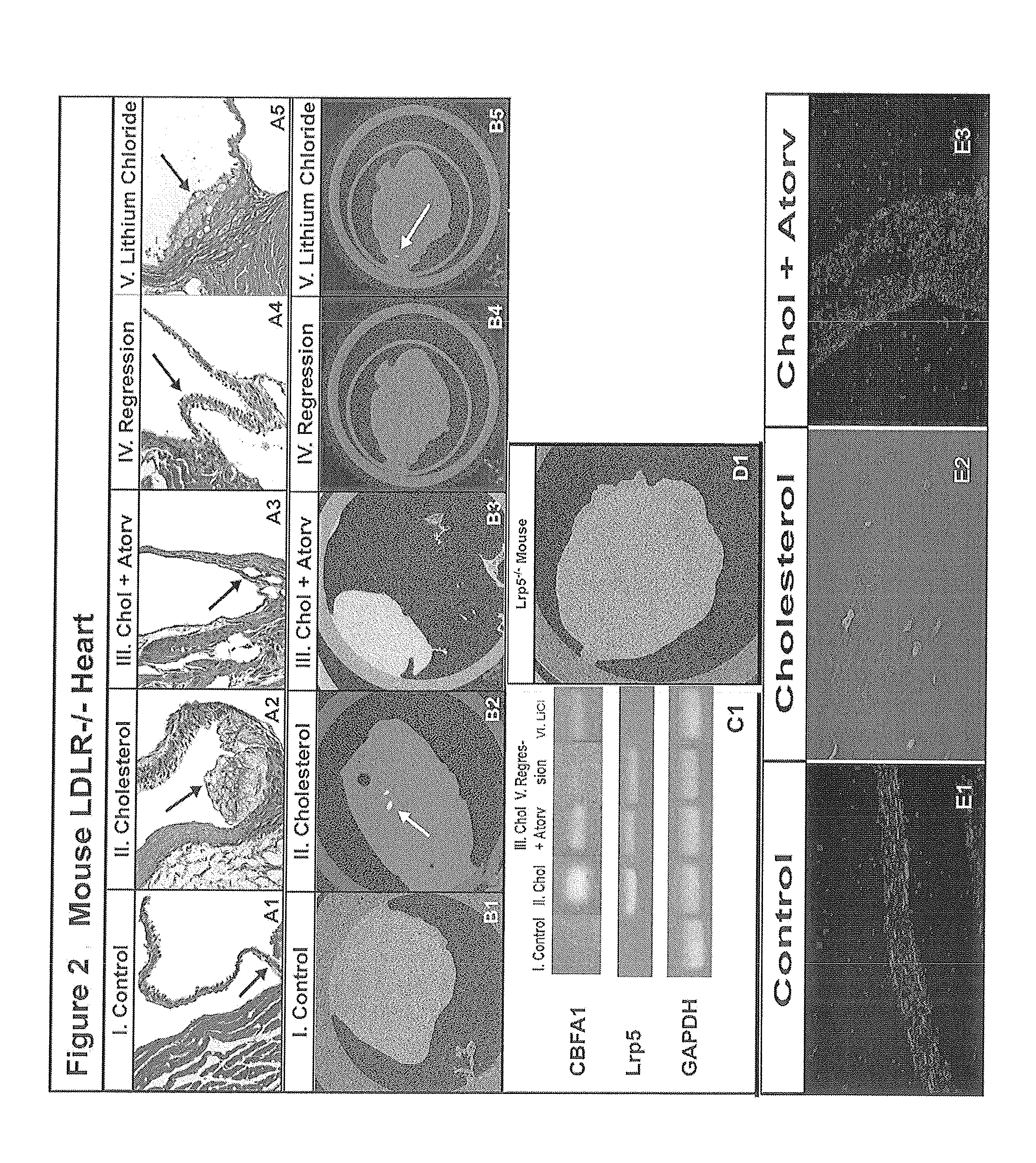
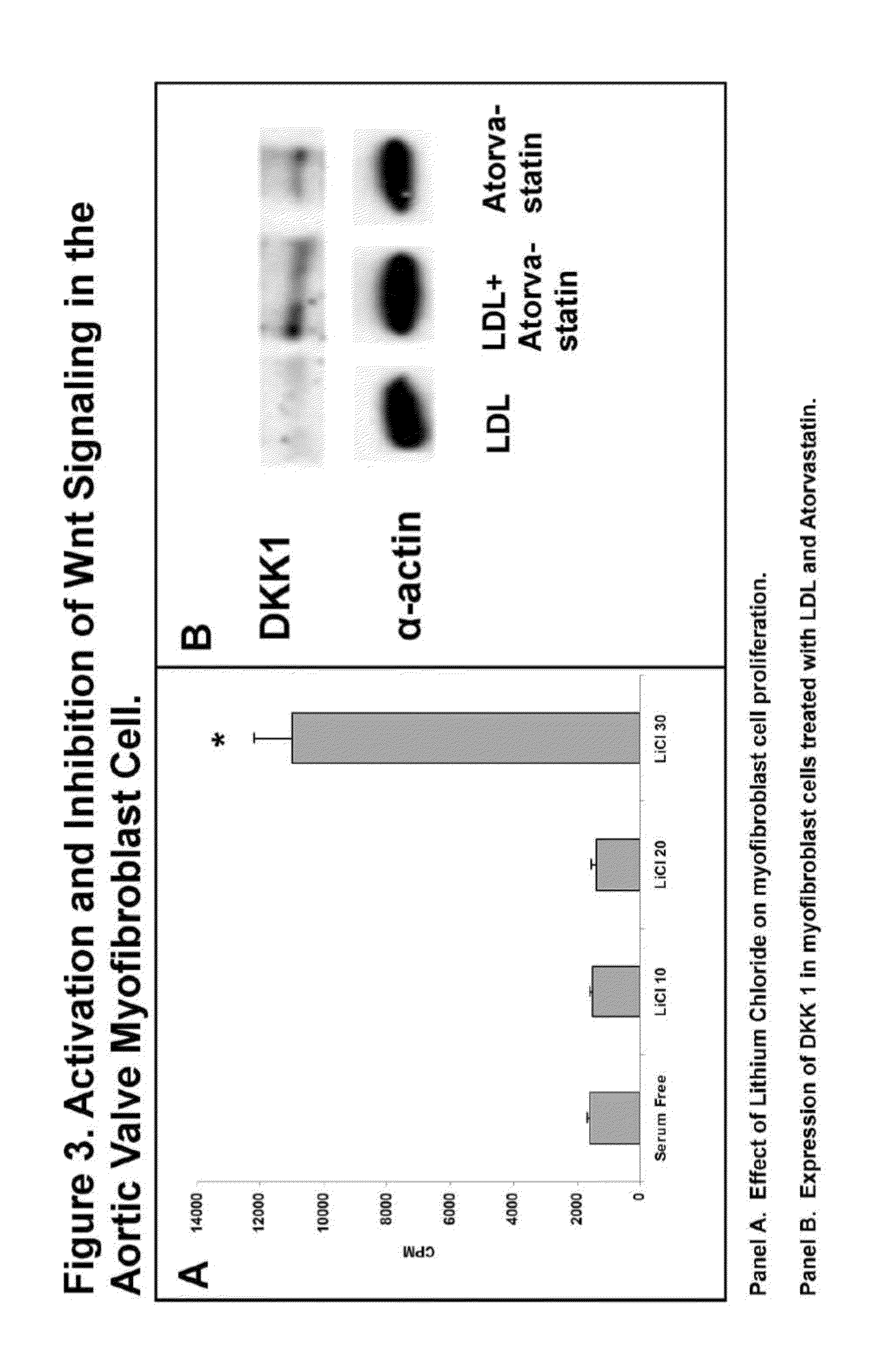
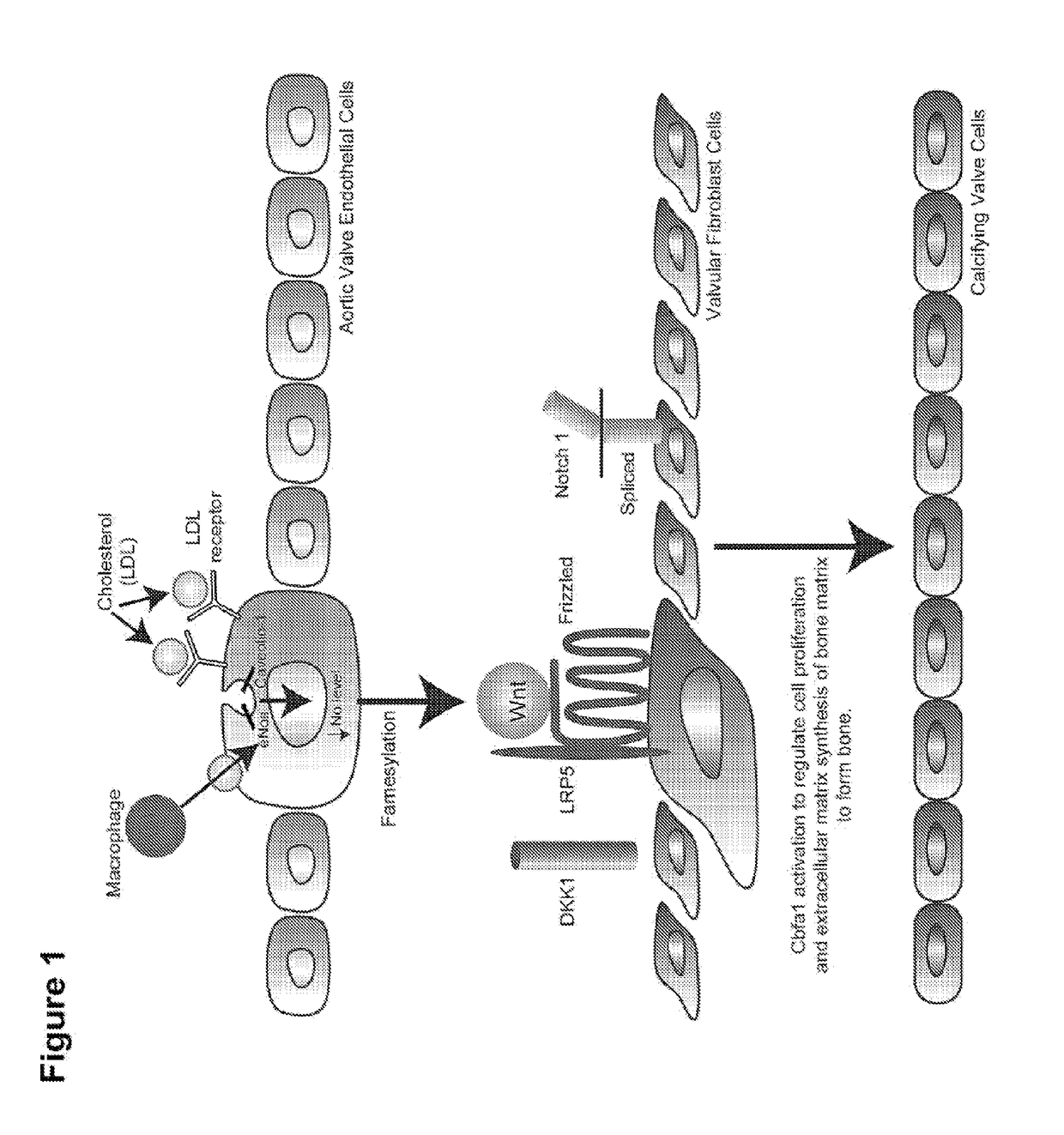
Devices and methods for inhibiting stenosis, obstruction, or calcification of a native heart valve, stented heart valve or bioprosthesis
InactiveUS20150306281A1Heart valvesPharmaceutical delivery mechanismDiseaseCombined Modality Therapy
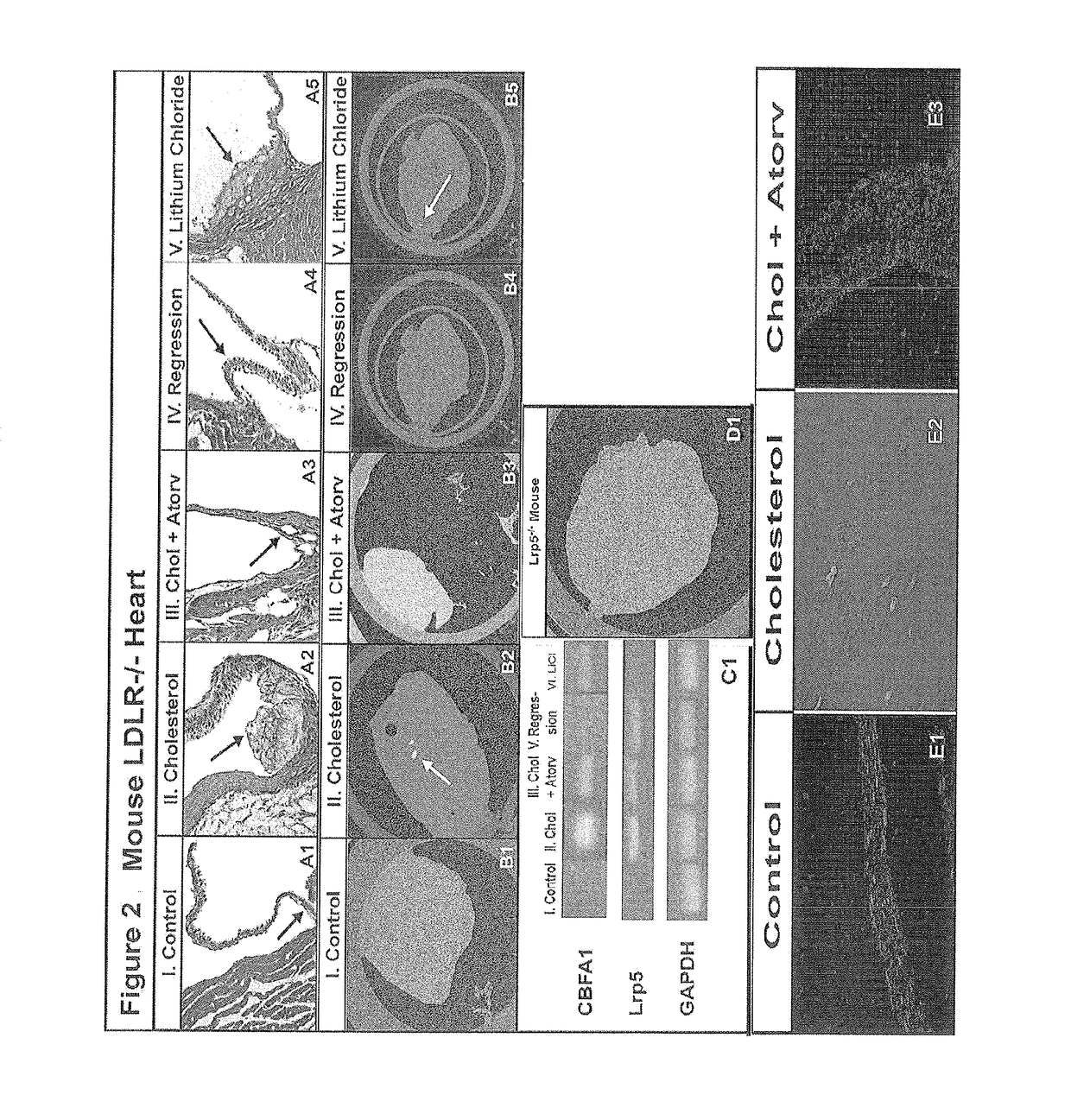
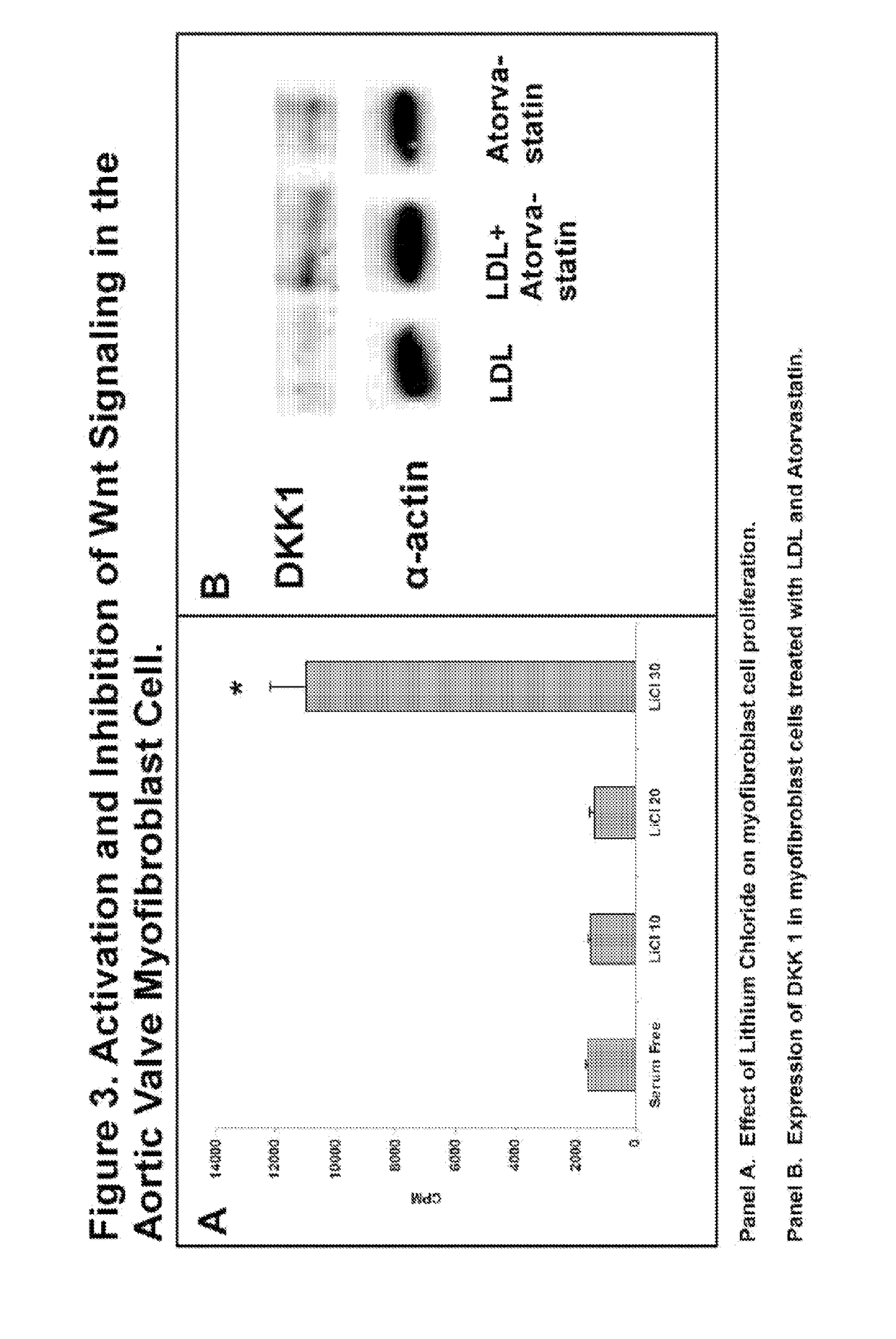
The present invention relates to methods for inhibiting stenosis, obstruction, or calcification of a valve following implantation of a valve prosthesis or a native valve which develops disease via the Lrp5 / Wnt Pathway in the presence of elevated lipids due to elevated Low Density Lipoprotein. This invention involves dispensing a combination of medications to target inflammation and attachment of the target cell and the secondary drugs to inhibit proliferation and calcification on an elastical stent, gortex graft or valve leaflet. The combination therapy inhibits bioprosthesis and native valve calcification with improvement of the longevity of the prosthetic material including the stent, the native valve, and the gortex covering. The valve prosthesis and or gortex graft is mounted on the elastical stent or prosthesis such that the elastical stent is connected to the valve.
Owner:CONCIEVALVE
Mammary stem cell marker
ActiveUS20070280948A1High expressionGenetic material ingredientsMicrobiological testing/measurementSurface markerLow-density lipoprotein
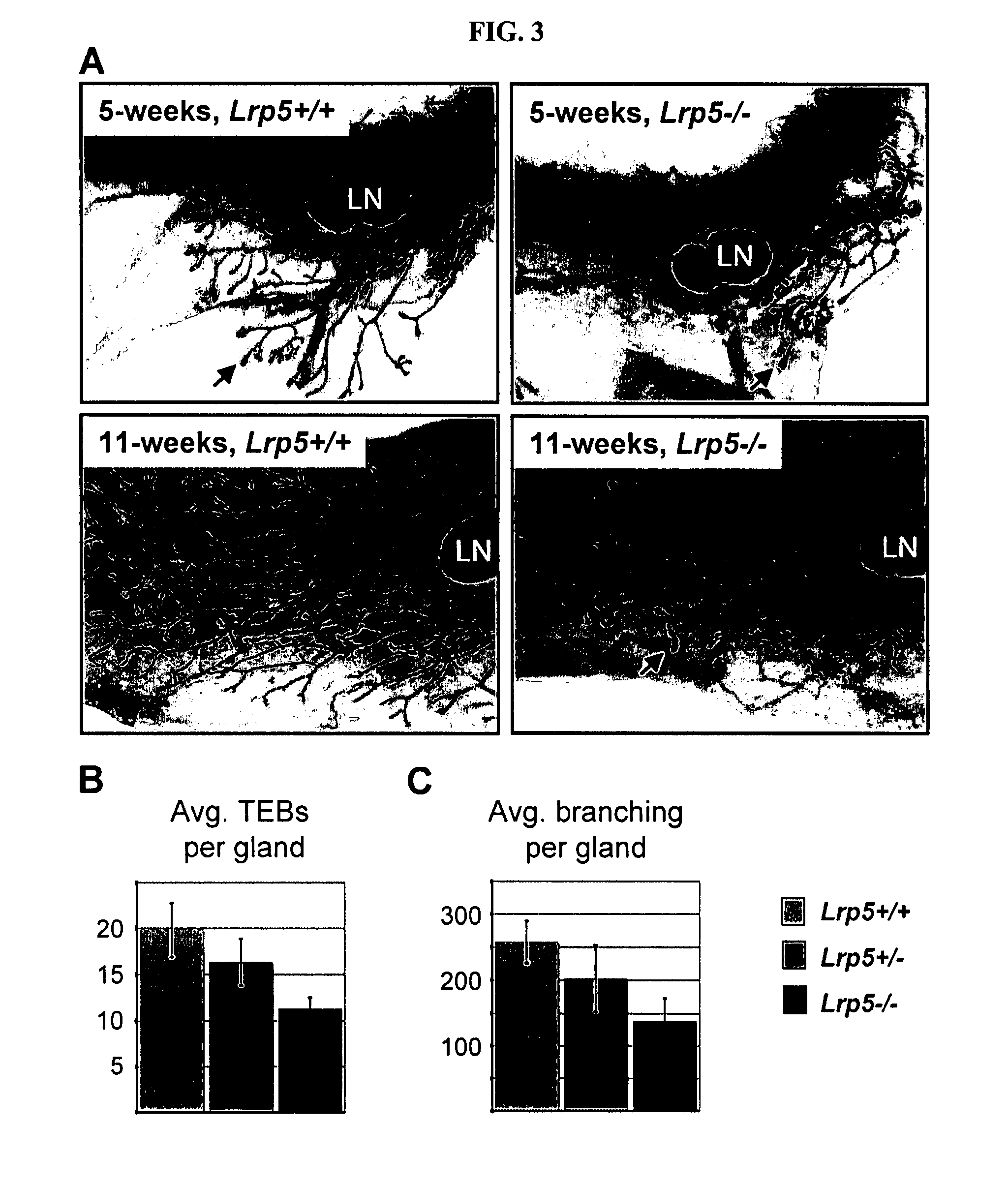
It is disclosed here that low density lipoprotein receptor-related protein 5 (LRP5) is a cell surface marker for somatic mammary stem cells and mammary tumor stem cells. The disclosure here provides new tools for enriching somatic mammary stem cells and mammary tumor stem cells. Methods of screening for agents that modulate LRP5 activity, of treating mammary tumor or breast cancer, of monitoring somatic mammary stem cells and mammary tumor stem cells in vivo are also provided, and of assessing prognosis of human breast cancer.
Owner:VAN ANDEL RES INST +1
Sulfation of wnt pathway proteins
ActiveUS20120071399A1Reduce inhibitionPeptide/protein ingredientsSkeletal disorderCrystallographySulfation
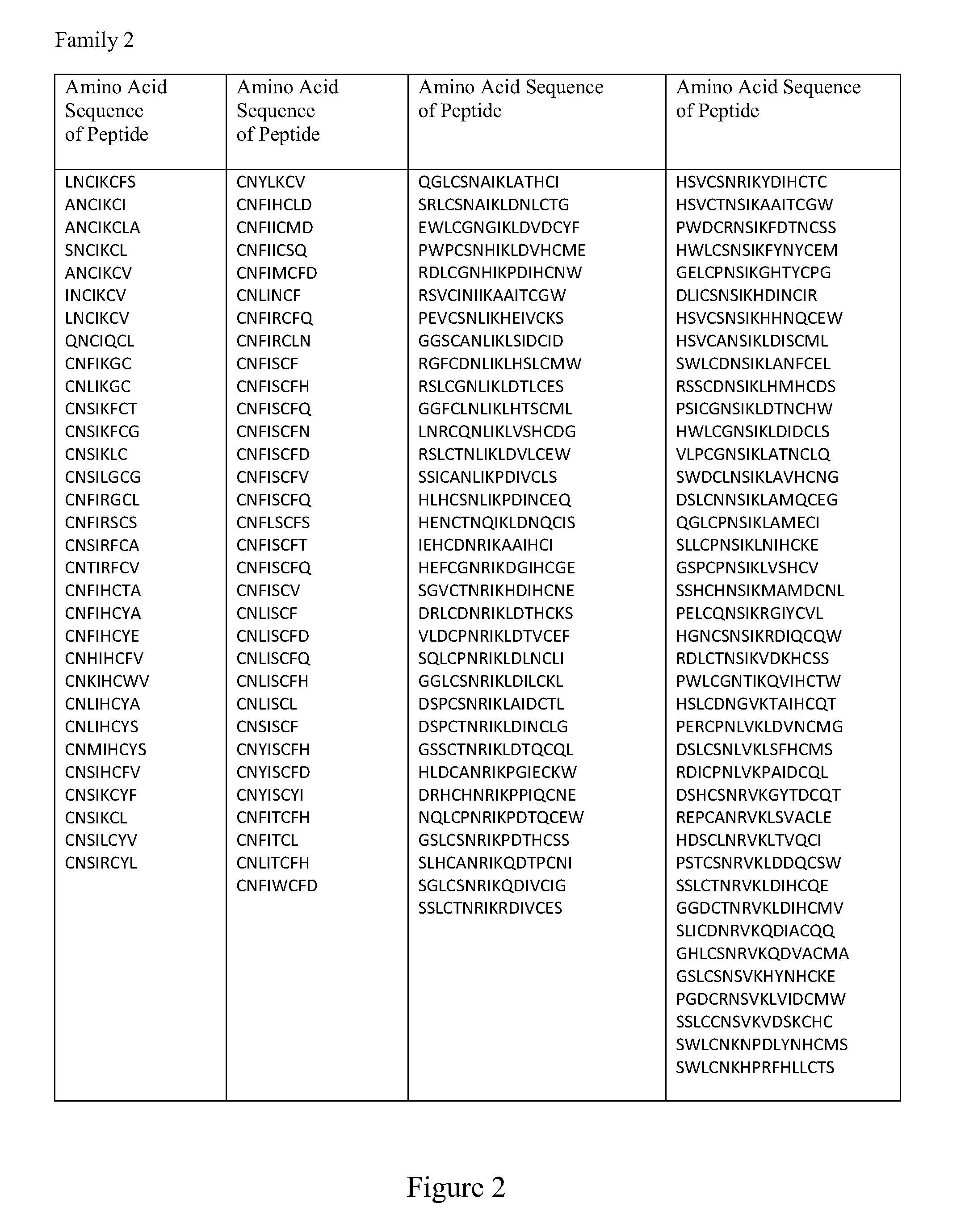
Provided is a composition comprising a peptide comprising amino acids and / or amino acid analogs comprising a continuous sequence of a sclerostin fragment comprising Tyr43 or Tyr213. Also provided is a composition comprising a peptide comprising less than about 75 amino acids and / or amino acid analogs including an amino acid or amino acid analog capable of being sulfated, where the composition is capable of inhibiting sclerostin binding to an LRP. Further provided is a composition comprising a peptide comprising less than about 75 amino acids and / or amino acid analogs including an amino acid or amino acid analog capable of being post-translationally sulfated, where the composition is capable of inhibiting binding of a protein ligand comprising a sulfation site to its binding partner. Additionally provided is a method of enhancing a Wnt signaling pathway comprising contacting an LRP5 / 6 receptor in the Wnt signaling pathway with either of the above-described compositions that comprise a sequence of a sclerostin fragment or is capable of inhibiting sclerostin binding to an LRP, where the tyrosine or tyrosine analog is not sulfated, in a manner sufficient to enhance the Wnt signaling pathway. Further provided is a method of treating a subject having a disease exacerbated by inhibition of a Wnt signaling pathway comprising administering either of the above-described compositions that comprise a sequence of a sclerostin fragment or is capable of inhibiting sclerostin binding to an LRP, where the tyrosine or tyrosine analog is not sulfated, to the subject in a manner sufficient to reduce the inhibition of the Wnt signaling pathway. Also, a method of inhibiting binding of a protein ligand comprising a sulfation site to its binding partner is provided. The method comprises adding the above-described composition that is capable of inhibiting binding of a protein ligand to its binding partner to the protein ligand and its binding partner in a manner sufficient to inhibit binding of the protein ligand to its binding partner.
Owner:ENZO THERAPEUTICS +1
Sulfation of Wnt pathway proteins
Provided is a composition comprising a peptide comprising amino acids and / or amino acid analogs comprising a continuous sequence of a sclerostin fragment comprising Tyr43 or Tyr213. Also provided is a composition comprising a peptide comprising less than about 75 amino acids and / or amino acid analogs including an amino acid or amino acid analog capable of being sulfated, where the composition is capable of inhibiting sclerostin binding to an LRP. Further provided is a composition comprising a peptide comprising less than about 75 amino acids and / or amino acid analogs including an amino acid or amino acid analog capable of being post-translationally sulfated, where the composition is capable of inhibiting binding of a protein ligand comprising a sulfation site to its binding partner. Additionally provided is a method of enhancing a Wnt signaling pathway comprising contacting an LRP5 / 6 receptor in the Wnt signaling pathway with either of the above-described compositions that comprise a sequence of a sclerostin fragment or is capable of inhibiting sclerostin binding to an LRP, where the tyrosine or tyrosine analog is not sulfated, in a manner sufficient to enhance the Wnt signaling pathway. Further provided is a method of treating a subject having a disease exacerbated by inhibition of a Wnt signaling pathway comprising administering either of the above-described compositions that comprise a sequence of a sclerostin fragment or is capable of inhibiting sclerostin binding to an LRP, where the tyrosine or tyrosine analog is not sulfated, to the subject in a manner sufficient to reduce the inhibition of the Wnt signaling pathway. Also, a method of inhibiting binding of a protein ligand comprising a sulfation site to its binding partner is provided. The method comprises adding the above-described composition that is capable of inhibiting binding of a protein ligand to its binding partner to the protein ligand and its binding partner in a manner sufficient to inhibit binding of the protein ligand to its binding partner.
Owner:ENZO THERAPEUTICS +1
Compositions and methods for bone formation and remodeling
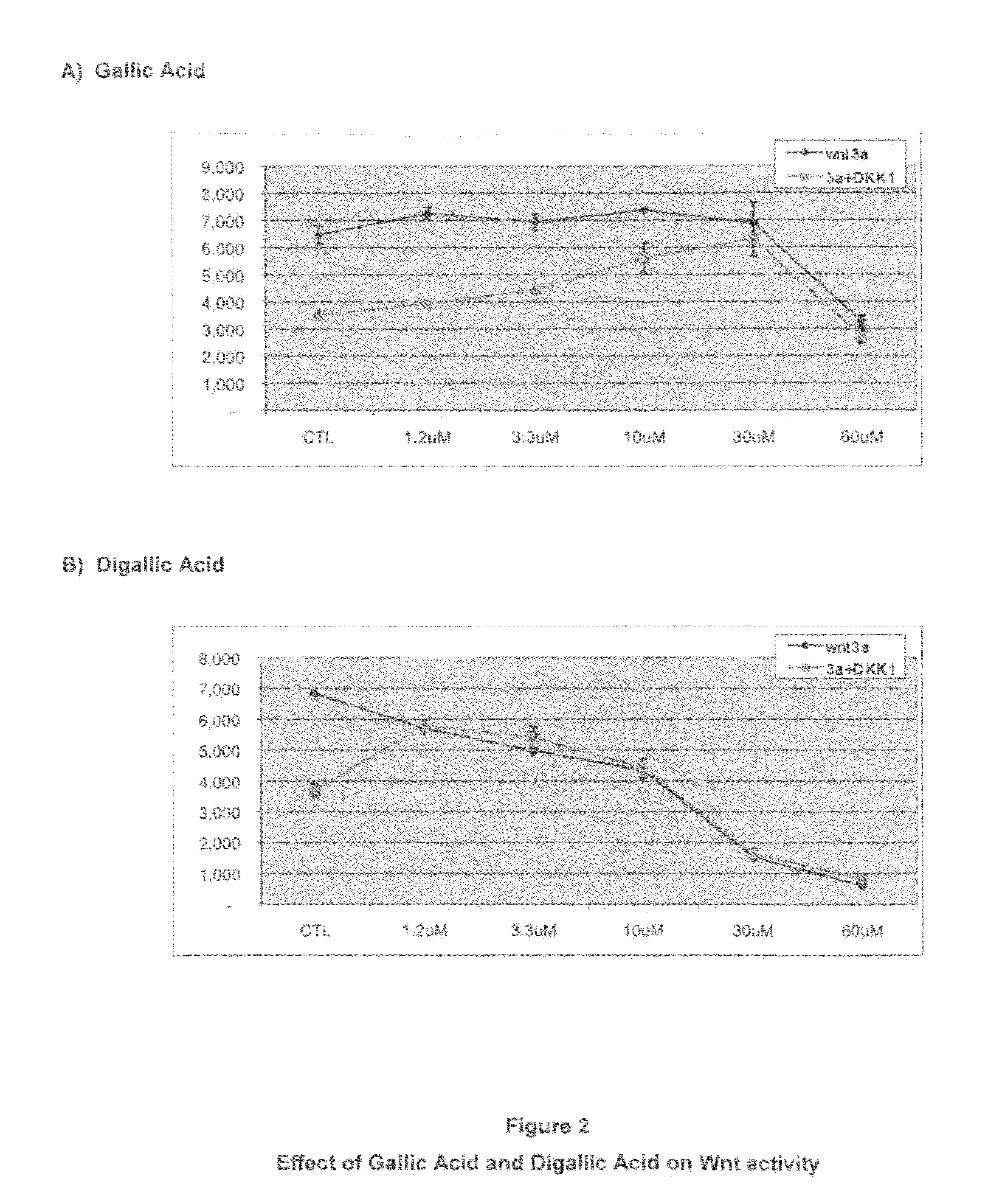
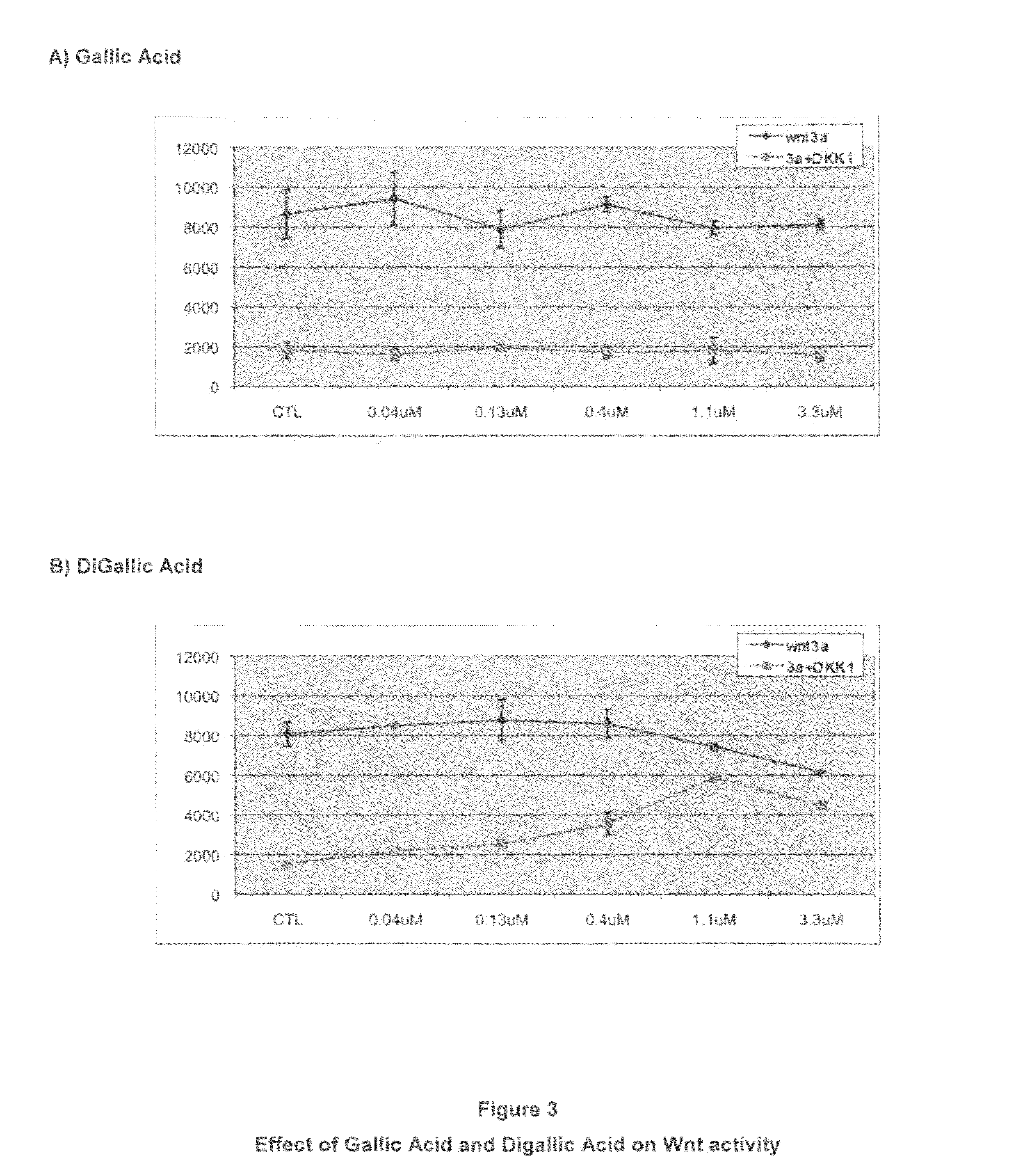
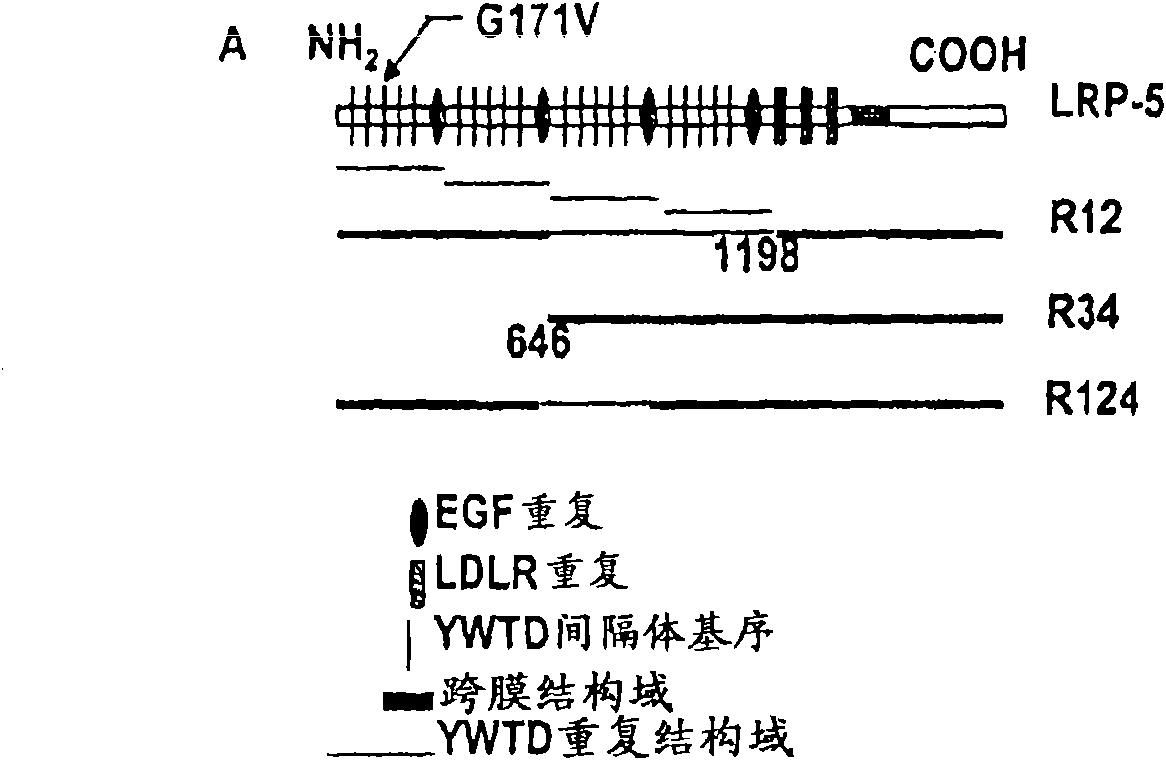
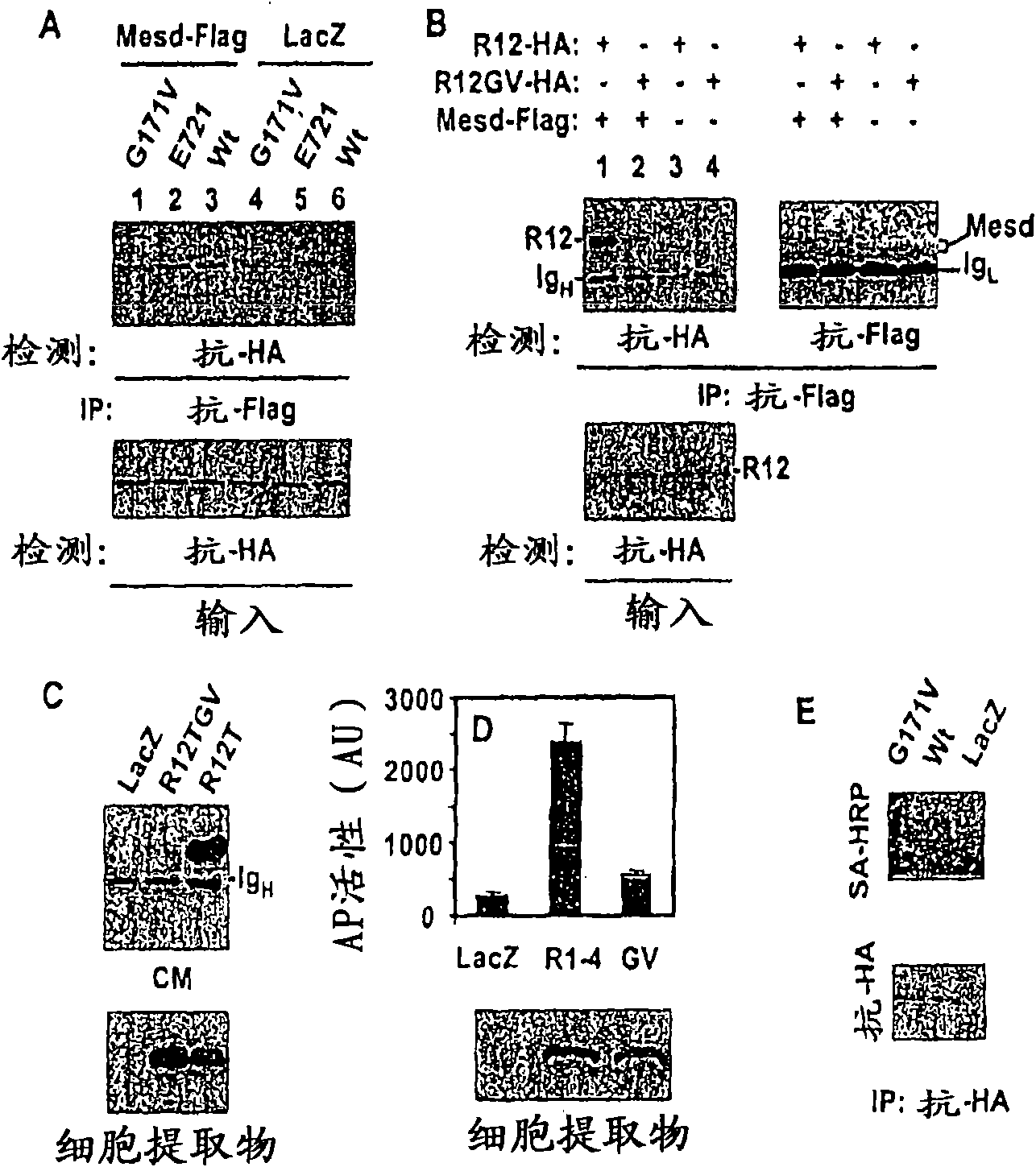
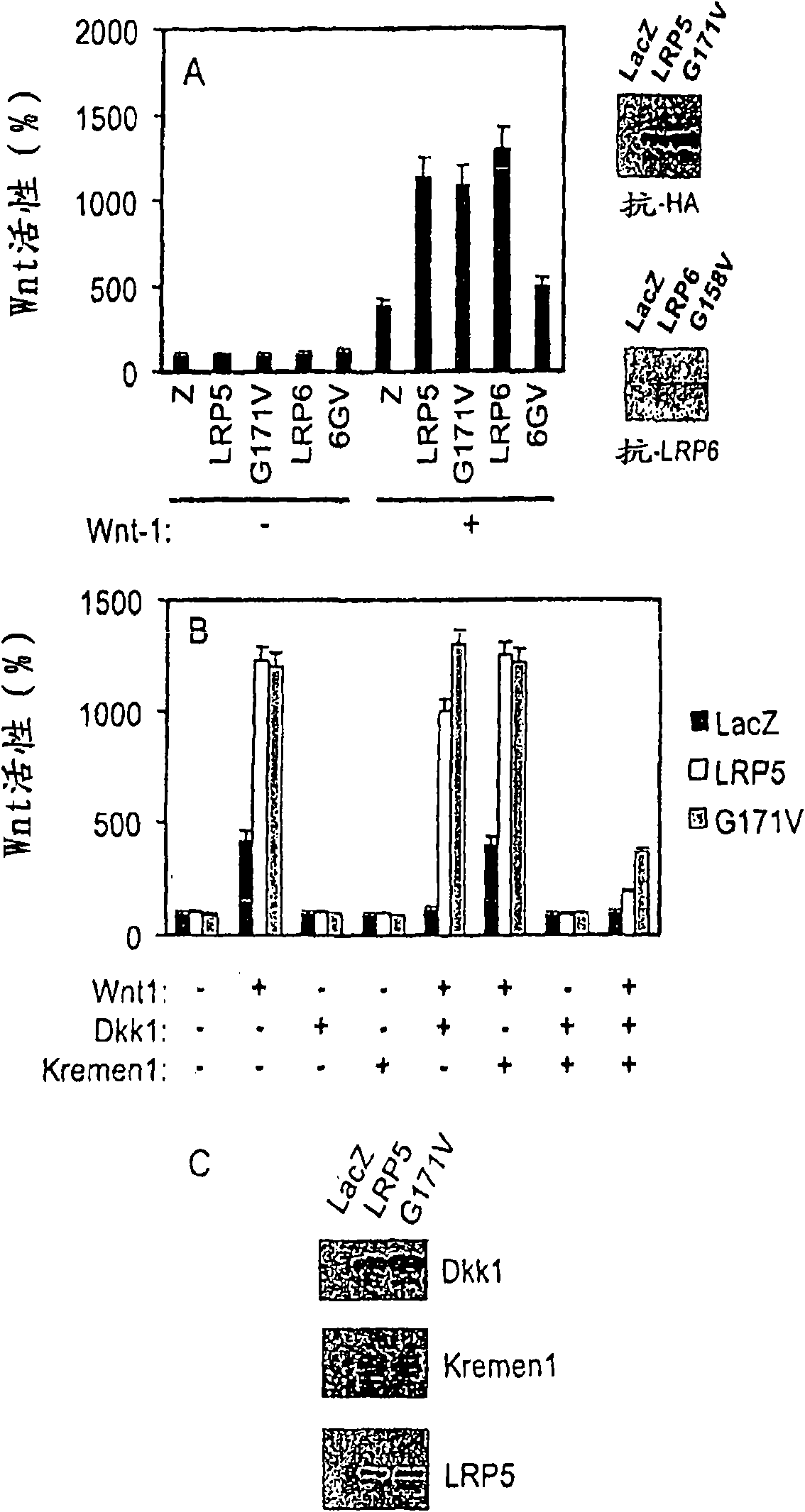
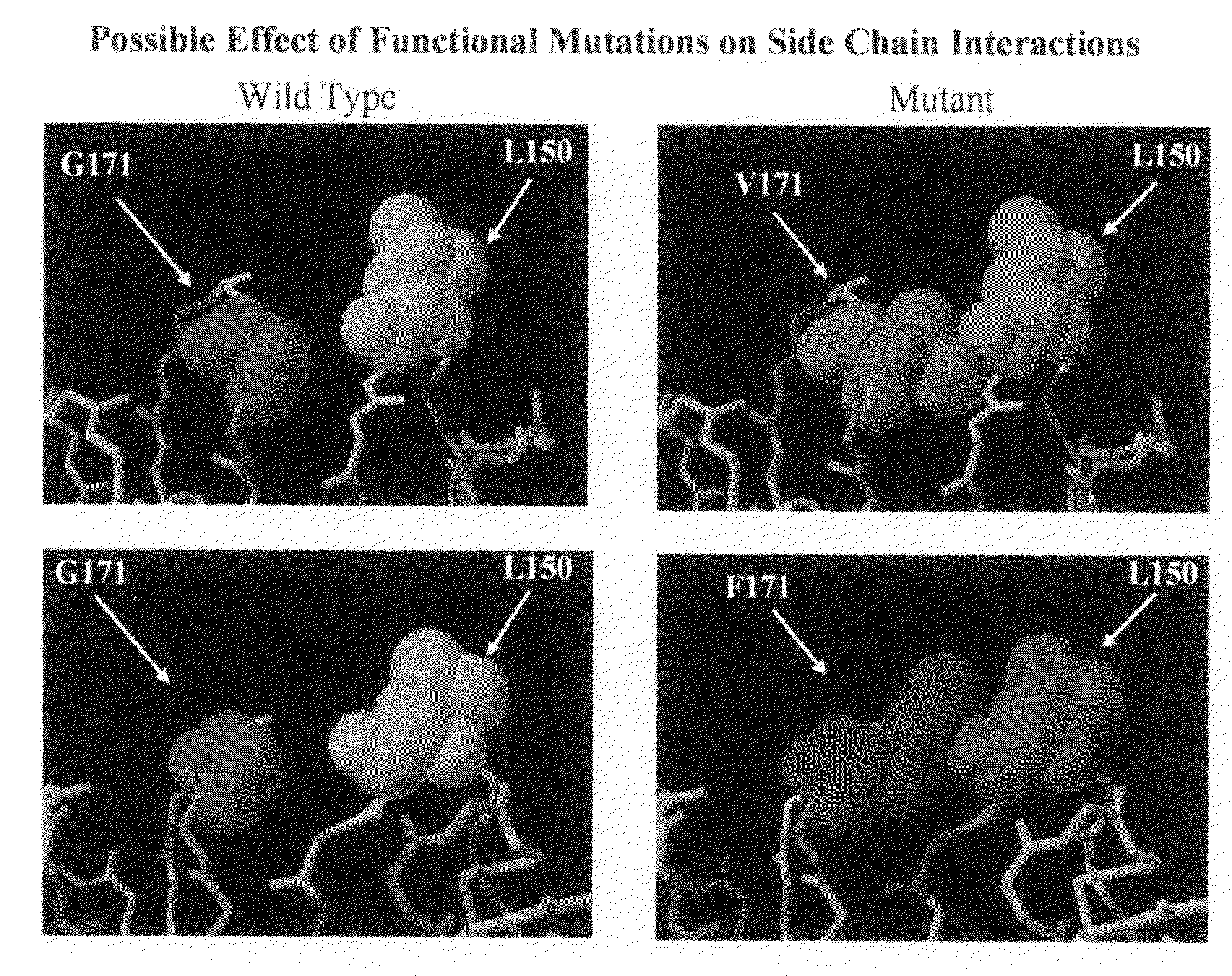
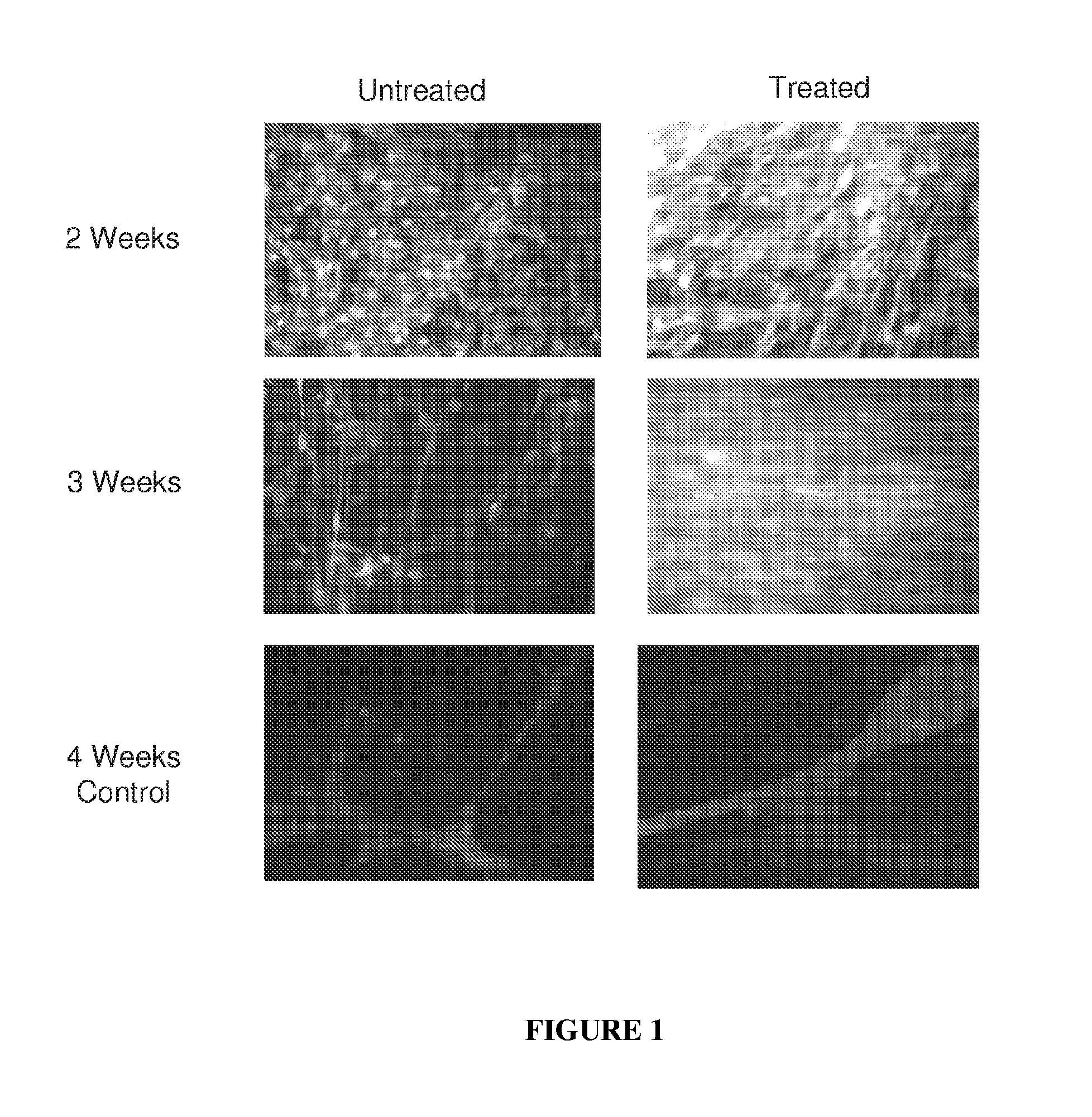
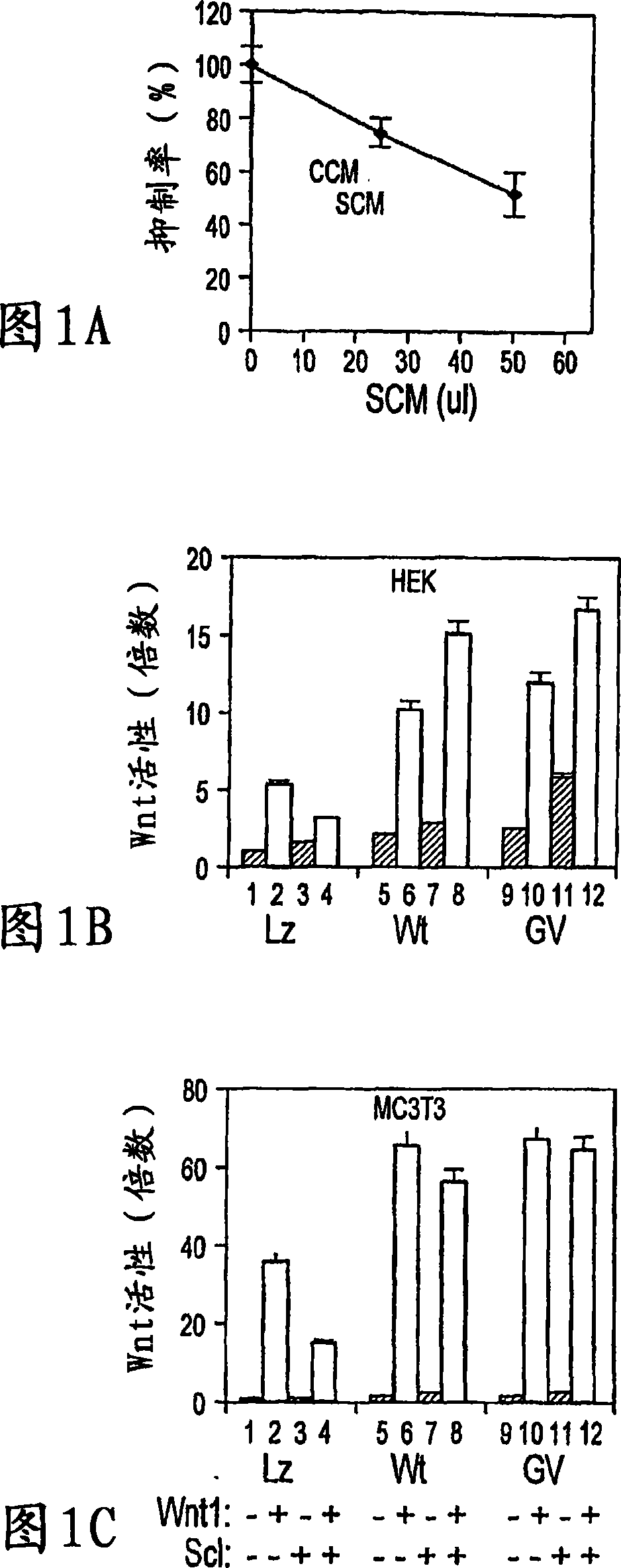
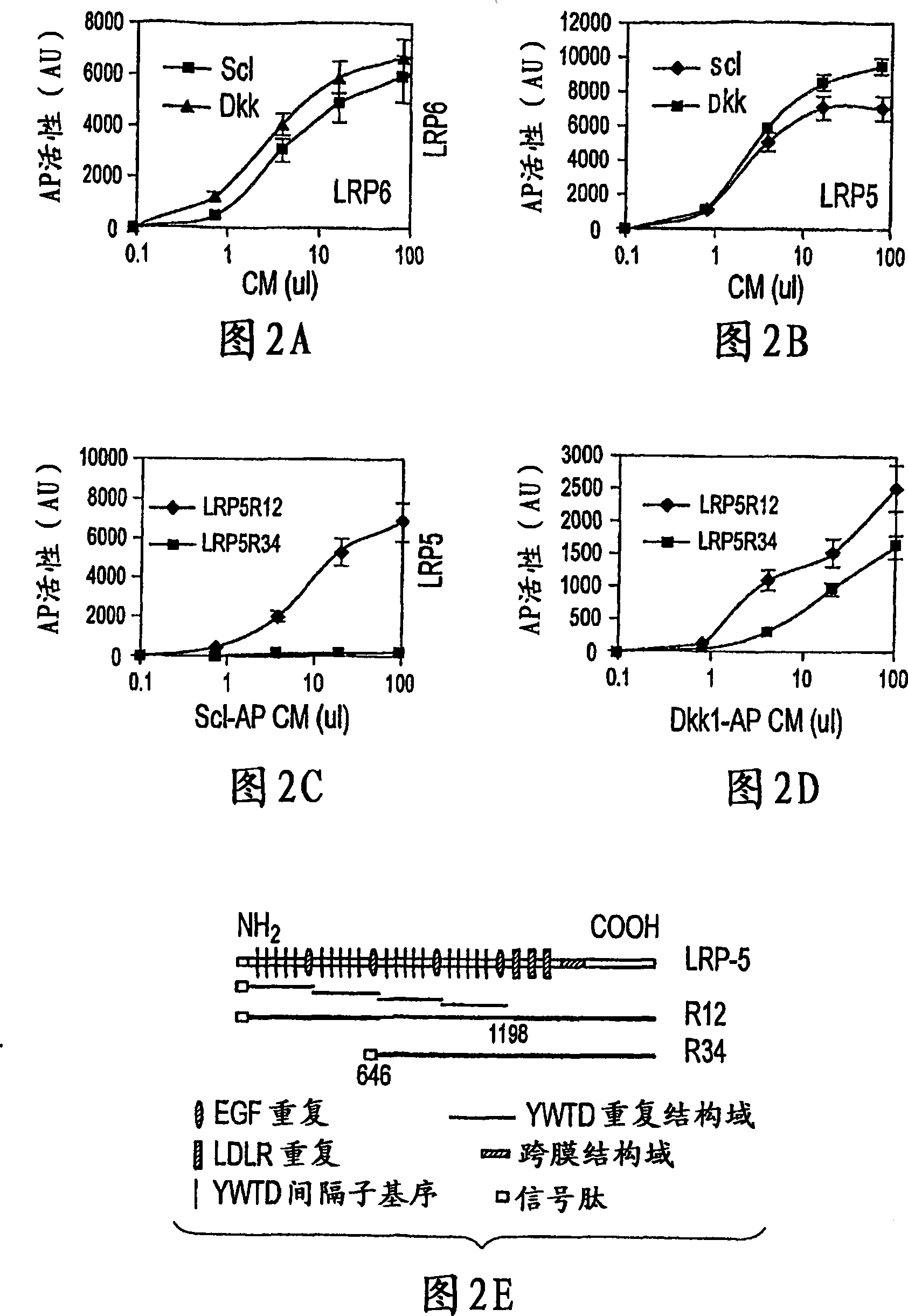
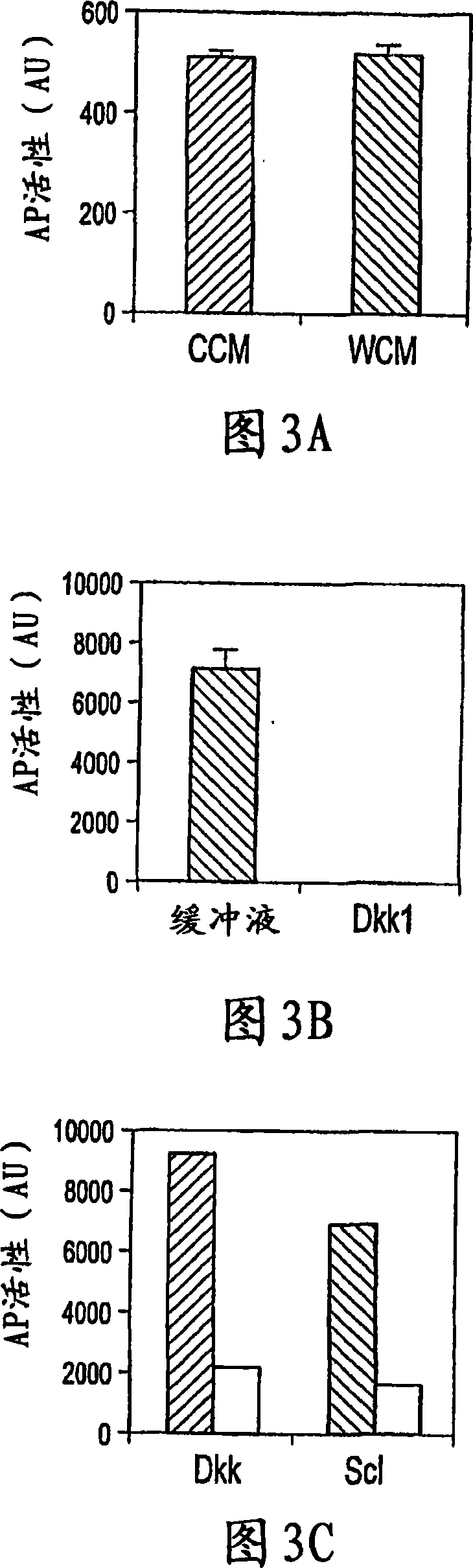
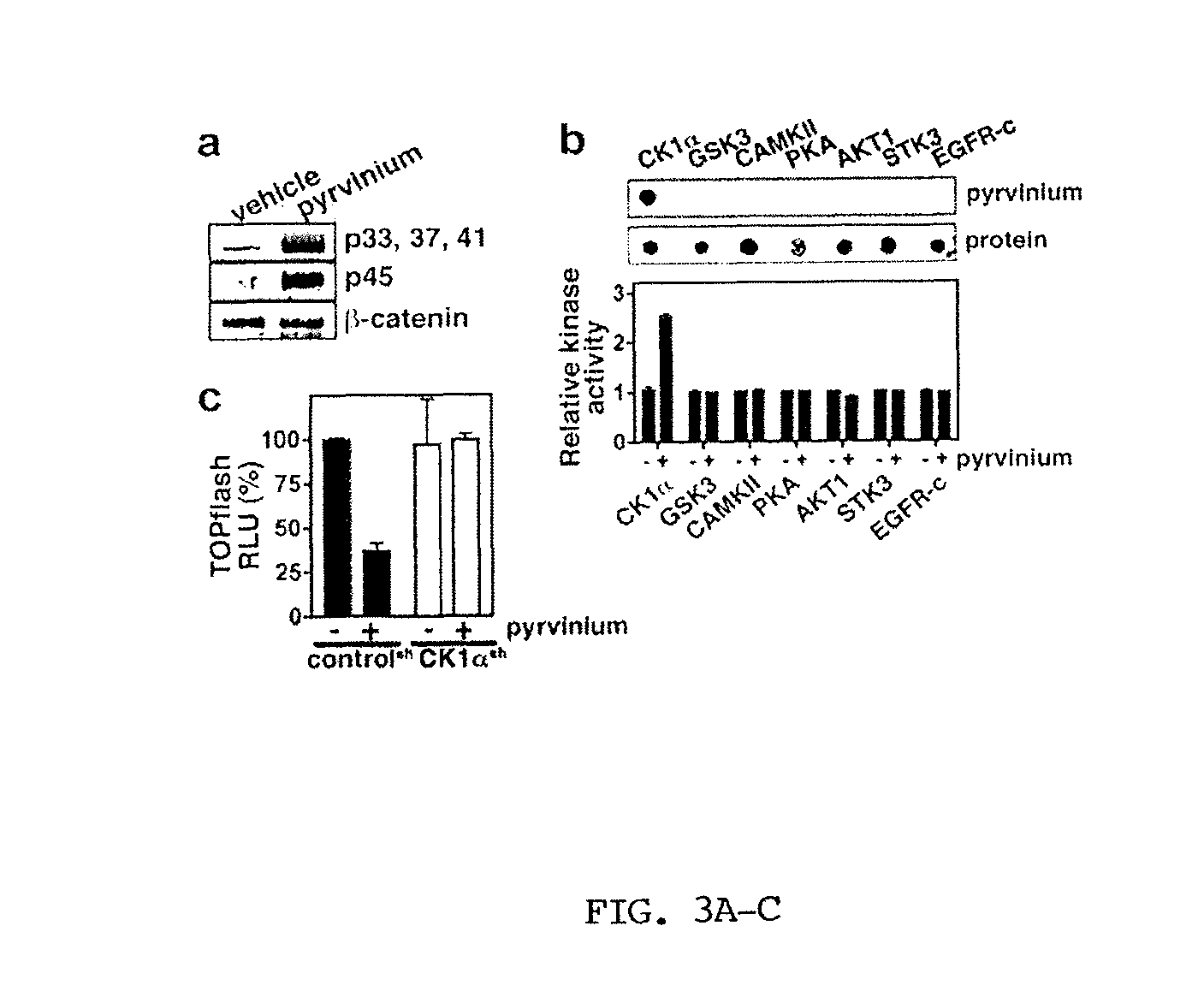
The mechanism by which the high bone mass (HBM) mutation (G171V) of the Wnt coreceptor LRP5 regulates the canonical Wnt signaling was investigated. The mutation was previously shown to reduce Dkk protein-1-mediated antagonism, suggesting that the first YWTD repeat domain where G171 is located may be responsible for Dkk protein-mediated antagonism. However, we found that the third YWTD repeat, but not the first repeat domain, is required for DKK1-mediated antagonism. Instead, we found that the G171V mutation disrupted the interaction of LRP5 with Mesd, a chaperon protein for LRP5 / 6 that is required for the coreceptors' transport to cell surfaces, resulting in less LRP5 molecules on the cell surface. Although the reduction in the level of cell surface LRP5 molecules led to a reduction in Wnt signaling in a paracrine paradigm, the mutation did not appear to affect the activity of coexpressed Wnt in an autocrine paradigm. Together with the observation that osteoblast cells produce autocrine canonical Wnt, Wnt7b, and that osteocytes produce paracrine Dkk1, we believe that the G171V mutation may cause an increase in Wnt activity in osteoblasts by reducing the number of targets for paracrine Dkk1 to antagonize without affecting the activity of autocrine Wnt.
Owner:ENZO THERAPEUTICS
Antibodies specific for Dkk-1
Owner:MEDIMMUNE LTD
HBM variants that modulate bone mass and lipid levels
The present invention relates to methods and materials used to express an HBM-like polypeptide derived from HBM, LRP5 or LRP6 in animal cells and transgenic animals. The present invention also relates to transgenic animals expressing the HBM-like polypeptides. The invention provides nucleic acids, including coding sequences, oligonucleotide primers and probes, proteins, cloning vectors, expression vectors, transformed hosts, methods of developing pharmaceutical compositions, methods of identifying molecules involved in bone development, and methods of diagnosing and treating diseases involved in bone development and lipid modulation. In preferred embodiments, the present invention is directed to methods for treating, diagnosing and preventing osteoporosis.
Owner:GENOME THERAPEUTICS +1
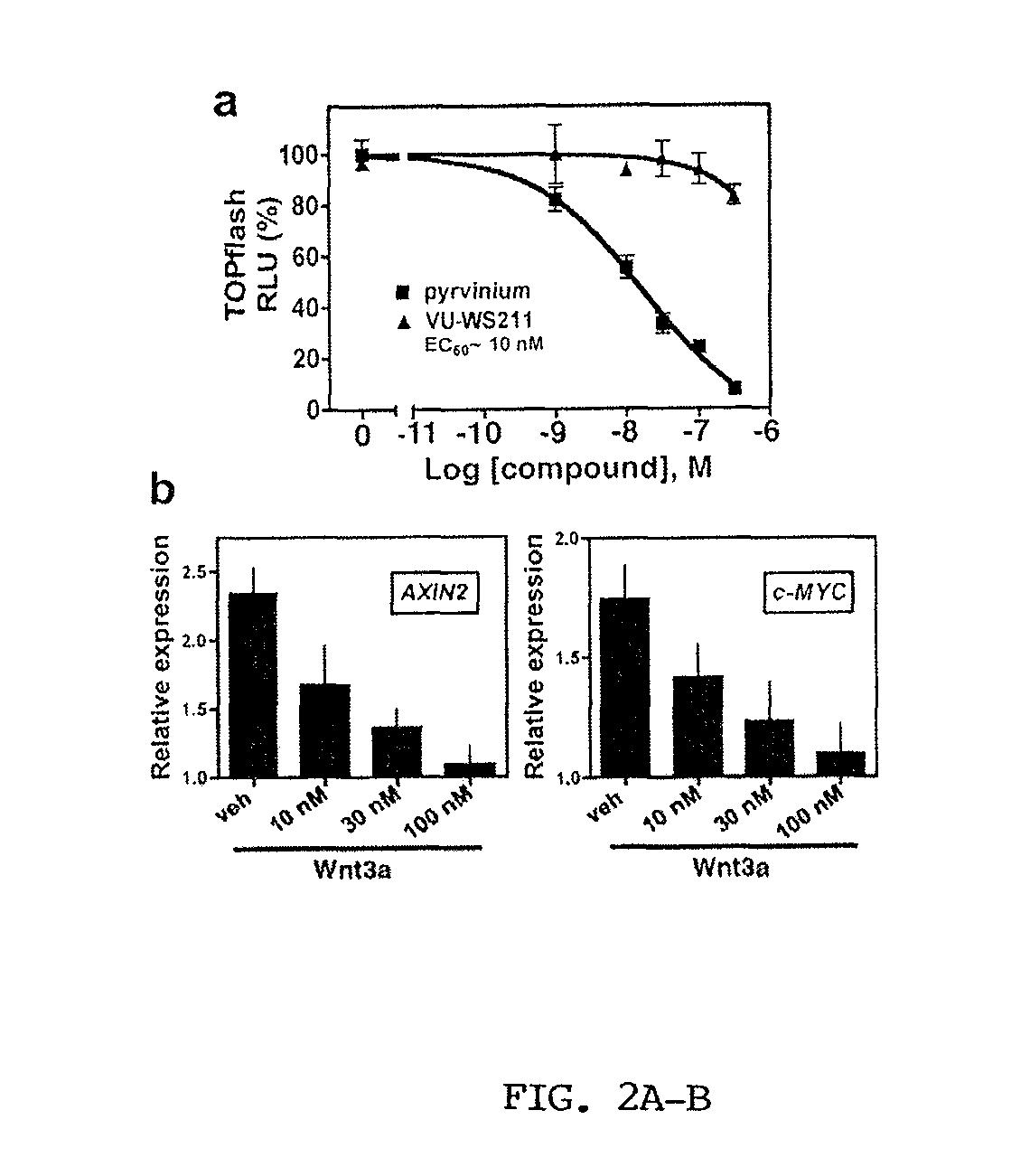
Screening method of drugs influencing classic Wnt signal pathway, and its application
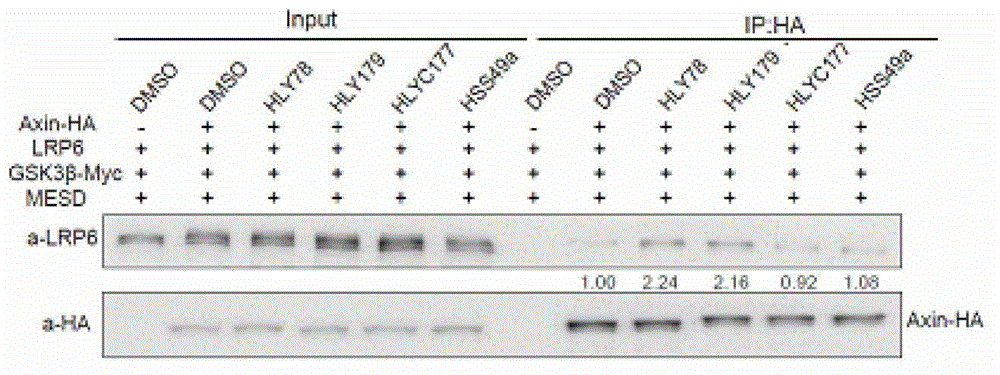
The invention provides a screening method of drugs influencing a classic Wnt signal pathway, and its application. The screening method of drugs influencing a classic Wnt signal pathway treats Axin and LRP5 / 6 interaction as a drug screening target. The drug screening method can be used for screening drugs for preventing or treating diseases or imbalance caused by the abnormity of the classic Wnt signal pathway, drugs for preventing or treating diseases or imbalance needing specific activation or inhibition of the classic Wnt signal pathway, or stem cell number amplification drugs.
Owner:CENT FOR EXCELLENCE IN MOLECULAR CELL SCI CHINESE ACAD OF SCI +1
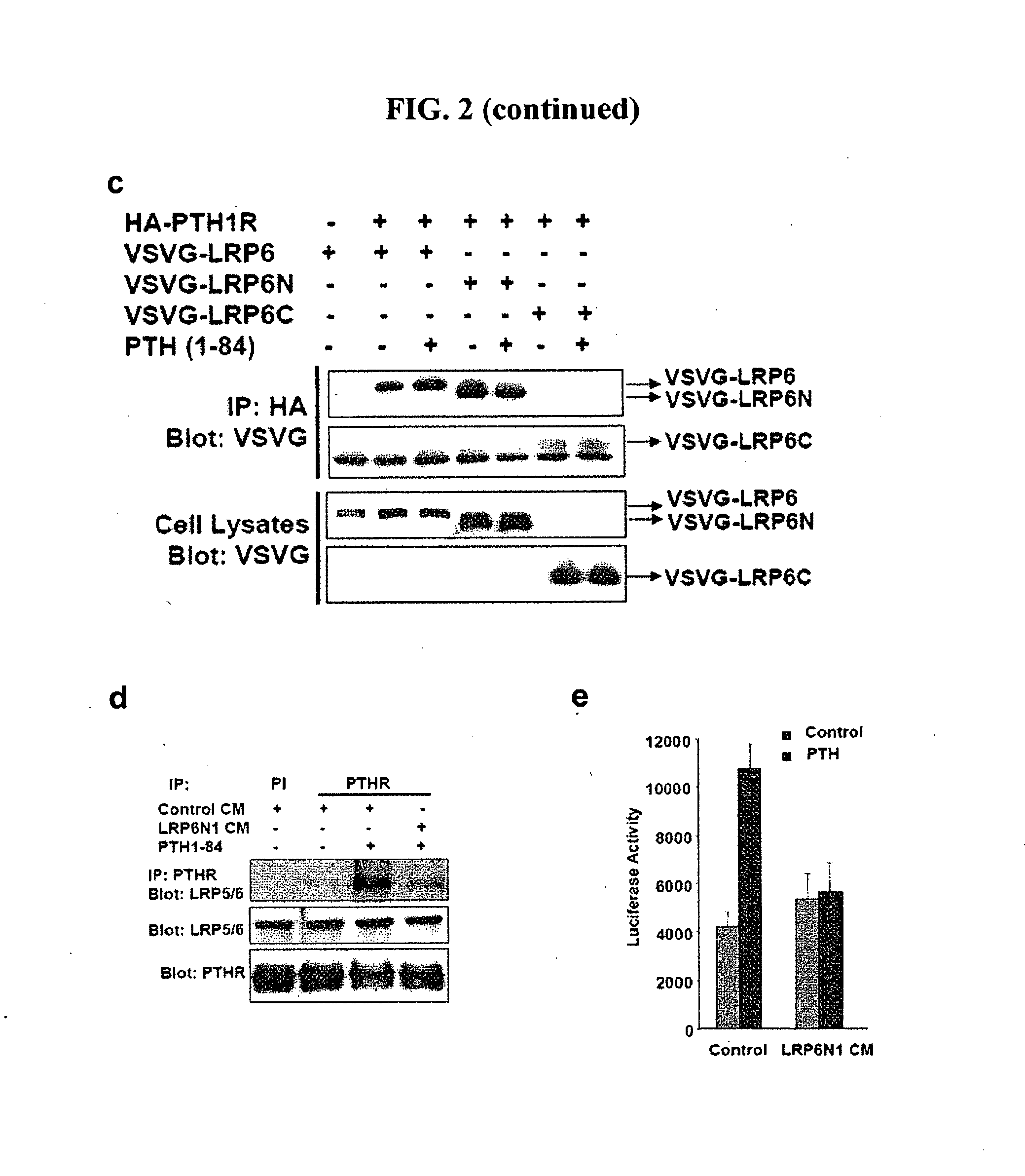
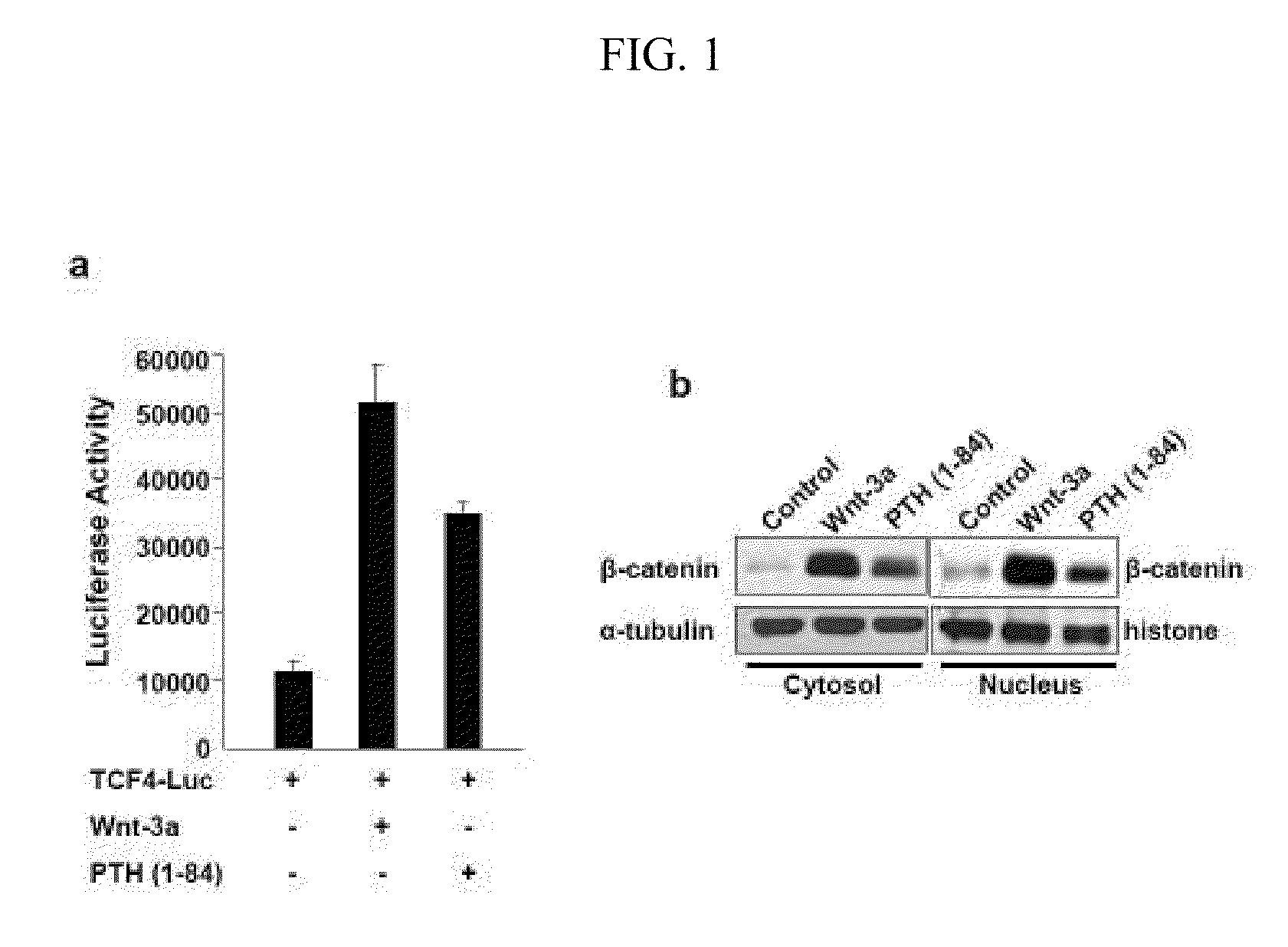
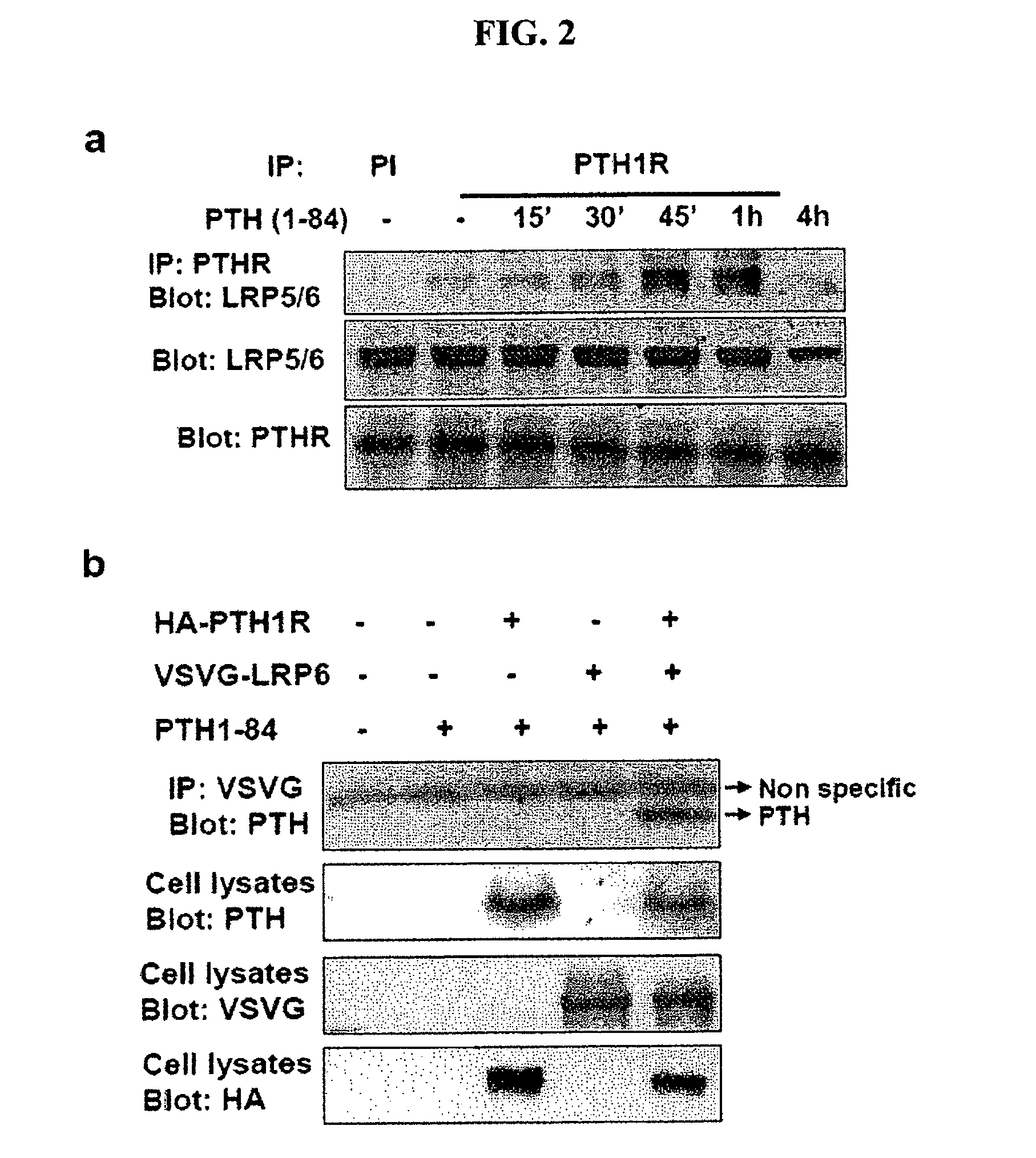
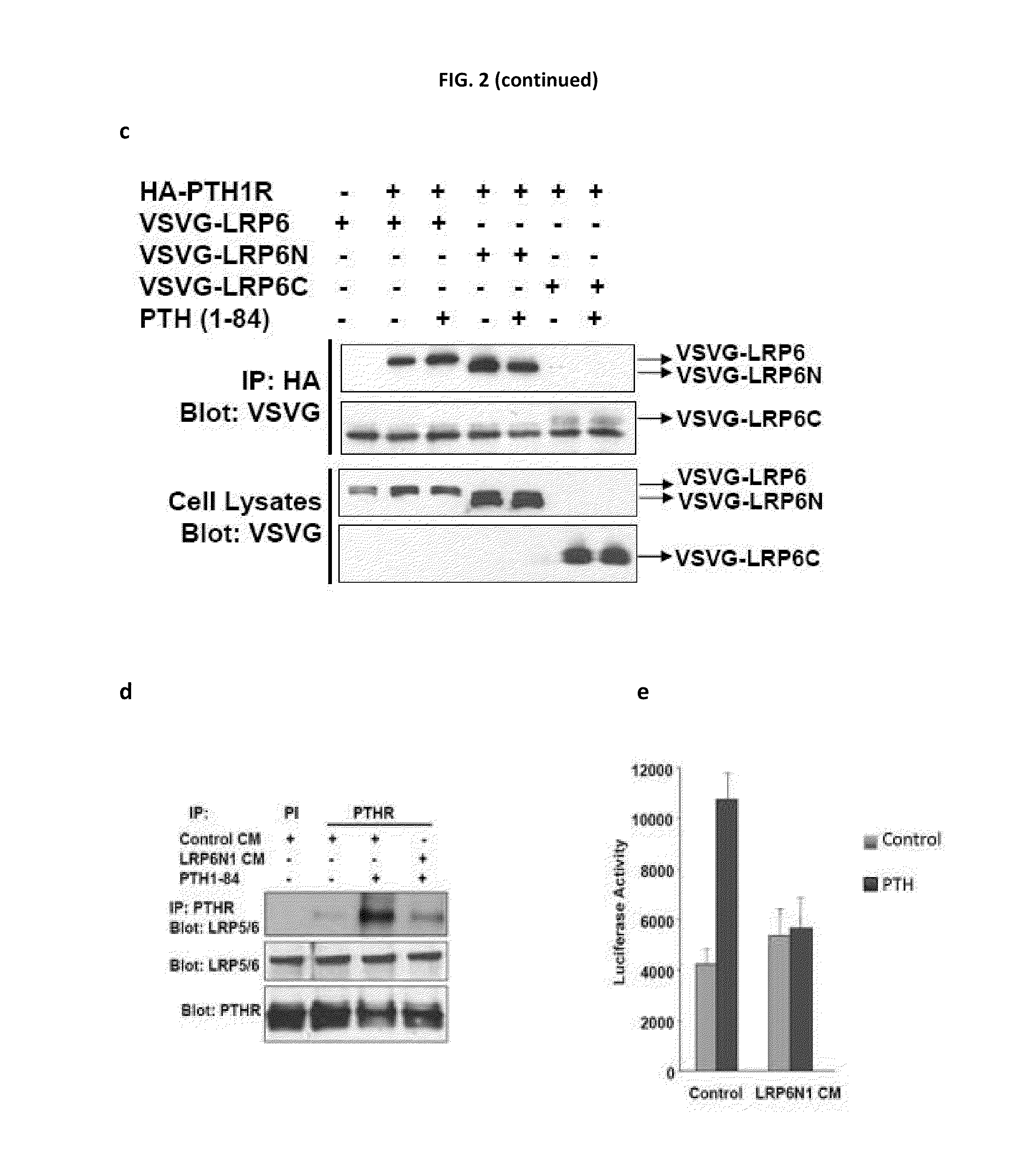
Compositions and Methods for Improving Bone Mass Through Modulation of Novel Receptors of PTH and Fragments Thereof
InactiveUS20100160220A1Enhanced interactionReduce interactionCompound screeningApoptosis detectionNephropathyLRP5
The present invention relates to the discovery of novel receptors for the signaling of PTH and / or fragments of PTH, and the role of cPTH in bone development. The novel PTH receptors identified are selected from the group consisting of LRP5 / 6, TGFβRII, BMPRII (long form and short form), ActRIIA, and ActRIIB. Specifically, the present invention provides a novel screening tool for identifying compounds that improve bone mass by affecting certain pathways that promote or downregulate bone-forming activity. This promotion of bone-forming activity could provide for treatments for bone-loss or bone density disorders and / or kidney disease. The invention further encompasses the compounds, PTH ligands, and fragments of PTH ligands described herein; pharmaceutical compositions comprising the compounds, PTH ligands, or fragments of PTH ligand; and methods of increasing bone density using the compounds, PTH ligands, or fragments of PTH ligands.
Owner:UAB RES FOUND
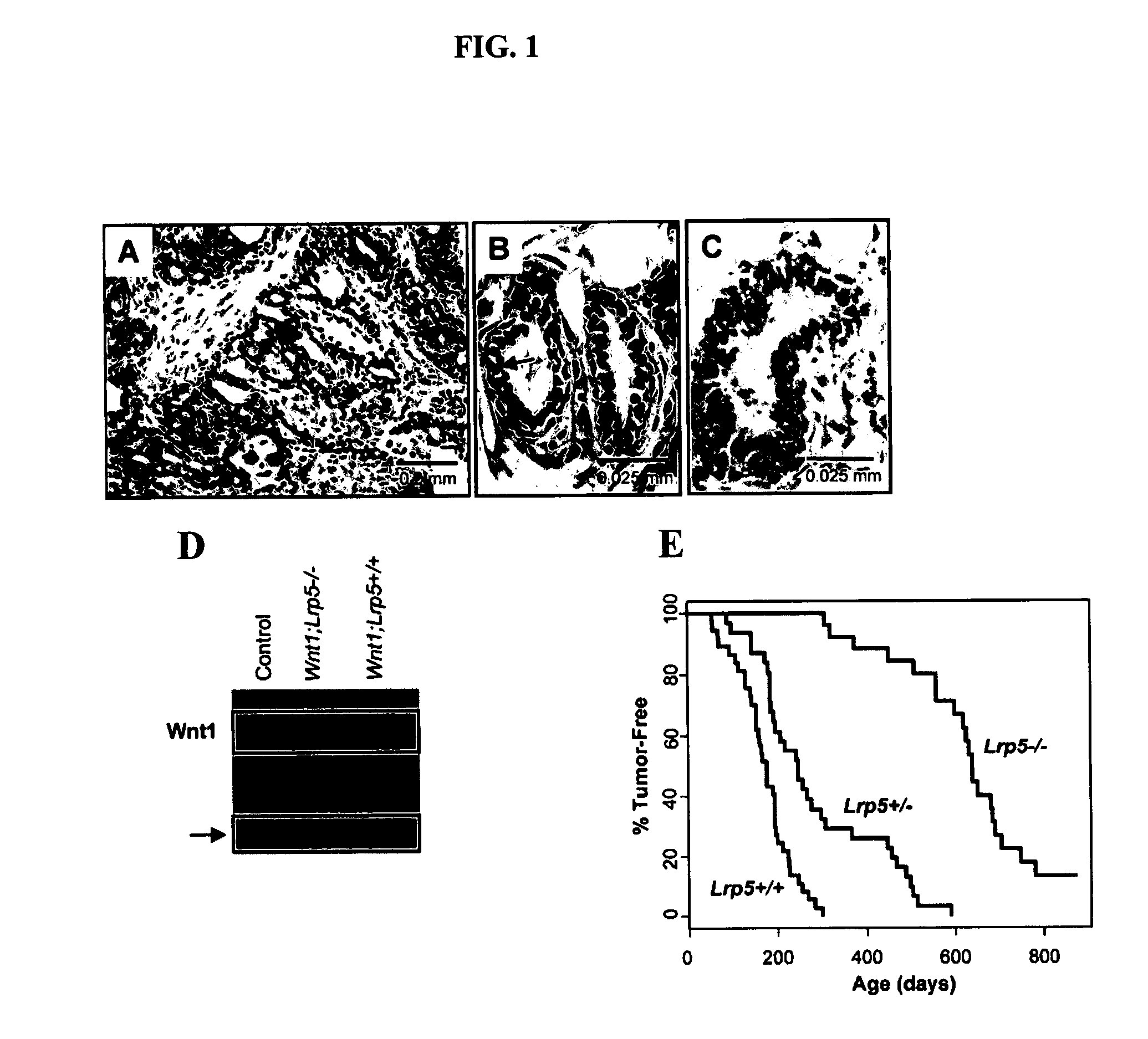
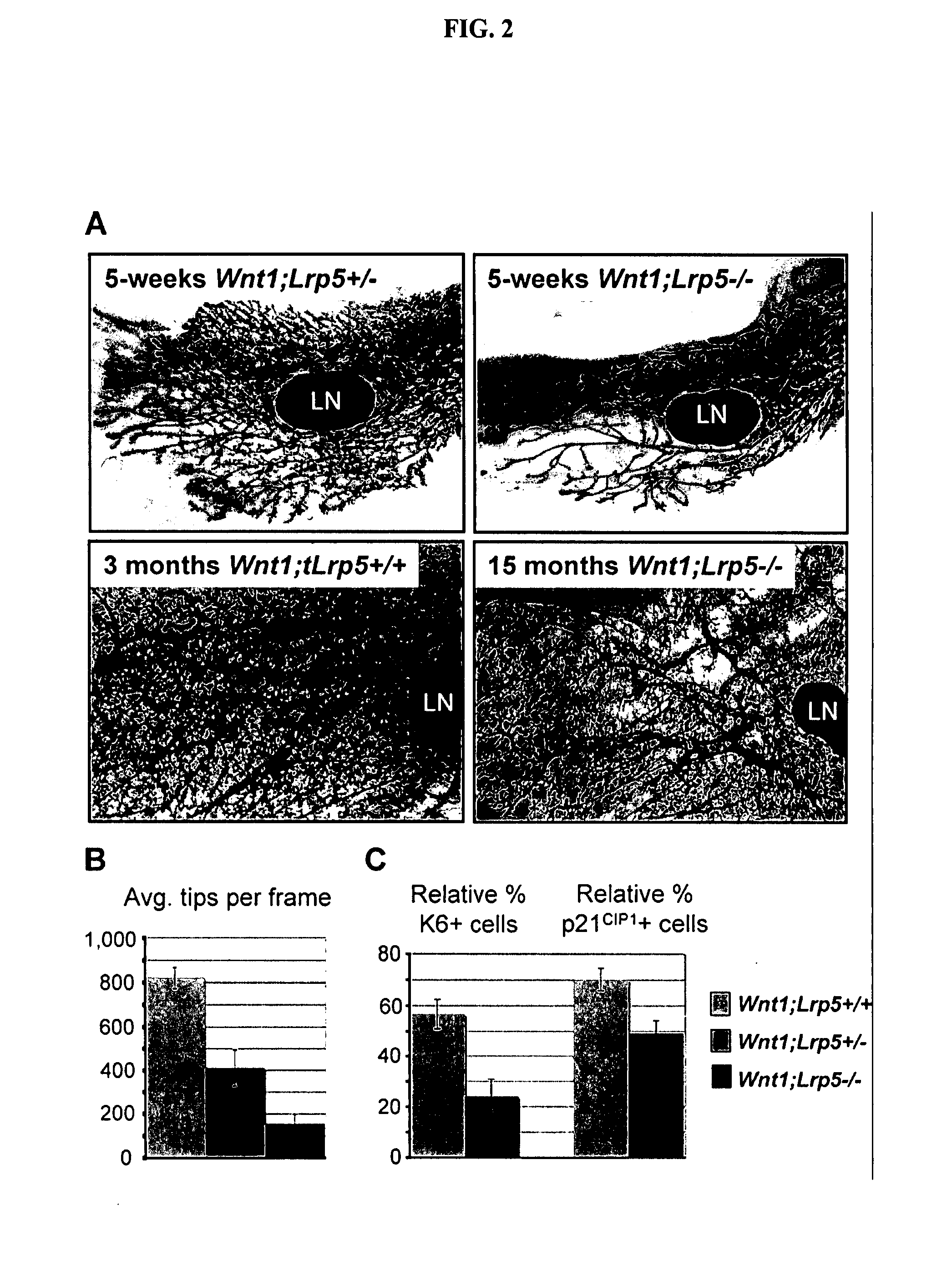
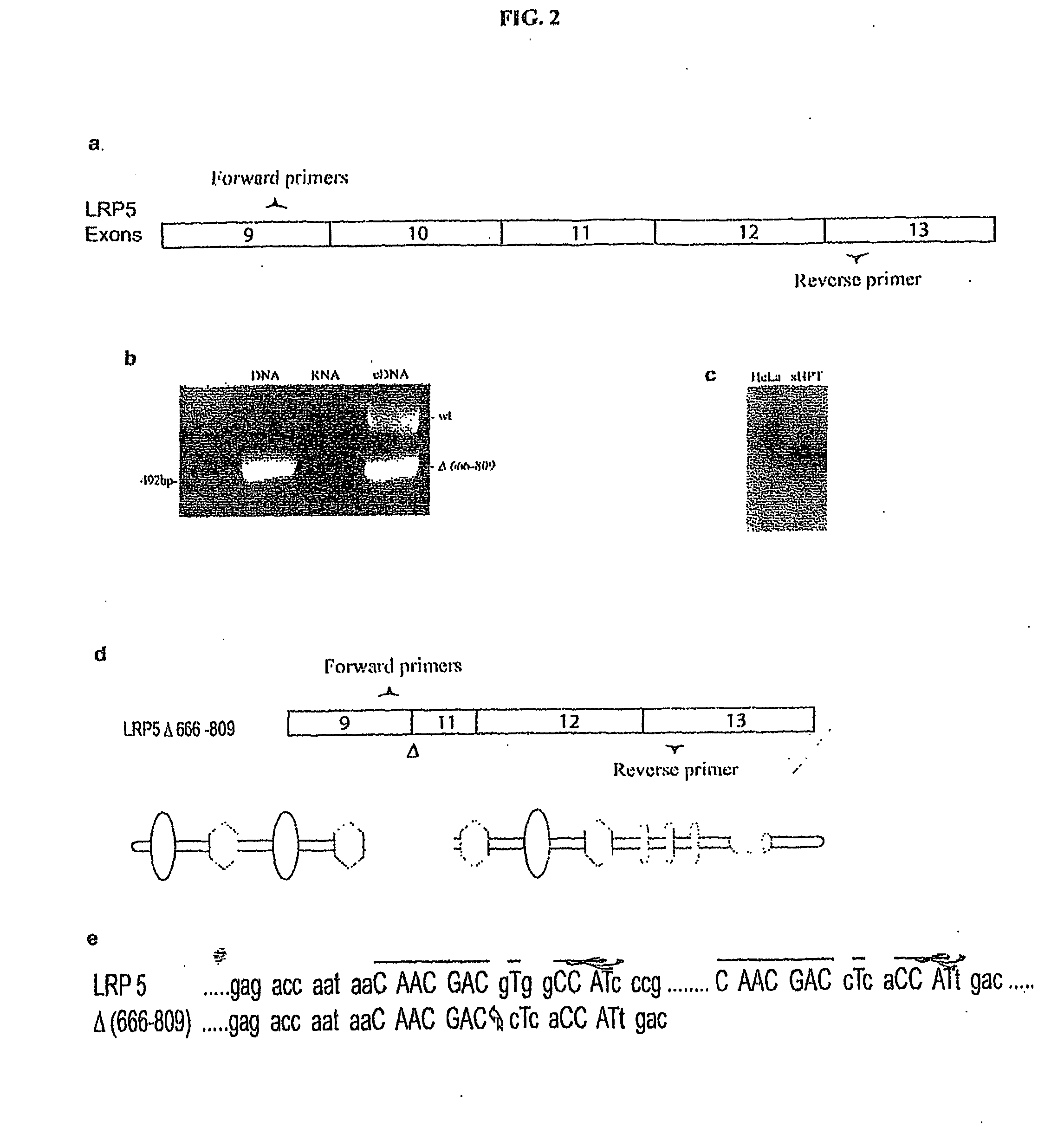
Mutant Lrp5/6 Wnt-Signaling Receptors in Cancer Diagnosis, Prognosis, and Treatment
A novel mutant form of lrp5 and lrp6 genes, the mutant LRP5 and LRP6 receptor proteins expressed therefrom, and a cell line which expresses the mutant LRP5 and / or LRP6 receptor proteins. Methods of diagnosing, prognosing and treating LRP5 related diseases, specifically hyperthyroidism and parathyroid tumors, and kits suitable for rapid on-site testing. Finally, methods of screening for agents capable of modulating the mutant LRP5 or LRP6 receptor proteins and pharmaceutical compositions comprising the selected agents.
Owner:BIOINVENT INT AB
Methods and compositions for diagnosis and treatment of genetic and retinal disease
A process of detecting the presence of or susceptibility to a disease involving the Frizzled-4 receptor is provided. The inventive method determines the presence or absence of one or more mutations in Frizzled-4 alone or in conjunction with other proteins such as Norrin and LRP5. The presence of a mutation predicts the presence of a disease or susceptibility to a disease. The inventive process further provides correction or prevention of a disease by administration of frizzled-4 to a subject to alter or maintain a physiological function.
Owner:DRENSER KIMBERLY
Polypeptides antagonizing wnt signaling in tumor cells
ActiveUS20180344868A1Improve propertiesLess side effectsHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseIntravenous gammaglobulin
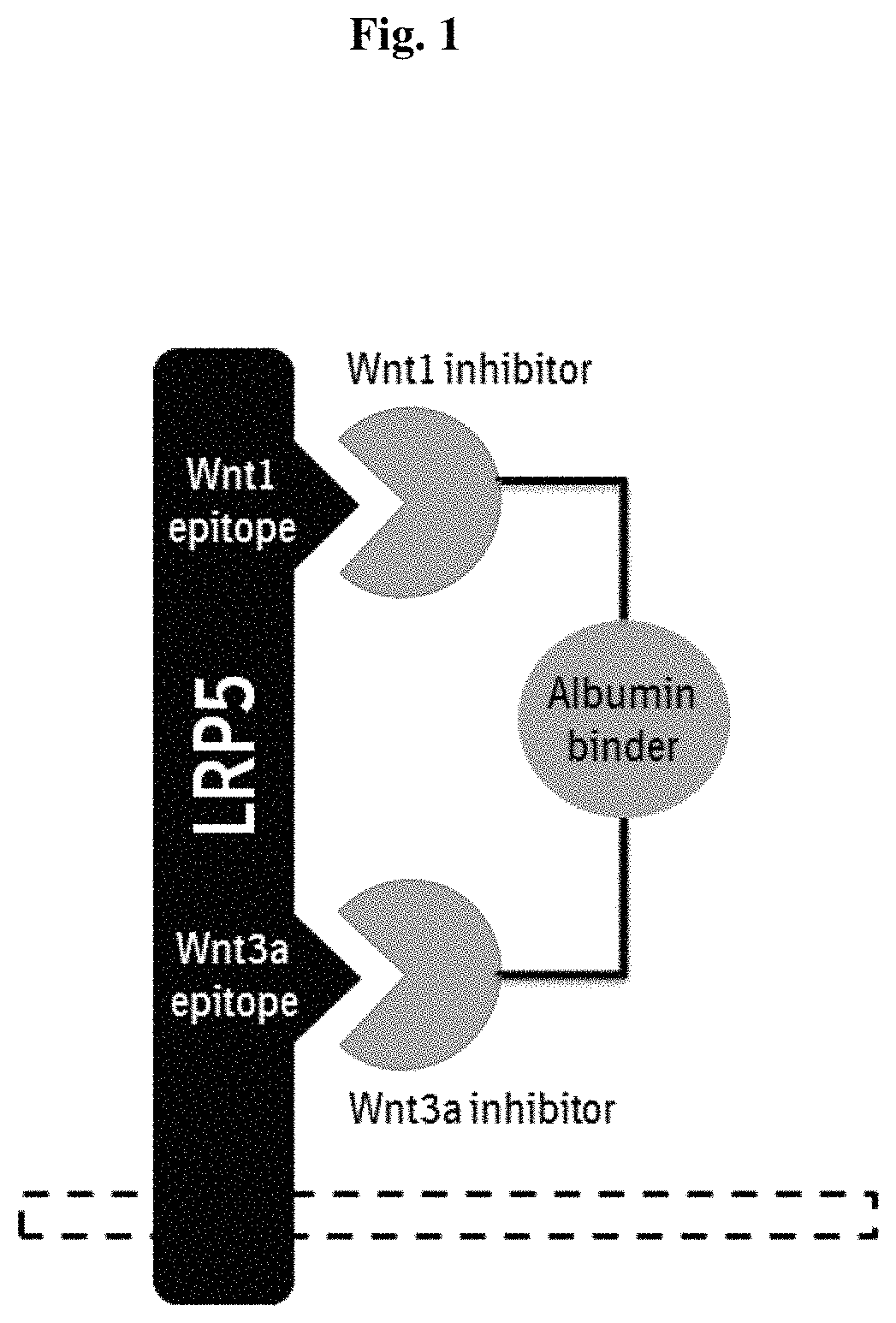
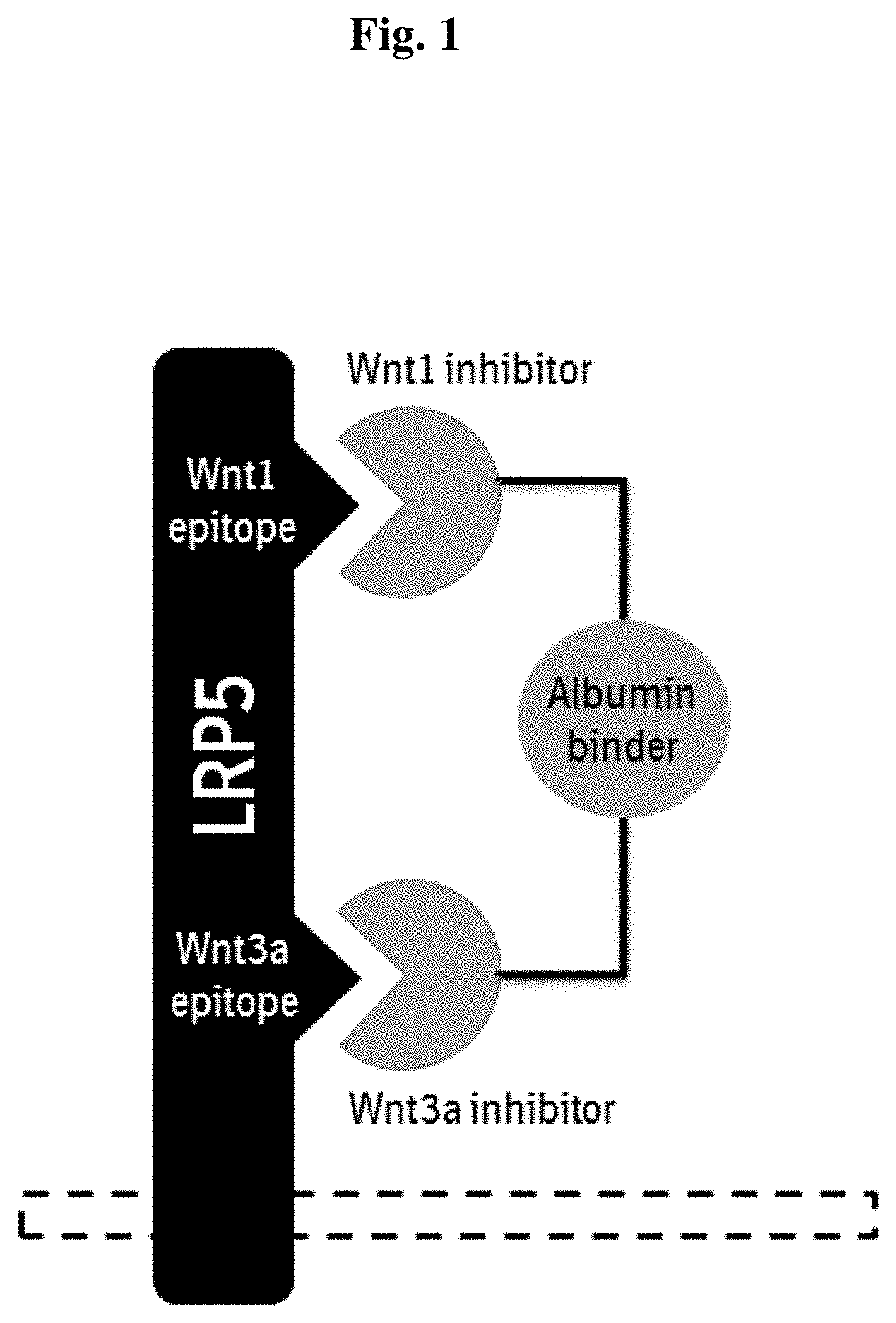
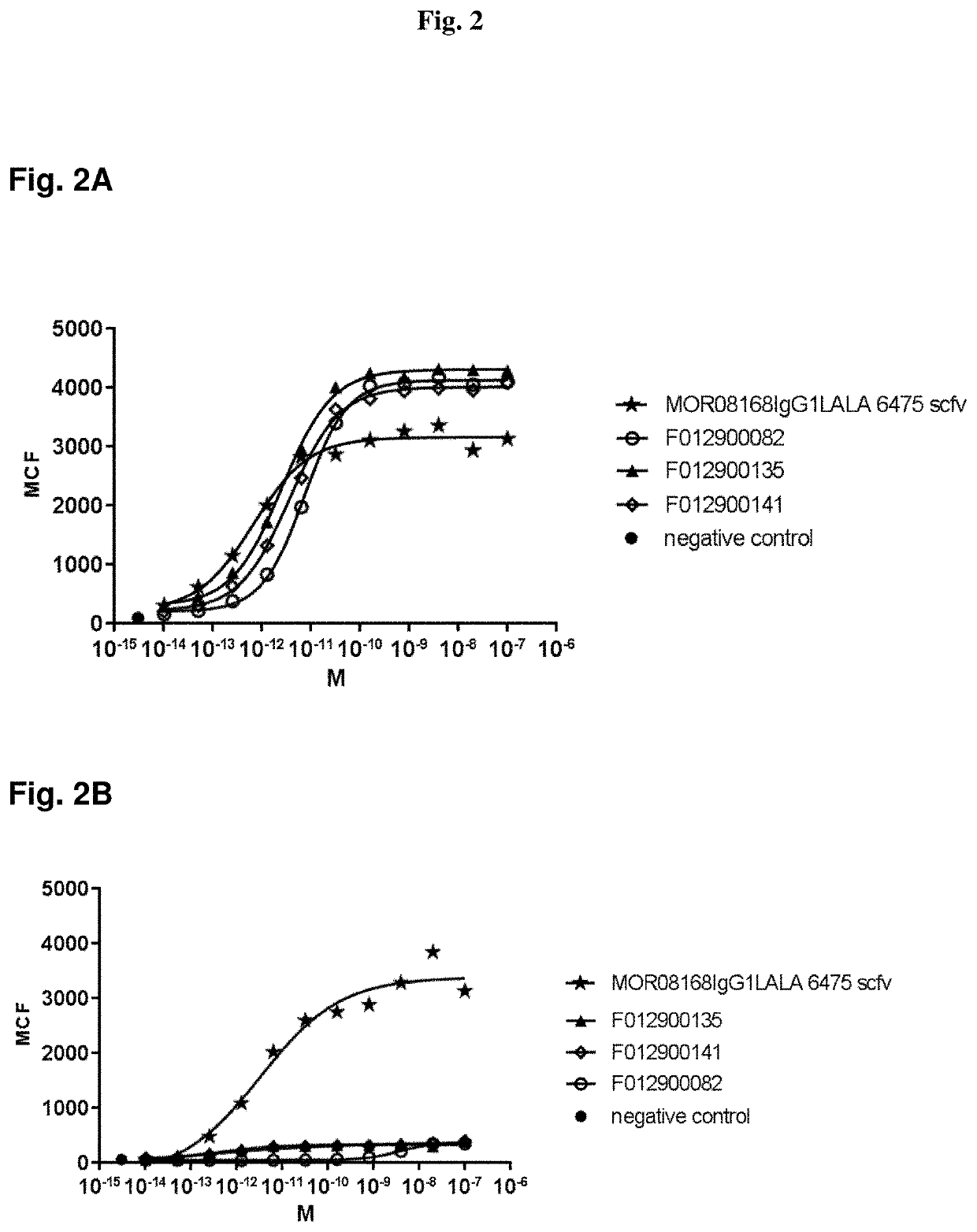
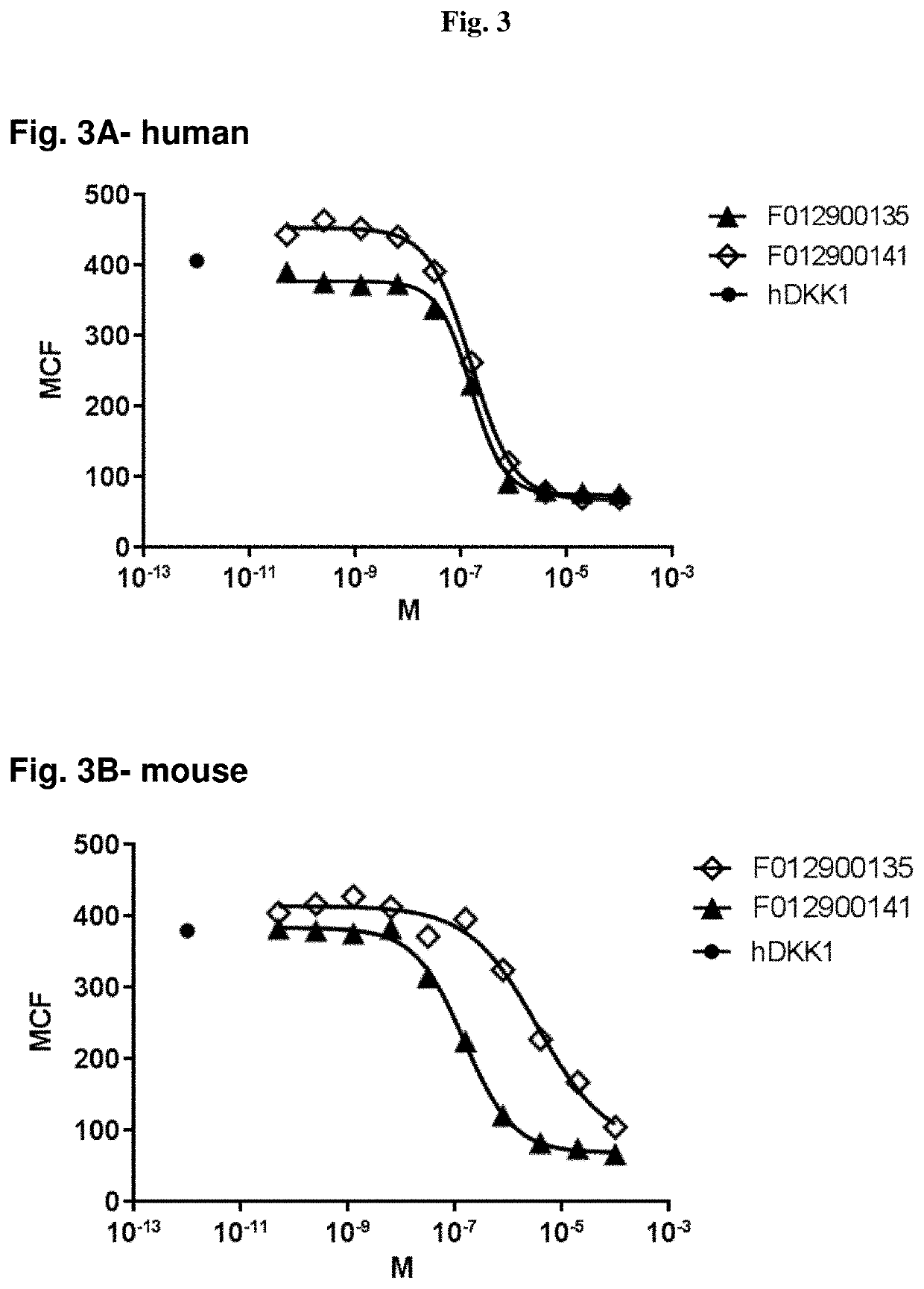
The invention provides novel LRP5-binding polypeptides, and more specifically novel LRP5-binding immunoglobulin single variable domain constructs which can inhibit Wnt signaling pathways. The invention also relates to specific sequences of such polypeptides, methods of their production, and methods of using them, including methods of treatment of diseases such as cancer.
Owner:BOEHRINGER INGELHEIM INT GMBH
METHODS AND COMPOSITIONS FOR MODULATING THE Wnt PATHWAY
The invention provides methods and compositions for modulating the Wnt signaling pathway, in particular by interfering with binding of Dkk1 or SOST with LRP5 and / or LRP6.
Owner:GENENTECH INC
Sclerostin and the inhibition of wnt signaling and bone formation
InactiveCN101888849AAvoid combiningBinding blockCompound screeningApoptosis detectionLRP6Bone formation
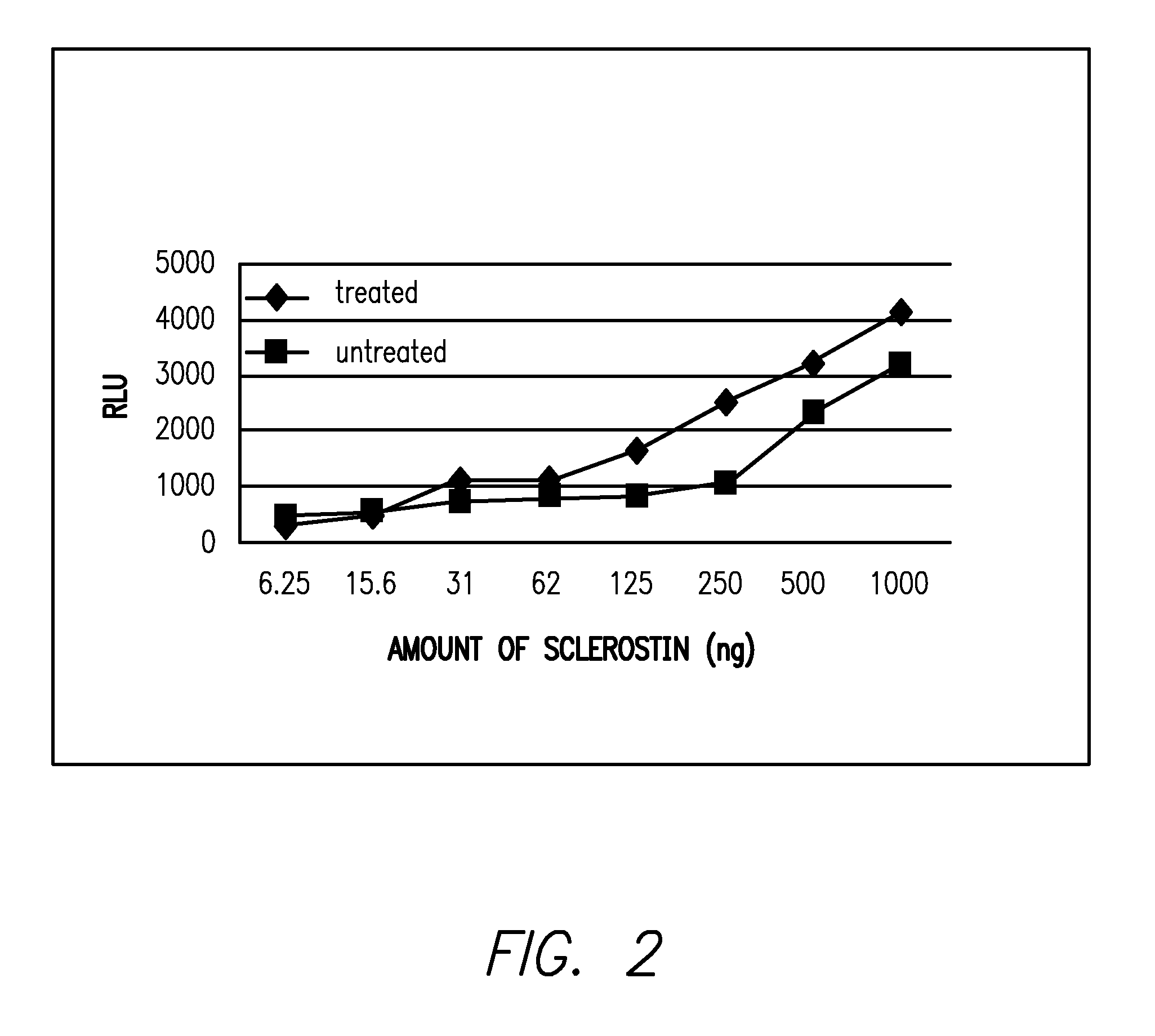
The loss of the SOST gene product sclerostin leads to sclerosteosis characterized byihigh bone mass (HBM). In this report, we found that sclerostin could antagonize canonical, Wnt signaling in human embryonic kidney A293 cells and mouse osteoblastic MC3T3 cells. This sclerostin-mediated antagonism could be reversed by over-expression of Wnt coreceptor LRP5. In addition, we found that sclerostin bound to LRP5 as well as LRP6 and identified the first two YWTD-EGF repeat domains of LRP5 as being responsible for the binding. Although these two repeat domains are required for transducing canonical Wnt signals, canonical Wnt did not appear to compete with sclerostin for binding to LRP5. Examination of the expression of sclerostin and Wnt7b, an autocrine canonical Wnt, during primary calvarial osteoblast differentiation revealed that sclerostin is expressed at the late stages of osteoblast differentiation coinciding with the expression of osteogenic marker osteocalcin and trailing after the expression of Wnt7b. Given the plethora of evidence indicating that canonical Wnt signaling stimulates osteogenesis, we believe that the HBM phenotype associated with the loss of sclerostin may at least in part be attributed to an increase in canonical Wnt signaling resulting from the reduction in sclerostin-mediated Wnt antagonism.
Owner:UNIV OF CONNECTICUT
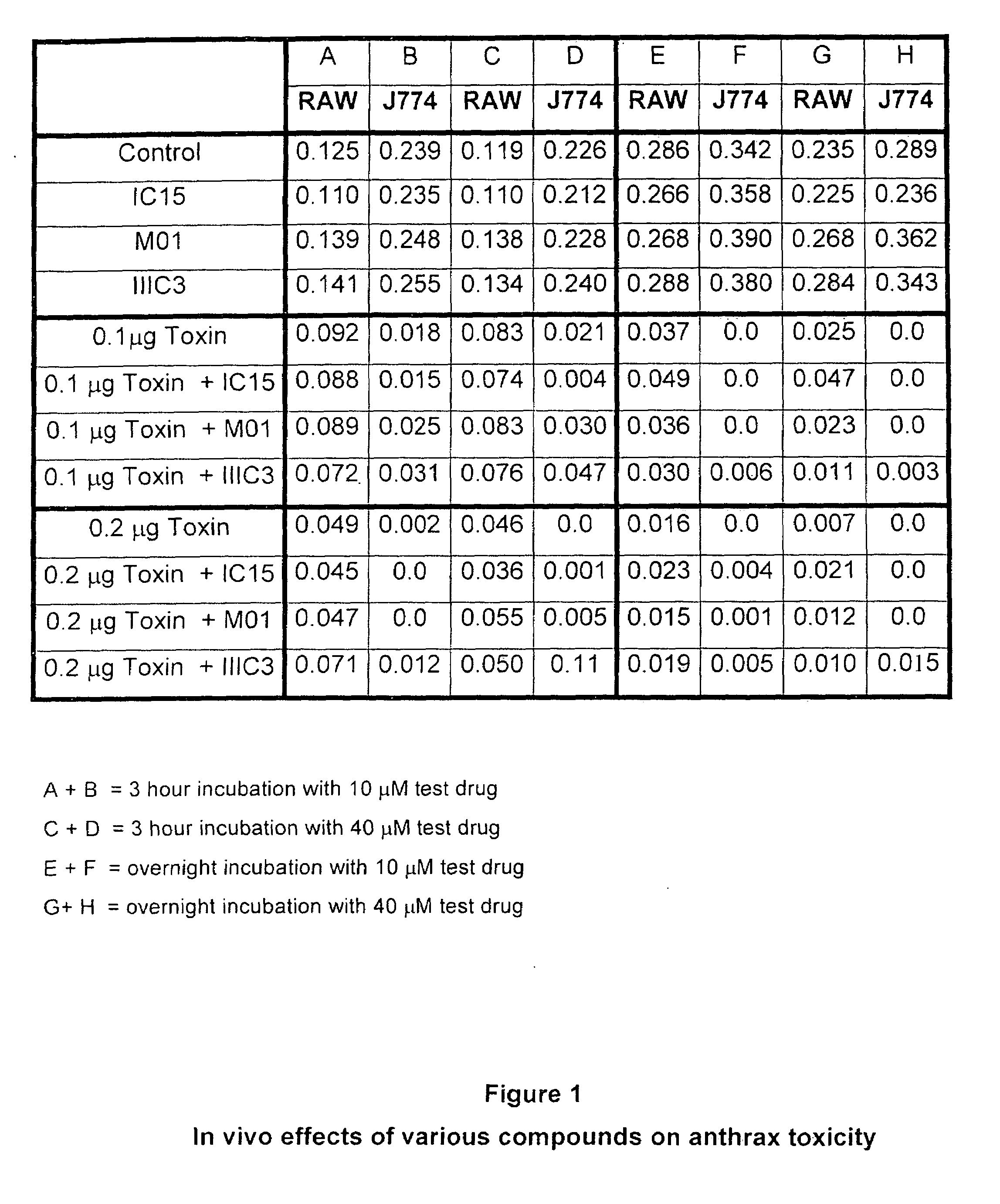
Compositions and methods for bone formation, bone remodeling and toxin protection
InactiveUS20100041599A1Intuitive effectReduce in quantityOrganic active ingredientsPeptide/protein ingredientsBone formationBone remodeling
The present invention identifies compounds that disrupt the interaction between anthrax proteins and LRP5 / 6 receptors, resulting in a reduction in anthrax toxicity. The compounds act to disrupt the intracellular transport of toxin complexes into a target cell. The present invention also provides methods for testing the effect of compounds on Wnt activity, through the use of in vitro experiments involving cells that have in at least one gene mutation involved in the Wnt pathway.
Owner:ENZO BIOCHEM
Biparatopic polypeptides antagonizing Wnt signaling in tumor cells
ActiveUS10597449B2Easy to manufactureIncrease concentrationSenses disorderImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseLRP6
Owner:BOEHRINGER INGELHEIM INT GMBH
Compositions and methods for improving bone mass through modulation of receptors of PTH and fragments thereof
InactiveUS8008074B2Enhanced interactionReduce interactionCompound screeningApoptosis detectionLRP5Increased Bone Density
The present invention relates to the discovery of novel receptors for the signaling of PTH and / or fragments of PTH, and the role of cPTH in bone development. The novel PTH receptors identified are selected from the group consisting of LRP5 / 6, TGFβRII, BMPRII (long form and short form), ActRIIA, and ActRIIB. Specifically, the present invention provides a novel screening tool for identifying compounds that improve bone mass by affecting certain pathways that promote or downregulate bone-forming activity. This promotion of bone-forming activity could provide for treatments for bone-loss or bone density disorders and / or kidney disease. The invention further encompasses the compounds, PTH ligands, and fragments of PTH ligands described herein; pharmaceutical compositions comprising the compounds, PTH ligands, or fragments of PTH ligand; and methods of increasing bone density using the compounds, PTH ligands, or fragments of PTH ligands.
Owner:UAB RES FOUND
Devices and methods for inhibiting stenosis, obstruction, or calcification of a native heart valve, stented heart valve or bioprosthesis
The present invention relates to methods for inhibiting stenosis, obstruction, or calcification of a valve following implantation of a valve prosthesis or a native valve which develops disease via the Lrp5 / Wnt Pathway in the presence of elevated lipids due to elevated Low Density Lipoprotein. This invention involves dispensing a combination of medications to target inflammation and attachment of the target cell and the secondary drugs to inhibit proliferation and calcification on an elastical stent, gortex graft or valve leaflet. The combination therapy inhibits bioprosthesis and native valve calcification with improvement of the longevity of the prosthetic material including the stent, the native valve, and the gortex covering. The valve prosthesis and or gortex graft is mounted on the elastical stent or prosthesis such that the elastical stent is connected to the valve.
Owner:CONCIEVALVE
Methods of using an antibody to inhibit WNT-mediated cardiac remodeling
InactiveUS8790650B2Reduced heart failure related hospitalizationsImprove athletic abilityPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseMonoclonal antibody
The present invention is directed to monoclonal antibodies and fragments thereof directed to LRP5 / 6 that find use in the prevention and treatment of cardiac remodeling and cancer. Also disclosed are methods for using such monoclonal antibodies in the prevention and treatment of such diseases.
Owner:VANDERBILT UNIV
Antibodies specific for dkk-1
Antibodies specific for Dkk-1, an inhibitor of the osteoanabolic Wnt / LRP5 signaling pathway, are described. The antibodies, which inhibit binding of Dkk-1 to LRP5, are useful in compositions for stimulating bone growth, in particular, compositions for treating bone disorders which result in a loss in bone, for example, osteoporosis.
Owner:MEDIMMUNE LTD
Polypeptides antagonizing Wnt signaling in tumor cells
ActiveUS11033636B2Easy to manufactureMore solubleHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseIntravenous gammaglobulin
The invention provides novel LRP5-binding polypeptides, and more specifically novel LRP5-binding immunoglobulin single variable domain constructs which can inhibit Wnt signaling pathways. The invention also relates to specific sequences of such polypeptides, methods of their production, and methods of using them, including methods of treatment of diseases such as cancer.
Owner:BOEHRINGER INGELHEIM INT GMBH
Biparatopic polypeptides antagonizing wnt signaling in tumor cells
ActiveUS20200199222A1Easy to manufactureMore solubleSenses disorderImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseLRP6
The invention provides novel biparatopic LRP5 / LRP6 cross-reactive binding polypeptides, and more specifically novel biparatopic LRP5 / LRP6 cross-reactive immunoglobulin single variable domain constructs which can inhibit Wnt signaling pathways. The invention also relates to specific sequences of such polypeptides, methods of their production, and methods of using them, including methods of treatment of diseases such as cancer.
Owner:BOEHRINGER INGELHEIM INT GMBH
Antibodies for modulating binding between lrp and wise
InactiveUS20190194314A1Inhibit bindingCell receptors/surface-antigens/surface-determinantsImmunoglobulins against cell receptors/antigens/surface-determinantsLRP6LRP5
Owner:STOWERS INST FOR MEDICAL RES
Polypeptides antagonizing wnt signaling in tumor cells
PendingUS20220362393A1Easy to manufactureIncrease concentrationHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseLRP5
The invention provides novel LRP5-binding polypeptides, and more specifically novel LRP5-binding immunoglobulin single variable domain constructs which can inhibit Wnt signaling pathways. The invention also relates to specific sequences of such polypeptides, methods of their production, and methods of using them, including methods of treatment of diseases such as cancer.
Owner:BOEHRINGER INGELHEIM INT GMBH
Detection method and application of chicken Salmonella enteritidis infection resistance molecular marker
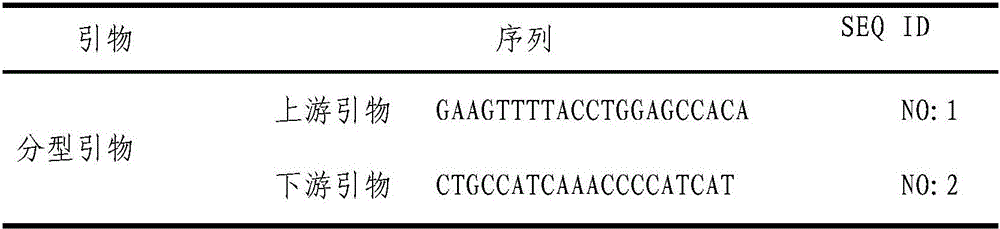
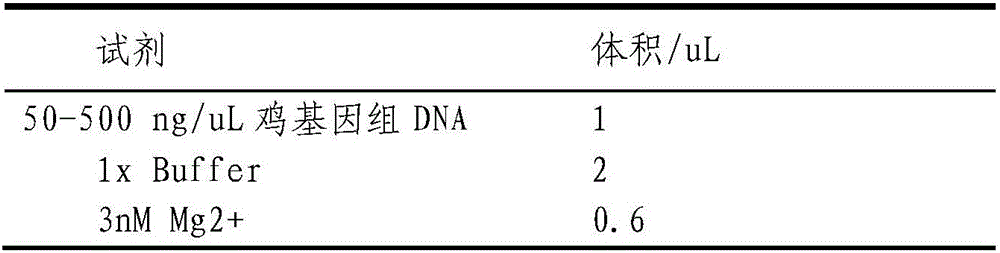
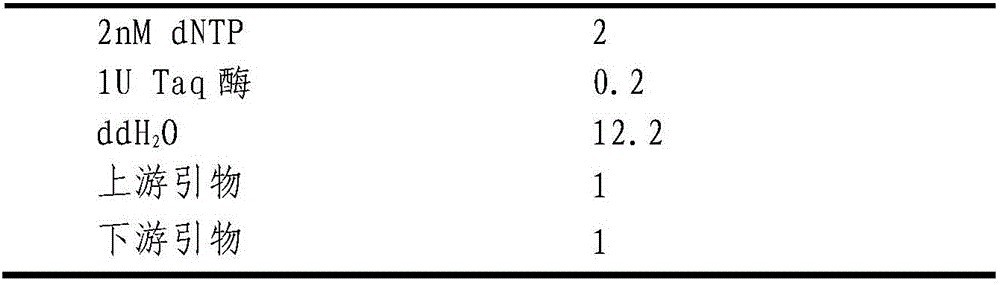
ActiveCN106636331AAccurate detectionMicrobiological testing/measurementAgainst vector-borne diseasesGenotypeLRP5
The invention relates to the technical field of gene engineering, and provides a detection method and application of a chicken Salmonella enteritidis infection resistance molecular marker-related gene LRP5. The method provides a chicken LRP5 gene rs80757564 site as the molecular marker, and correspondingly provides typing primers and corresponding PCR (polymerase chain reaction) conditions. The primers and method can be utilized to accurately detect the genotype of the LRP5 gene after the chickens are infected by Salmonella enteritidis. The individual with high Salmonella enteritidis infection resistance is selected to provide theoretical basis for chicken Salmonella-enteritidis-resistant breeding.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com