Methods for treating inflammation
a technology of inflammation and treatment, applied in the field of inflammation treatment, can solve the problems of impaired glucose-induced insulin secretion from the pancreatic islets, glucose tolerance defect when challenged, impaired chylomicron remnant clearance, etc., and achieve normal response level, reduced basal expression, and reduced inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
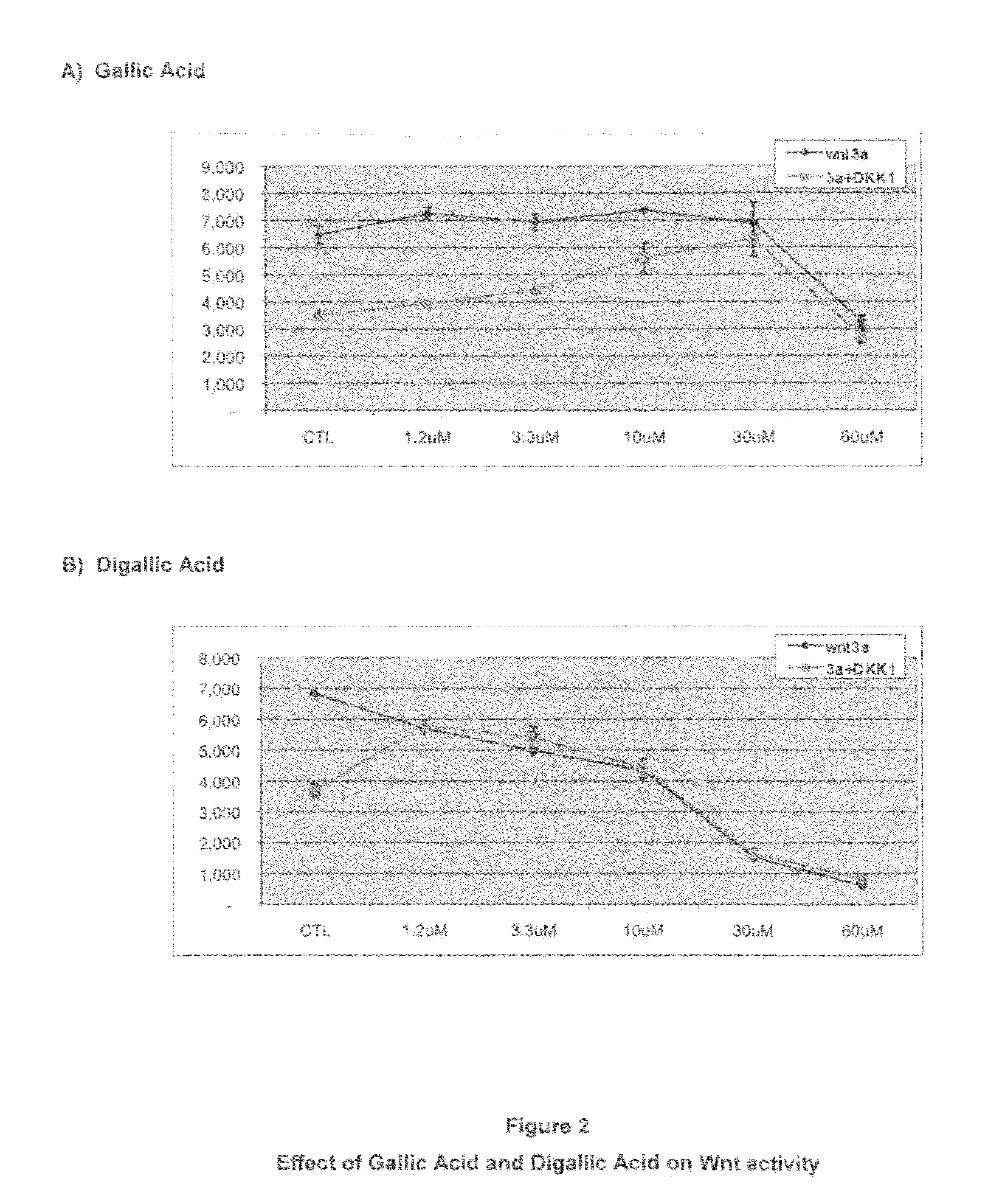
[0055]Effects of Gallic Acid and Digallic Acid on Wnt and Dkk Suppression of Wnt.
[0056]This experiment was carried out as previously described in U.S. Patent Application No. 20050196349 using Gallic Acid, a small molecule that represents a partial constituent of the IIIC3 molecule as well as Digallic Acid, which represents a dimeric form of Gallic Acid. The structures of Gallic Acid and Digallic Acid are provided below:
[0057]As seen in FIG. 2A, the small molecule derivative of IIIC3 is capable of providing protection against Dkk suppression when present at 30 mM. In this experiment, the Digallic Acid completely blocked Dkk at even the lowest (1.2 mM) value tested.
example 2
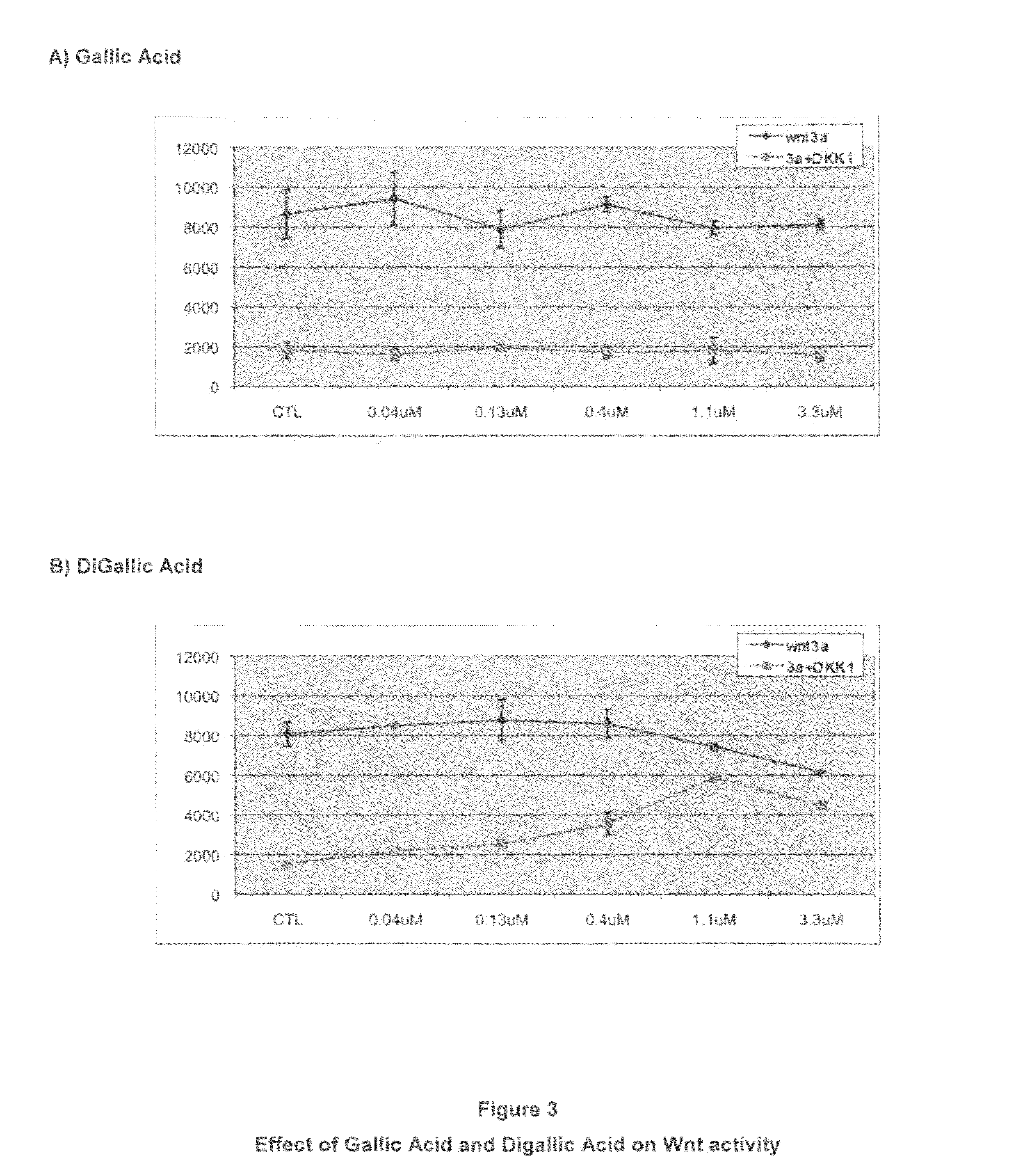
[0058]Effects of Digallic Acid on Wnt and Dkk Suppression of Wnt.
[0059]This experiment was carried out as described above except that a lower range of drug dosage was used as compared to Example 1. In FIG. 3A, there is essentially no effect upon either Wnt activity or suppression of Wnt activity by Dkk when up to 3.3 mM Gallic Acid was present (similar to what was seen with Example 1, FIG. 2A). In contrast, FIG. 3B shows that modest effects upon Wnt activity at the higher (1.1 and 3.3 uM) dosages and a dose dependent effect upon inhibition of Dkk suppression showing that the dimeric form is much more potent than the monomeric form. Both FIGS. 2 and 3 indicate that nearly 30 times as much Gallic Acid had to be present to achieve the same effect as the dimeric Gallic Acid.
example 3
[0060]Stimulatory Effects of Enzo IIC8 on Alveolar New Bone Formation in a Tooth Extraction Model.
[0061]A root extraction model (Lin et al., 1994 Anat Record 240; 492-506) was used to determine whether IIC8 (described in U.S. Patent Application No. 20050196349) is able to stimulate new bone formation. The bone regeneration process following tooth extraction is a complex phenomenon that involves wound healing, as well as bone formation. Briefly, the initial coagulum is followed by the formation of woven bone, lamellar bone, bone marrow, and cortical bone. At the cellular level this process involves induction and regulation of growth of several distinct cell types, as well as differentiation of stem cells into several cell types. The point of the experiment described below was to determine whether a drug could accelerate the process of bone growth without adversely affecting the end product of the process.
[0062]Procedure: 10 week old Sprague Dawley rats (˜300 gram body weight obtained...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| TNF-α levels | aaaaa | aaaaa |
| core structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com