Bi-functional monoclonal antibody capable of anti-human von willbrand factor-A3
A monoclonal antibody, hemophilia factor technology, application in medicine, bifunctional monoclonal antibody and its preparation field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
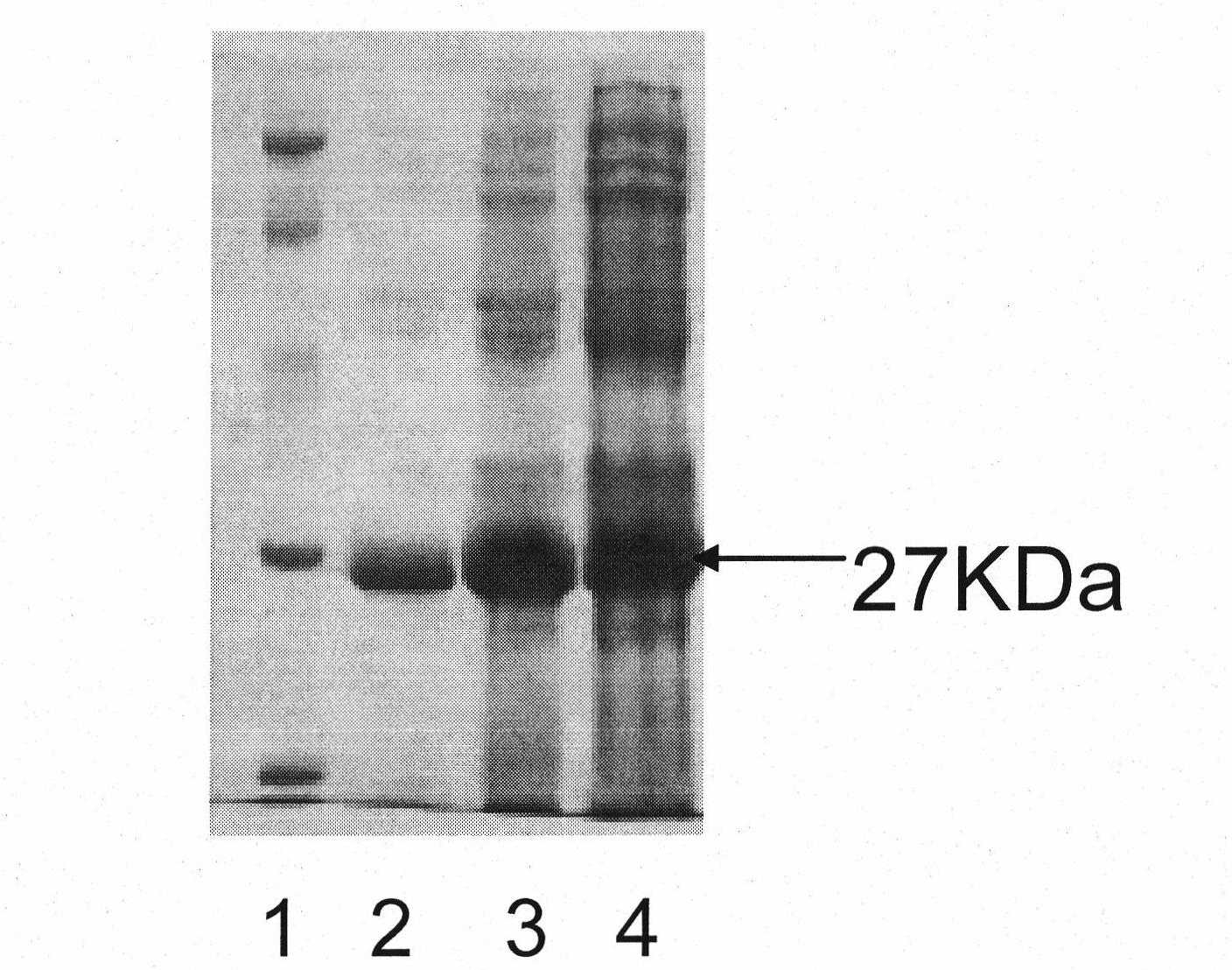
[0040] Example 1: Immunogen - Preparation of Recombinant Human Von Willebrand Factor A3 Region (rVWF-A3)
[0041] (1) Design and synthesis of PCR primers: design and synthesize a pair of primers respectively located on the upstream and downstream sides of the cDNA sequence (594bp) encoding the VWF-A3 region (amino acid 1681-1877),
[0042] For the upstream primer, see the base sequence described in SEQ ID NO.1: 5'-AT AAG CTT TCC CCTGCA CCT GAC TGC-3', with a Hind III restriction site at the 5' end;
[0043] Downstream primer, see the base sequence described in SEQ ID NO.2: 5'-TG CTC GAG CCT AACAAA TCC AGA GCA-3', 5' end contains Xho I restriction site; The cDNA was used as a template for PCR amplification to obtain PCR products.
[0044] (2) Cloning and sequence analysis of the target gene: under the action of T4 DNA ligase, the purified PCR product was directly connected to the pUCm-T vector to obtain the pUCm-T-VWF-A3 recombinant plasmid. The resulting recombinant plasmid ...
Embodiment 2
[0048] Example 2: Preparation of Monoclonal Antibody Specific Anti-Von Willebrand Factor A3 Region
[0049] We applied conventional immunological methods and hybridoma technology ( and Milstein, Nature, 215:495-497, 1975) to prepare the monoclonal antibodies of the present invention.
[0050] First, 8-week-old female Balb / c mice (Experimental Animal Center, Shanghai Academy of Sciences) were immunized with purified rVWF-A3 region protein for three times with four weeks between each time. The first two times were multi-point subcutaneous injection on the back plus intraperitoneal injection, and the third time was injection through the mouse tail vein plus intraperitoneal injection.
[0051] After the serum antibody titer of the immunized mice reached a high enough level, the animals were sacrificed and spleen cells were isolated. Using the standard monoclonal antibody cell fusion technology, the spleen cells of the immunized Balb / c mice were fused with the mouse SP2 / 0 myelo...
Embodiment 3
[0054] Example 3: Chemical properties of monoclonal antibodies of the present invention
[0055] (1) It was confirmed by double-diffusion immunoassay that the monoclonal antibodies SZ-123 and SZ-125 of the present invention both belong to IgG1 subclass.
[0056] (2) Inoculate monoclonal antibodies SZ-123 and SZ-125 in the peritoneal cavity of Balb / c mice to produce ascites, and use G-Sepharose-4B (Pharmacia company product) column to purify, and it is determined that every 1 mL of ascites can be purified to obtain IgG 3- 6mg.
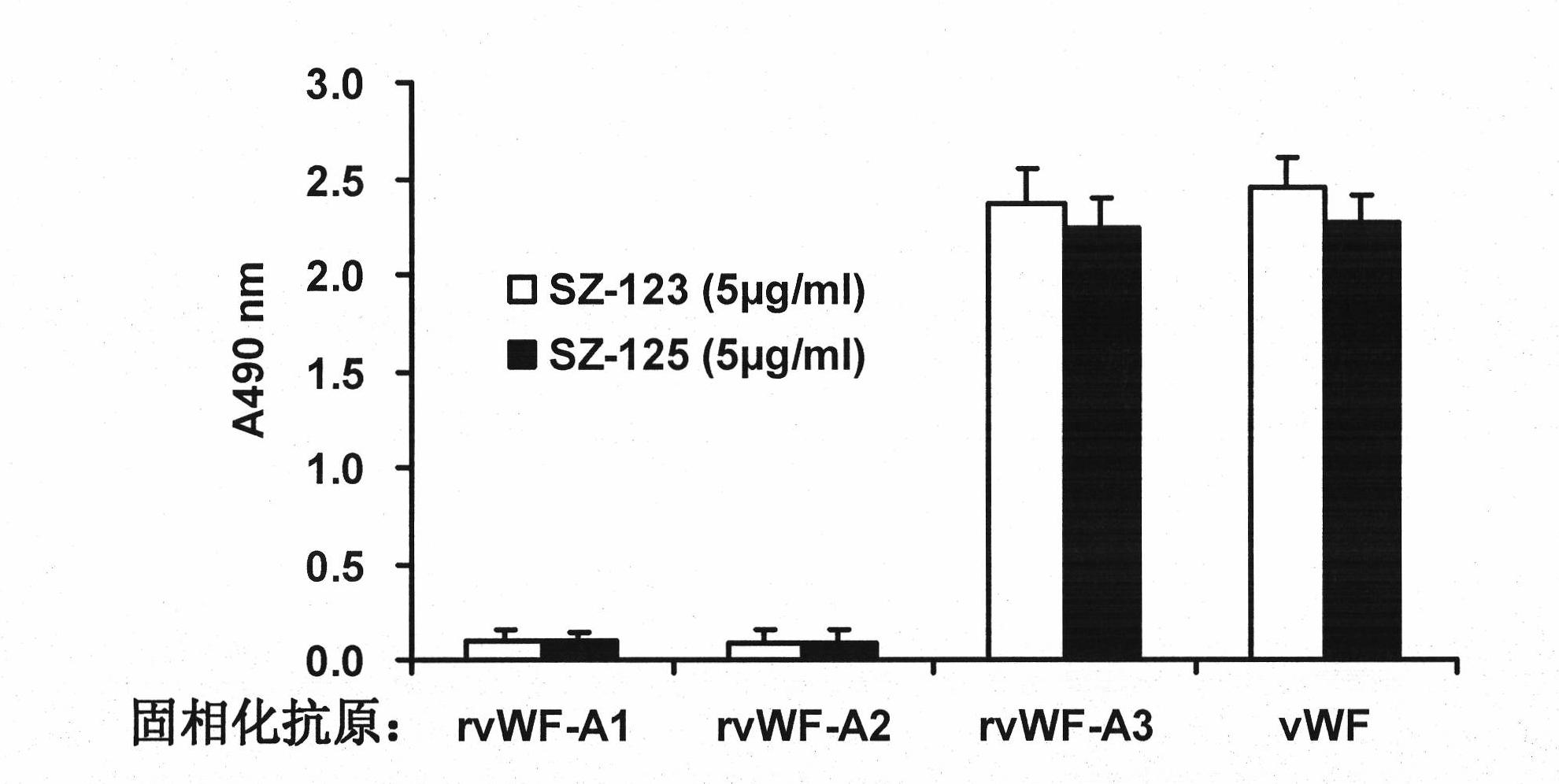
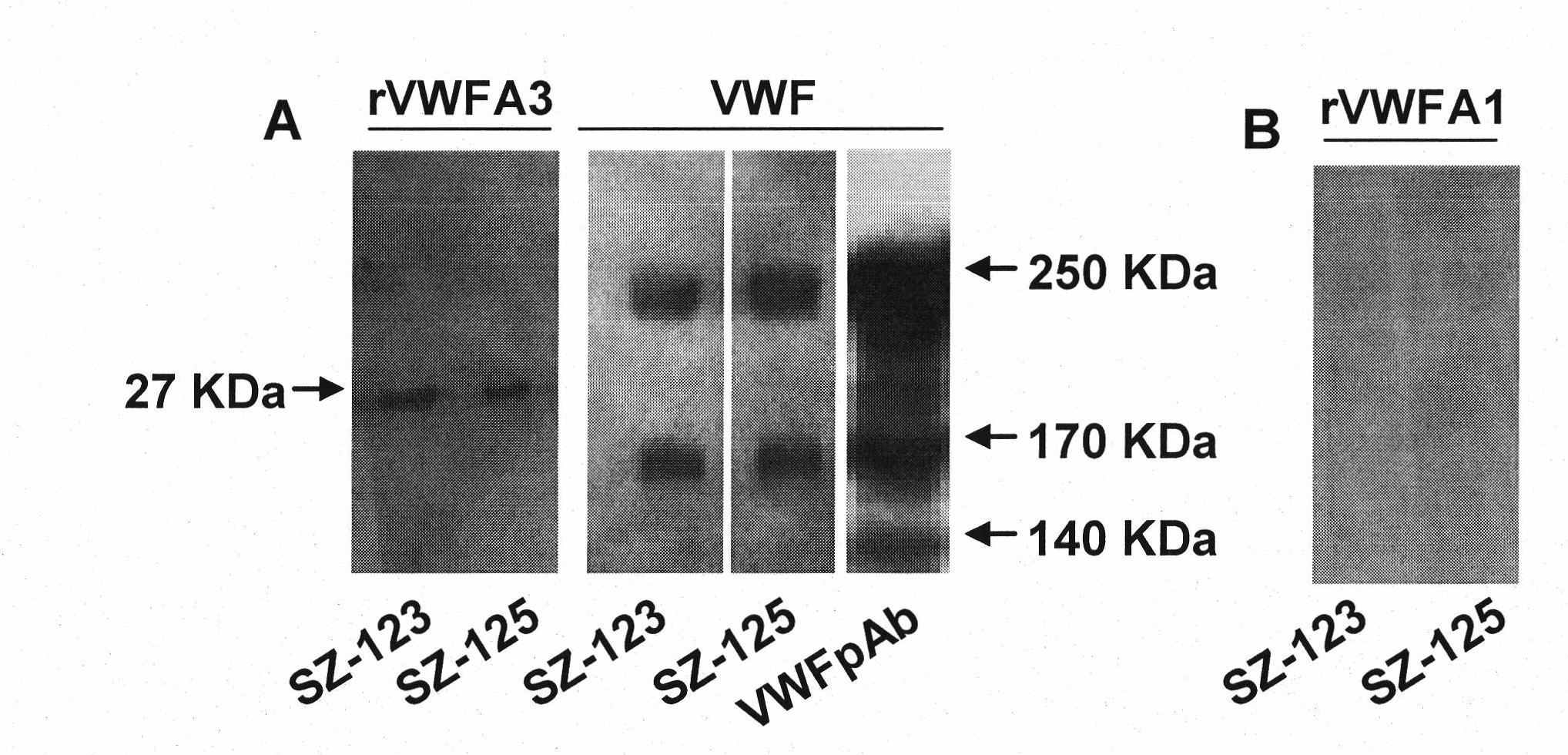
[0057] (3) ELISA assay to determine the specificity of SZ-123 and SZ-125: the recombinant VWF A1, A2, A3 (all expressed and purified in our laboratory) and pure human VWF (provided from Anhui Green Cross Biological Products Co., Ltd. FVIII concentrates, purified by Sepharose 4B gel chromatography) (2 μg / ml) were coated on 96-well plates. After overnight at 4°C and washed with PBS-0.05%-Tween 20, they were blocked with 2% BSA-PBS overnight. Purified m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| shear load | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com