Development and application of therapeutic agent for TSLP-related diseases
A sequence, CDR2 technology, used in applications, fermentation, anti-inflammatory agents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0179] The preparation of embodiment 1, TSLP and TSLPR recombinant protein
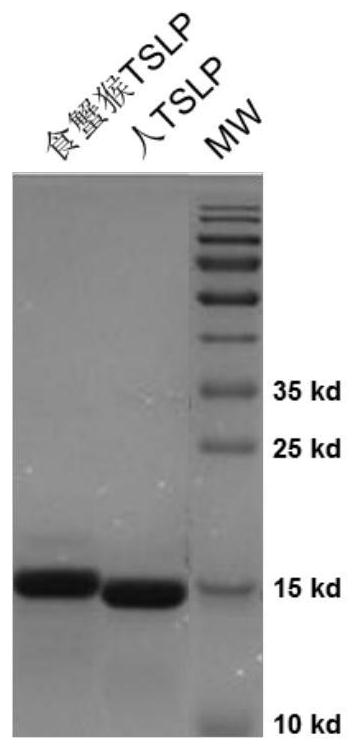
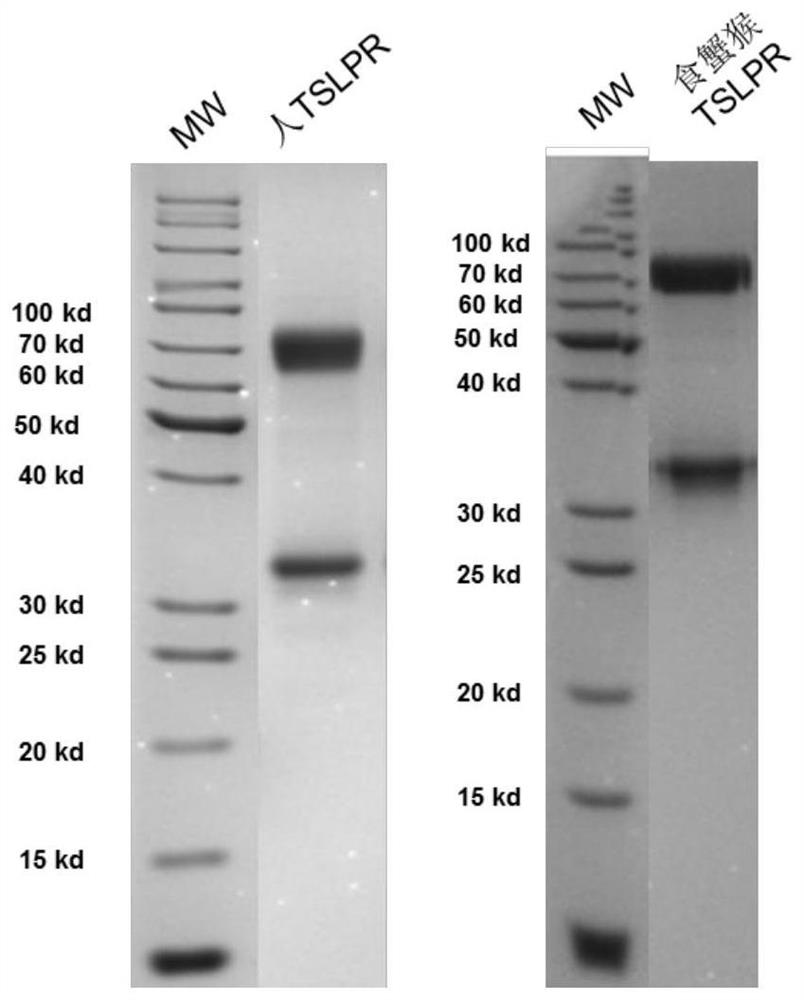
[0180]The cDNAs of human TSLP (Uniprot: Q969D9, SEQ ID NO: 1) and cynomolgus monkey TSLP (Uniprot: A0A2K5TXV0, SEQ ID NO: 2) were synthesized, and the sequences were cloned into the eukaryotic expression vector pCMV3 (purchased from Beijing Sinobiological, Cat. No. CG90911 -UT), insert the signal peptide MDMRVPAQLLGLLLLWLRGARS at the N-terminus, and express a tag containing 6 histidines at the carboxy-terminal of TSLP to obtain pSect-hTSLP-cHis and pSect-cyTSLP-cHis plasmids, and transfect HEK293 with these plasmids. 6E cells (ATCC CRL-1573), the cell supernatant was collected, but the recombinant protein of corresponding length was not obtained by nickel column affinity chromatography. Then the full-length fragment of TSLP was cloned into the prokaryotic expression plasmid pET-30a, and the plasmid was transformed into BL21 E.coli. After induction, the recombinant protein was found to be expressed in ...
Embodiment 2
[0183] Embodiment 2, TSLPR stably transfected cell construction
[0184] 1) Construction of Ba / F3-hTSLPR stably transfected cell line
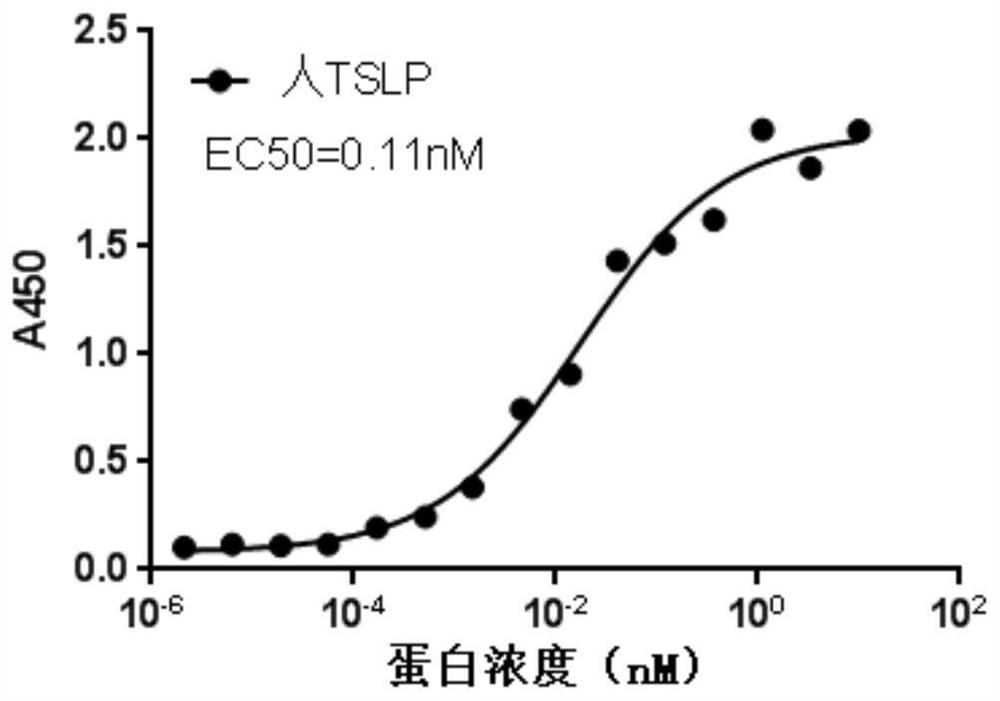
[0185] Ba / F3 cells were maintained in RPMI-1640 medium containing 10% fetal bovine serum, 50 μM 2-mercaptoethanol, 2 mM L-glutamine, 50 μg / mL penicillin-streptomycin and 10 ng / mL mouse IL-3 . The lentiviral expression vector expressing human TSLPR (Guangzhou Funeng Gene Co., Ltd., EX-W0156-Lv105-B) was packaged with the Lenti-Pac HIV lentiviral packaging kit (Guangzhou Funeng Gene Co., Ltd.) and transfected Ba / F3 cells were added with puromycin for resistance selection to obtain a cell line Ba / F3-hTSLPR capable of stably expressing human TSLPR. Figure 4 It was shown that by detecting the binding of biotinylated human TSLP protein to TSLPR on the cell surface, it was confirmed that the constructed cells expressed human TSLPR, and its EC 50 = 0.22 nM.
[0186] 2) Construction of Ba / F3-hTSLPR-IL7Rα stably transfected cell line
[0187] IL-7...
Embodiment 3
[0191] Embodiment 3, animal immunization
[0192] Select 6-week-old female Balb / C mice for immunization. Each mouse is immunized with 50 μg of antigen each time. The antigen is alternately immunized with human and cynomolgus TSLP. The antigen is mixed with Freund's adjuvant in equal volumes and subcutaneously immunized every two weeks. Immune once. After four times of immunization, blood was taken from the tail, and the titer of mouse serum and the inhibitory effect of serum on the combination of TSLP and TSLPR were detected by ELISA. Or after the initial immunization, use a gene gun to boost immunization every other week, mix the human TSLP expression plasmid with gold powder, and bombard the naked skin of the abdomen of the mouse with a pressure of 400 psi, and immunize nine times in total.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com