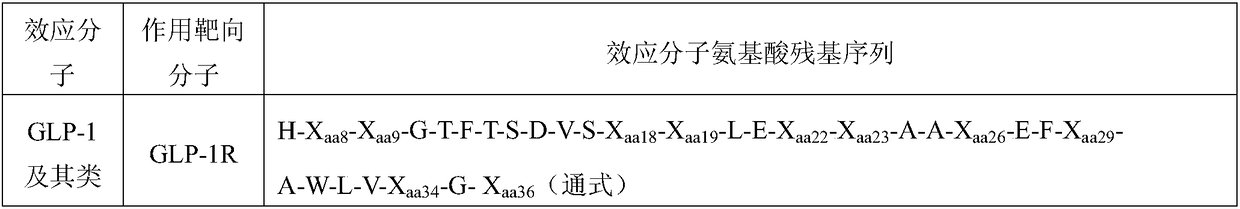
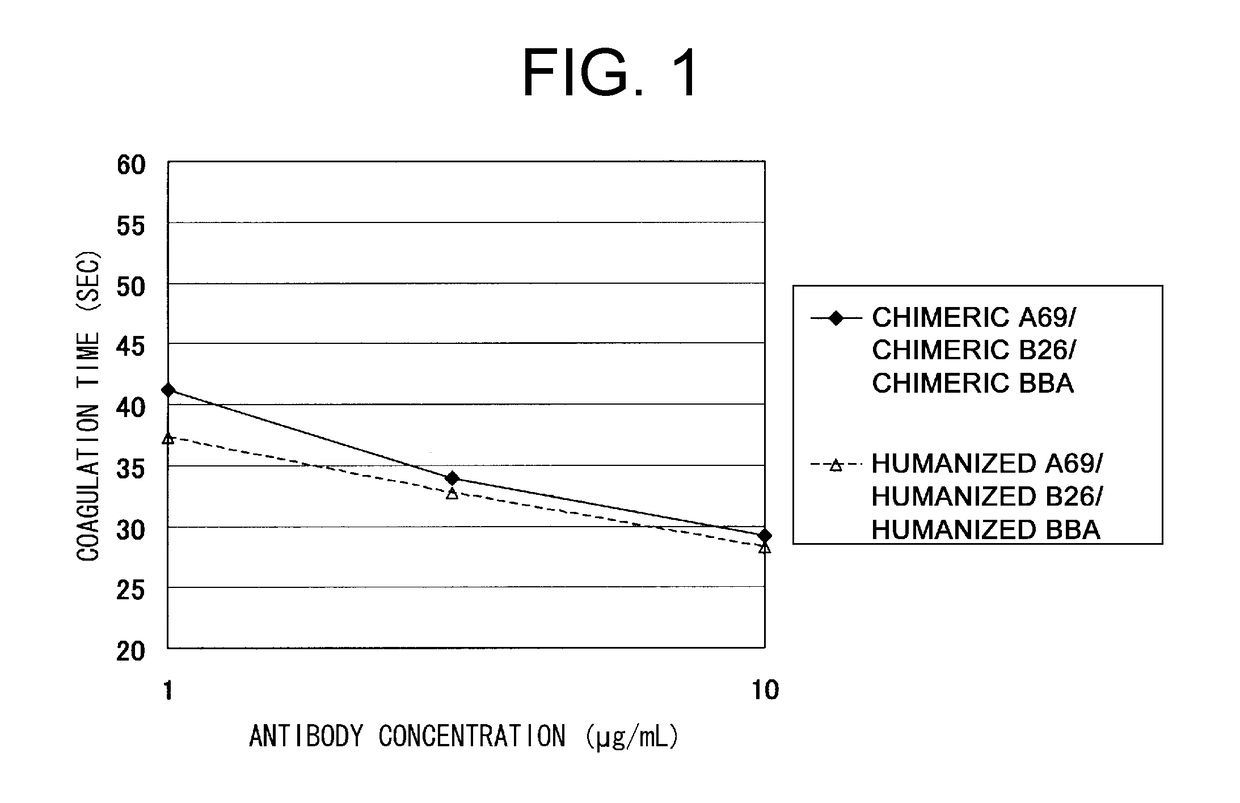
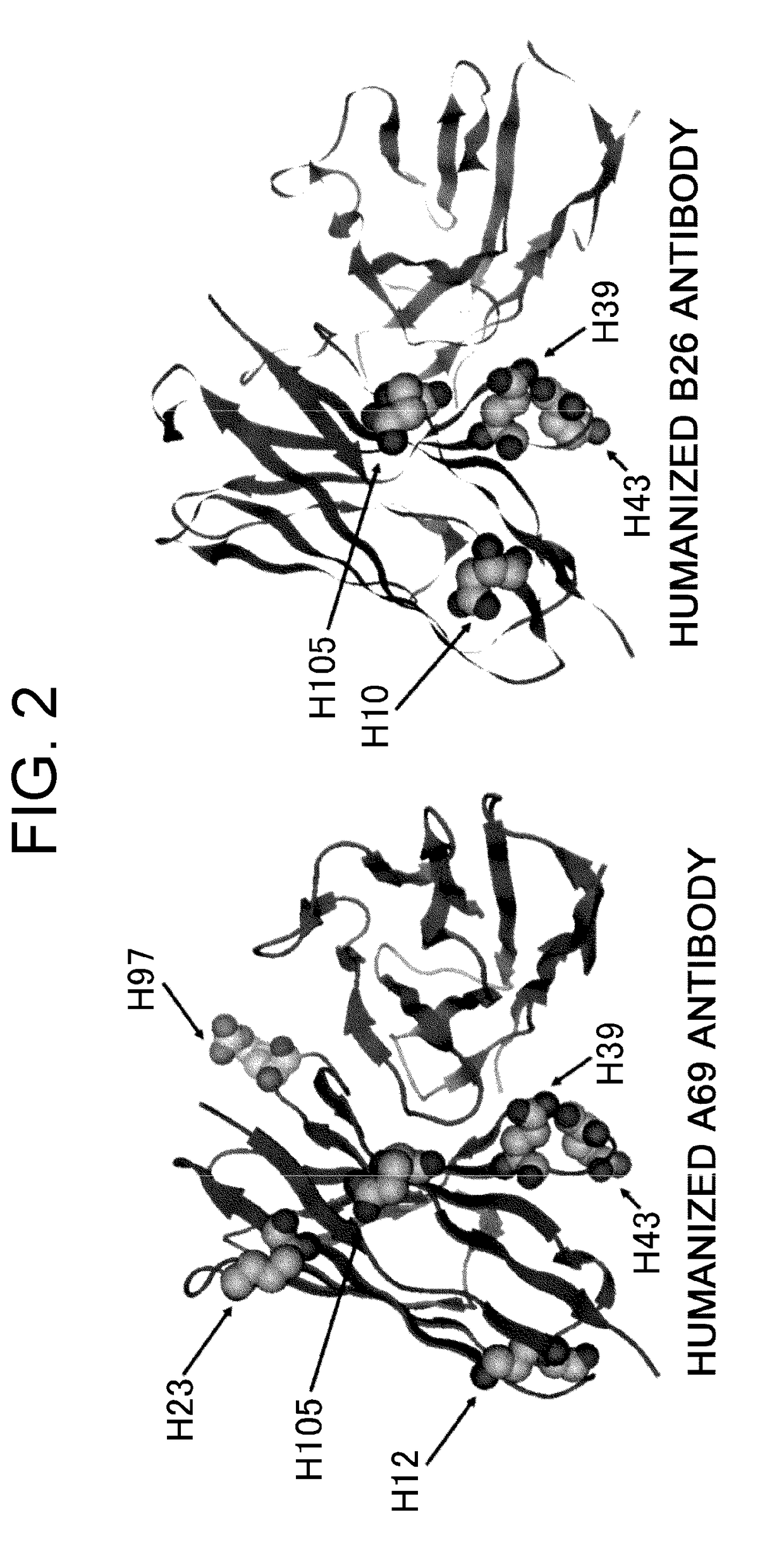
Patents
Literature
59 results about "Antibody constant region" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
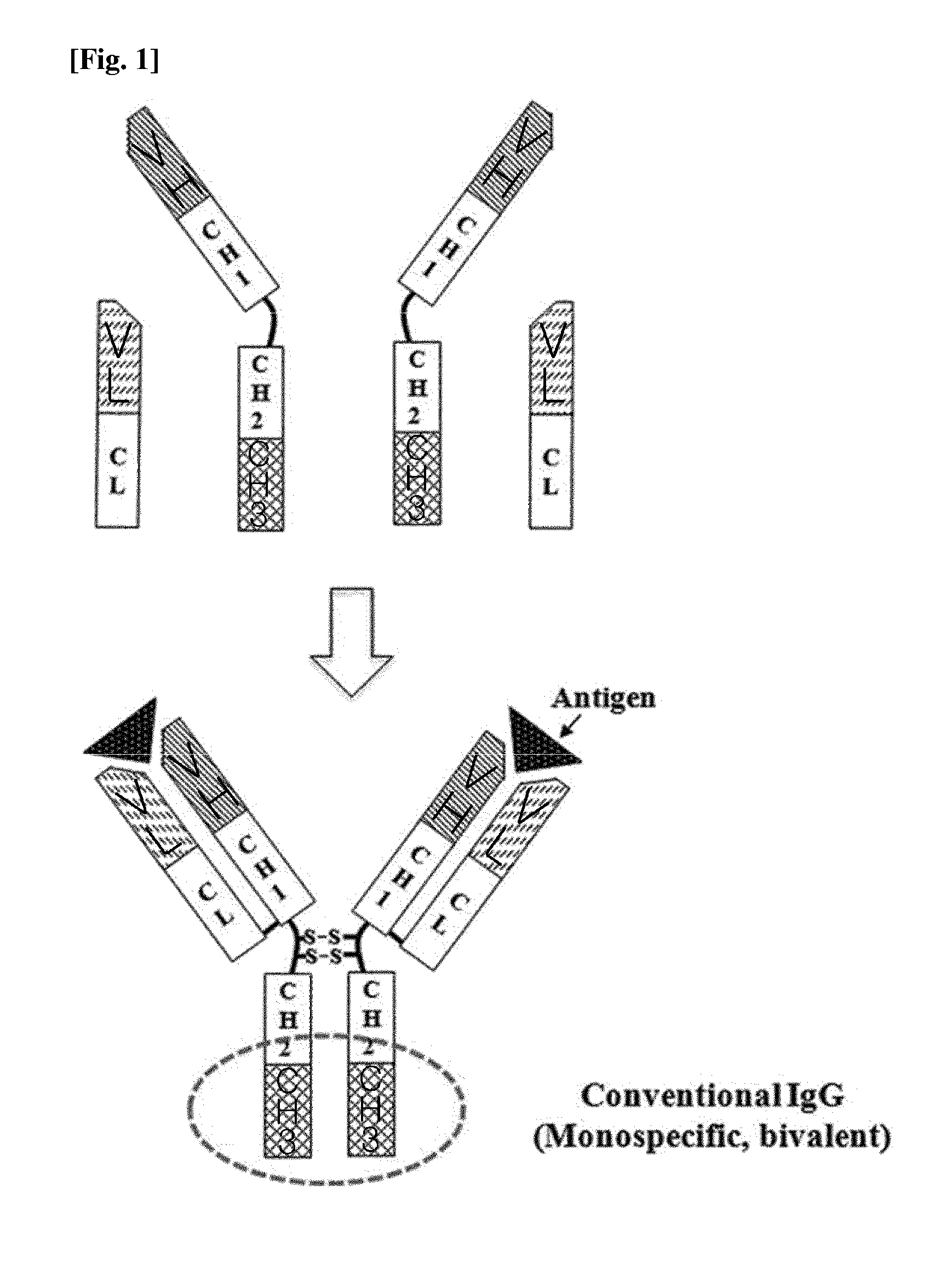
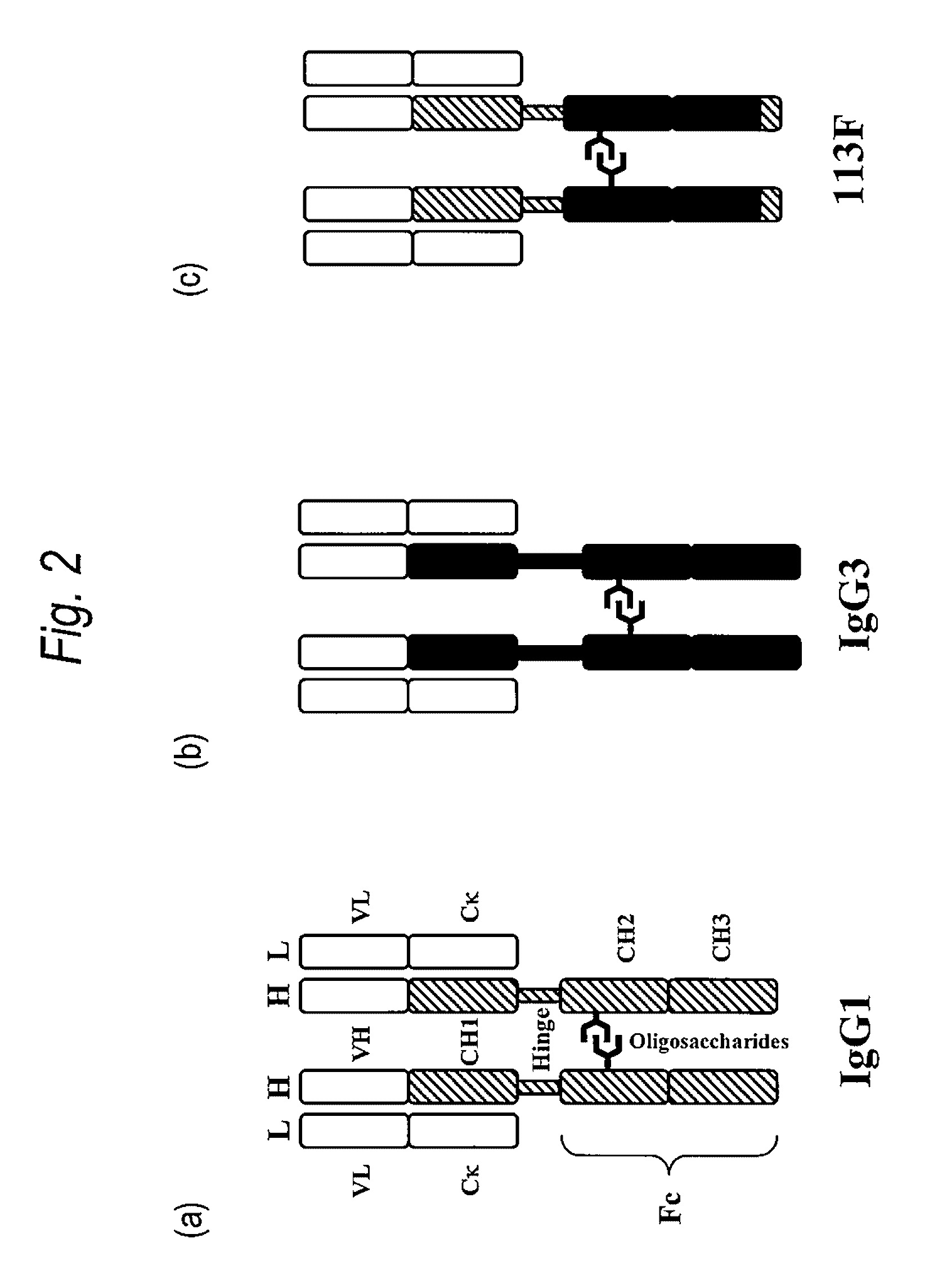
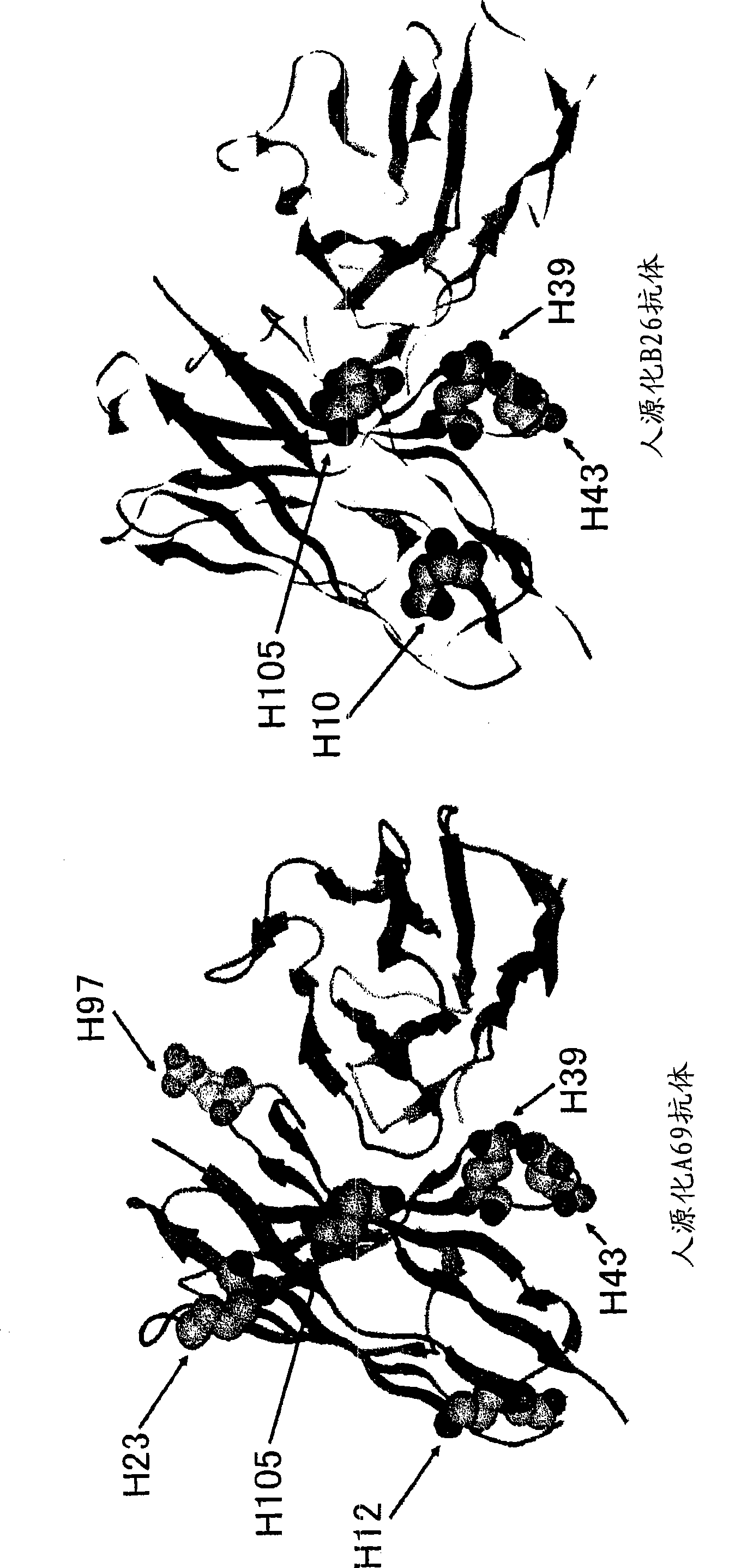
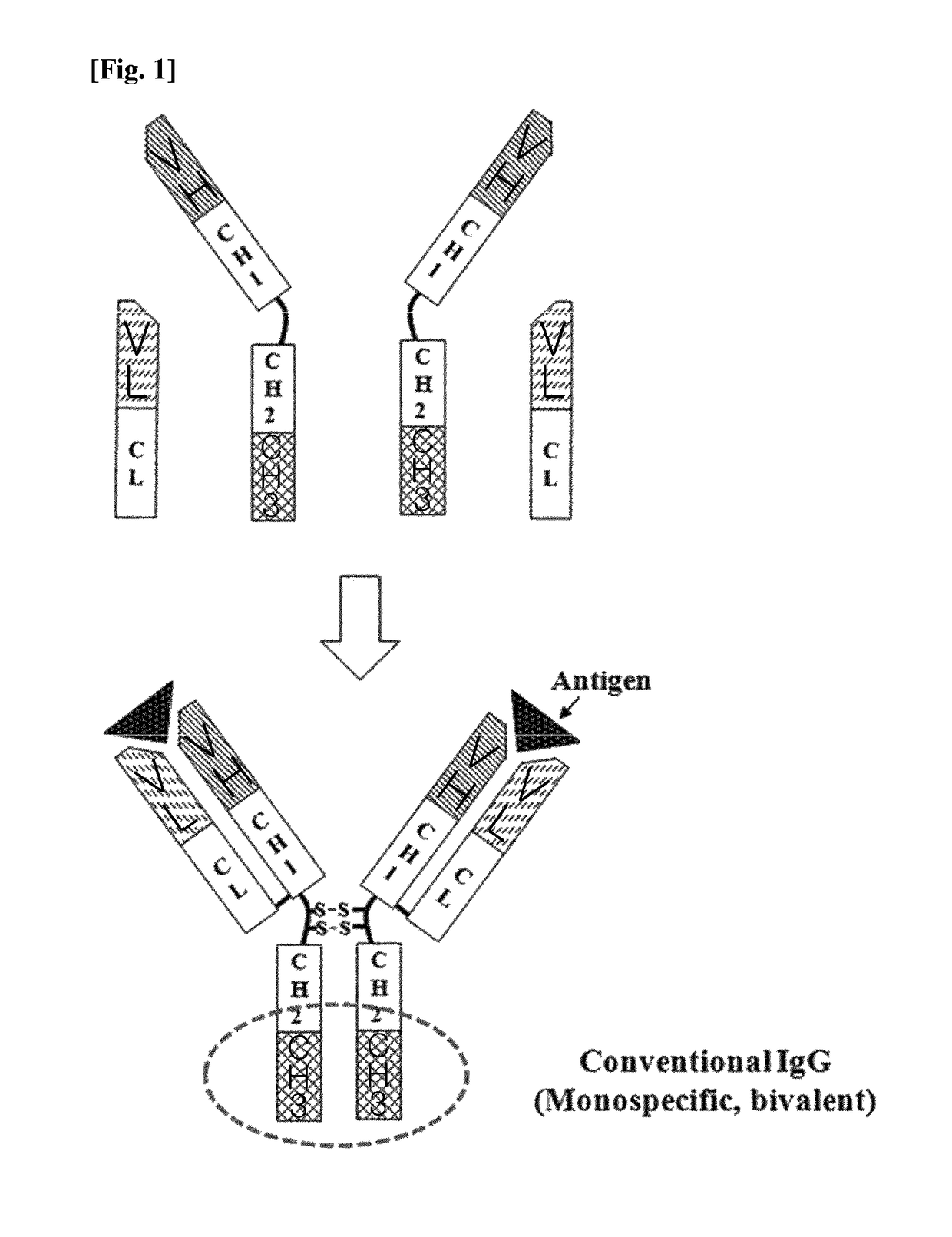
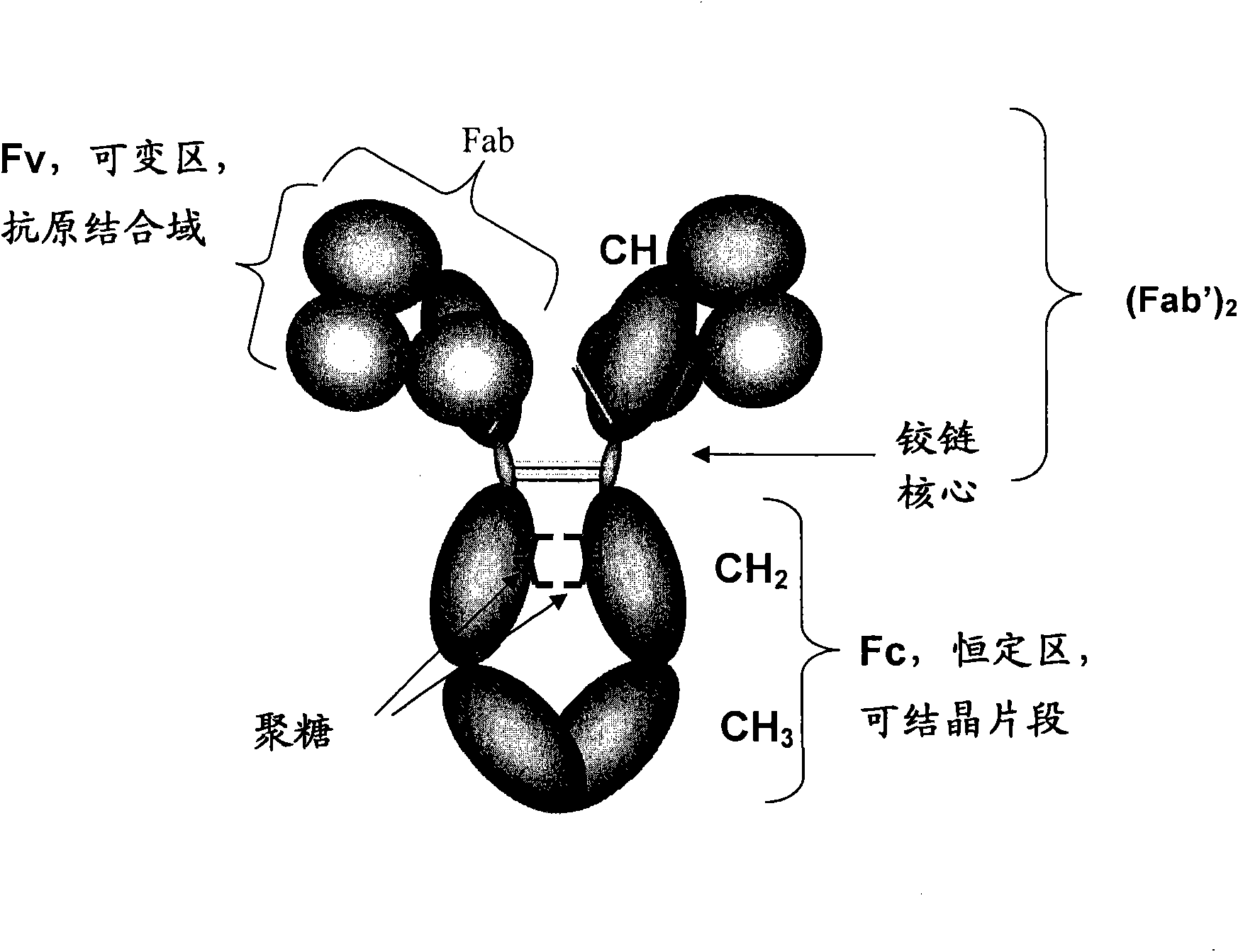
The constant region determines the mechanism used to destroy antigen. Antibodies are divided into five major classes, IgM, IgG, Iga, IgD, and IgE, based on their constant region structure and immune function. The variable region is further subdivided into hypervariable (HV) and framework (FR) regions.
Methods of modifying antibodies for purification of bispecific antibodies
ActiveUS20090263392A1Efficient purificationFunction increaseSugar derivativesAntibody ingredientsAntiendomysial antibodiesBinding site
The present inventors devised methods for efficiently purifying bispecific antibodies using a chromatography column based on the difference in isoelectric points between the H chains of two types of antibodies, wherein the difference is introduced by modifying the amino acids present on the surface of the antibody variable regions of two types of antibodies that constitute a bispecific antibody. Furthermore, the inventors devised methods for efficiently purifying bispecific antibodies using a chromatography column by linking respective antigen binding sites (heavy chain variable regions) to the antibody constant regions having different isoelectric points, and then coexpressing these antibodies.
Owner:CHUGAI PHARMA CO LTD
Modified Antibody Constant Region
ActiveUS20100298542A1Improving immunogenicityImprove propertiesAntipyreticAnalgesicsHigh concentrationHinge region
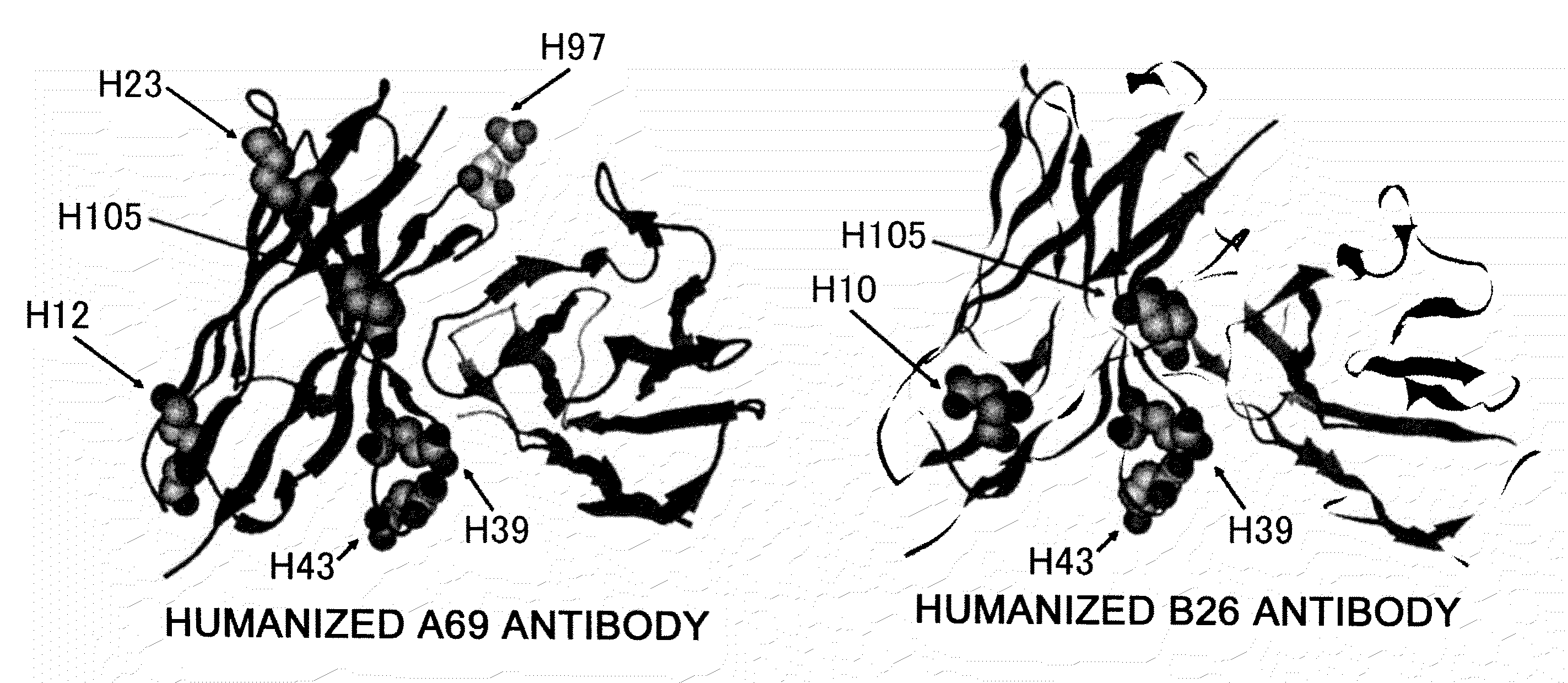
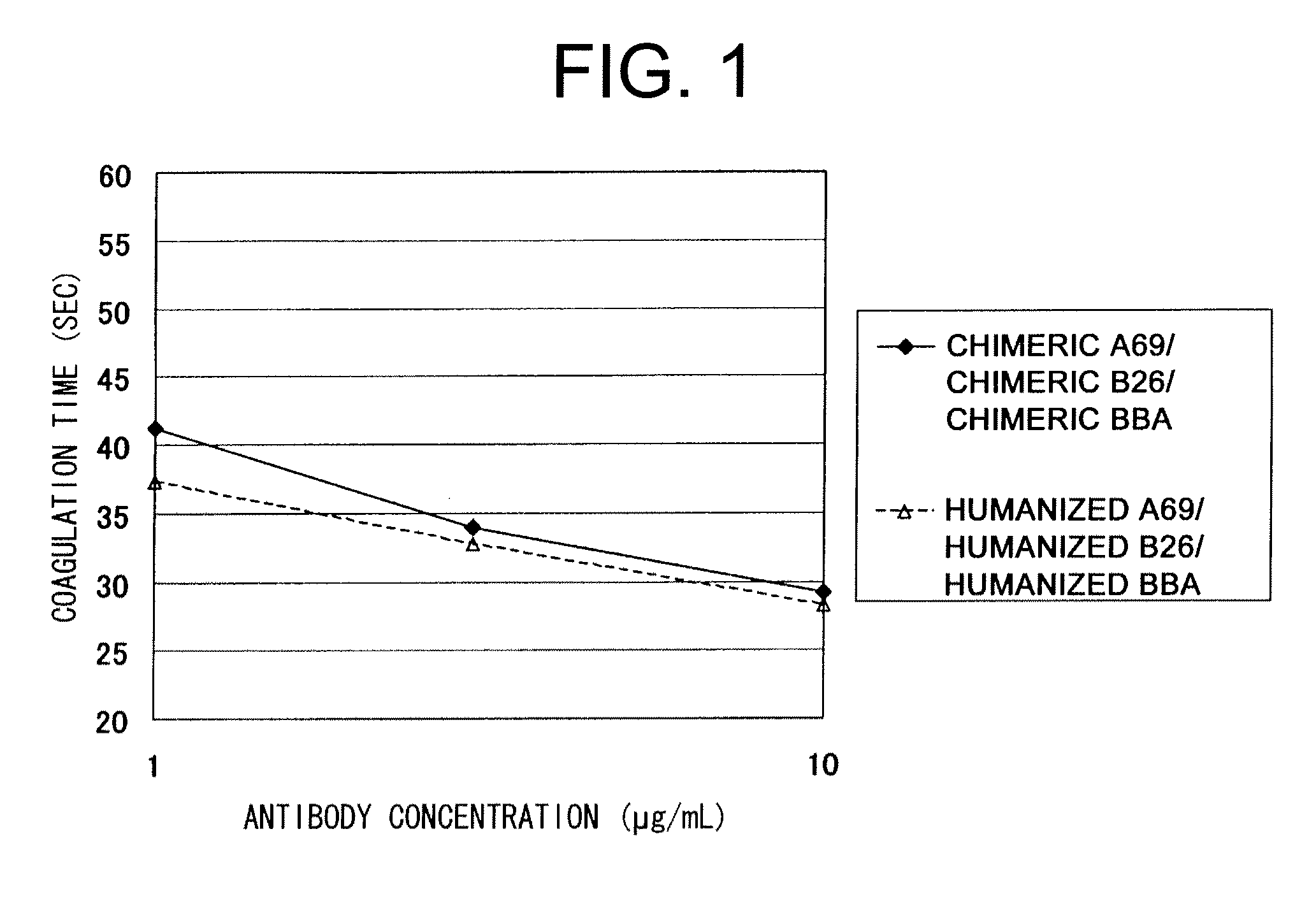
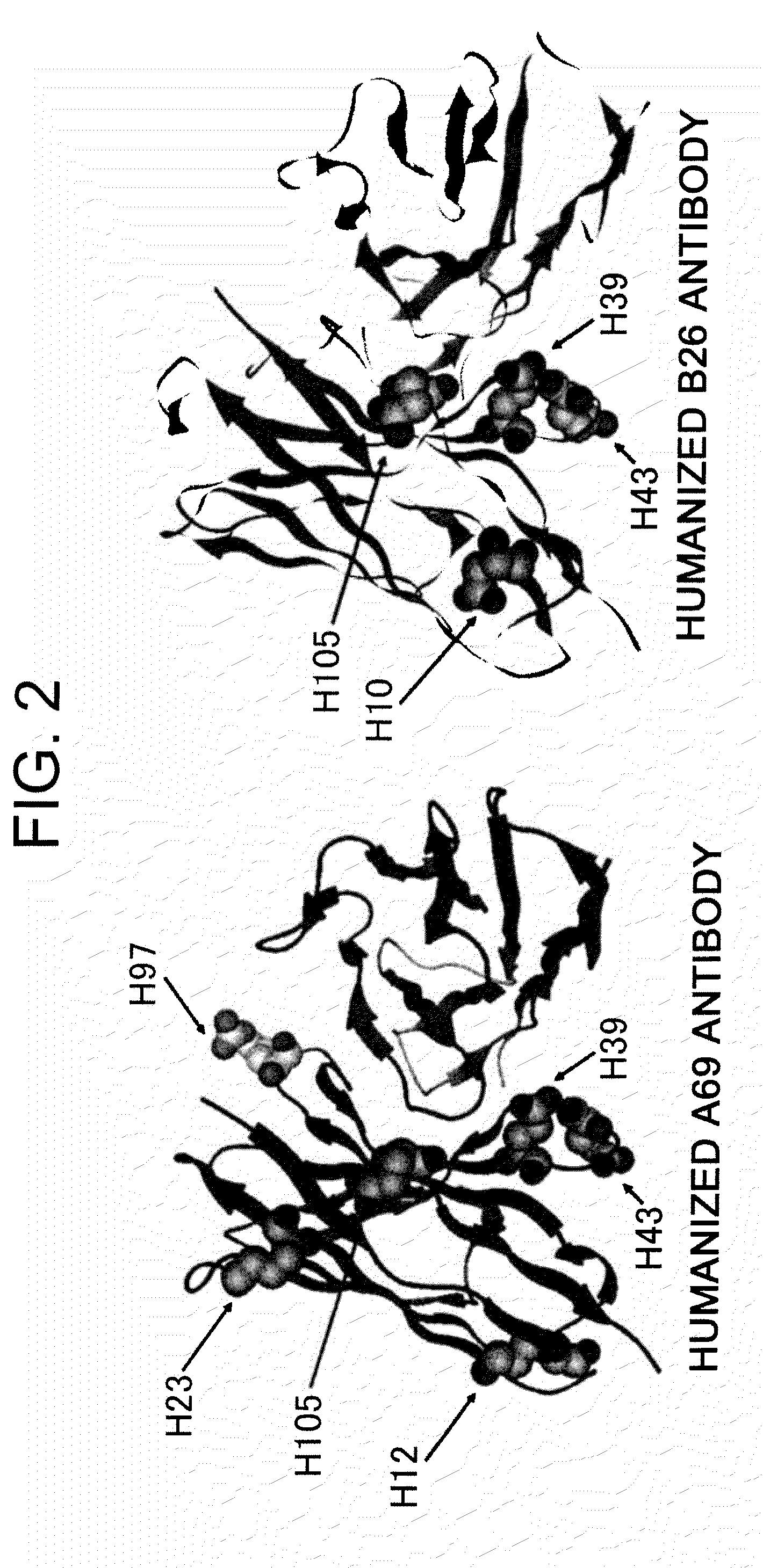
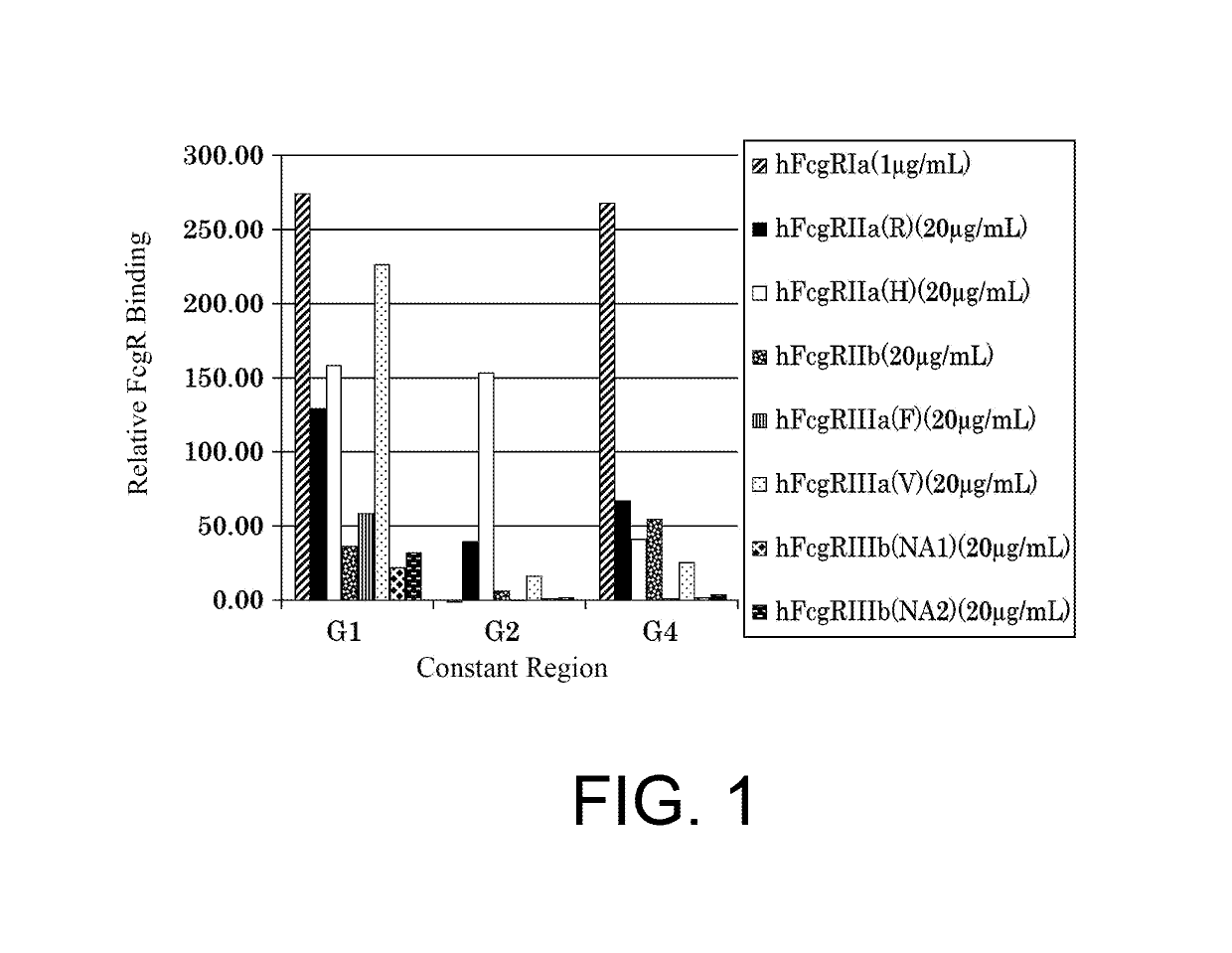
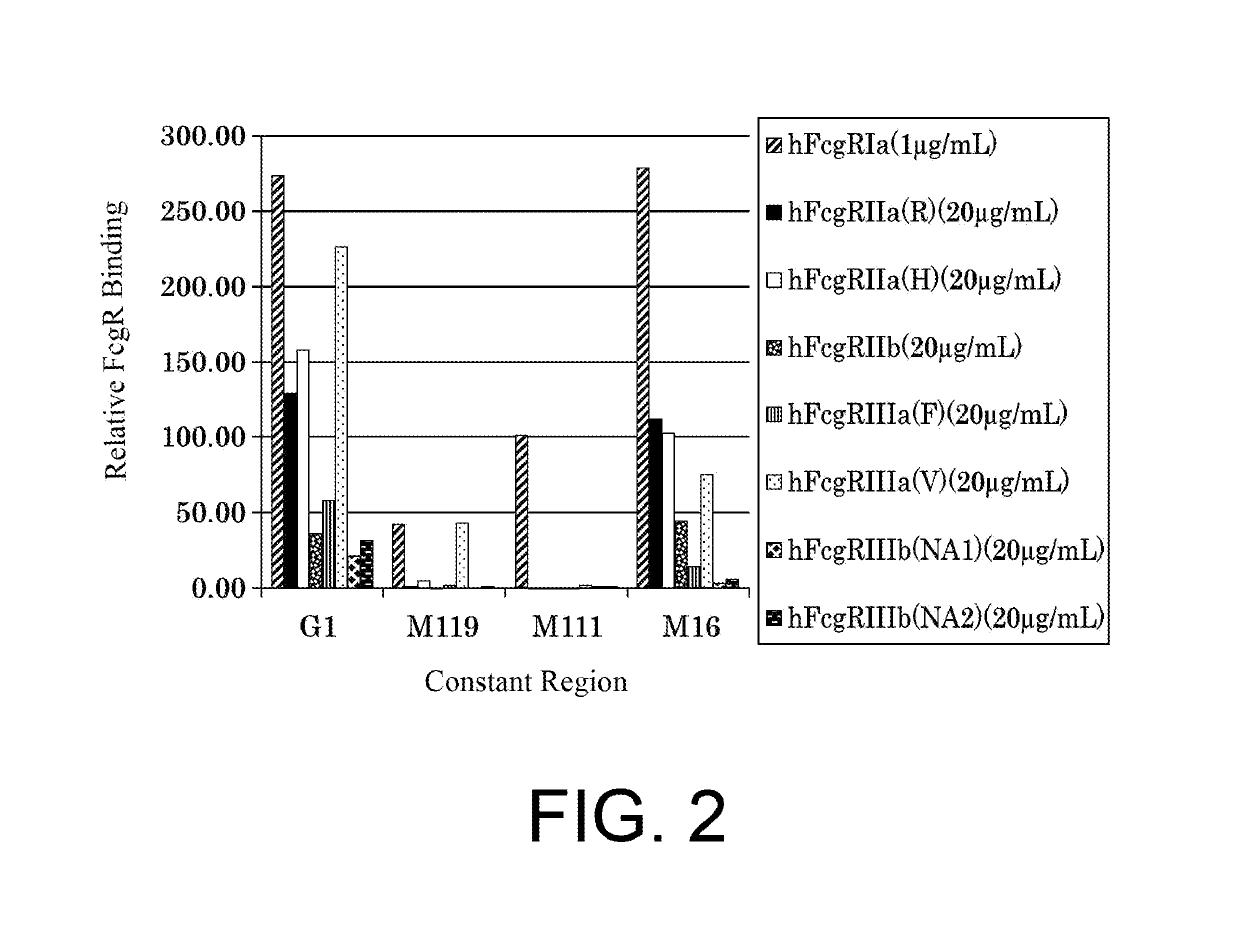
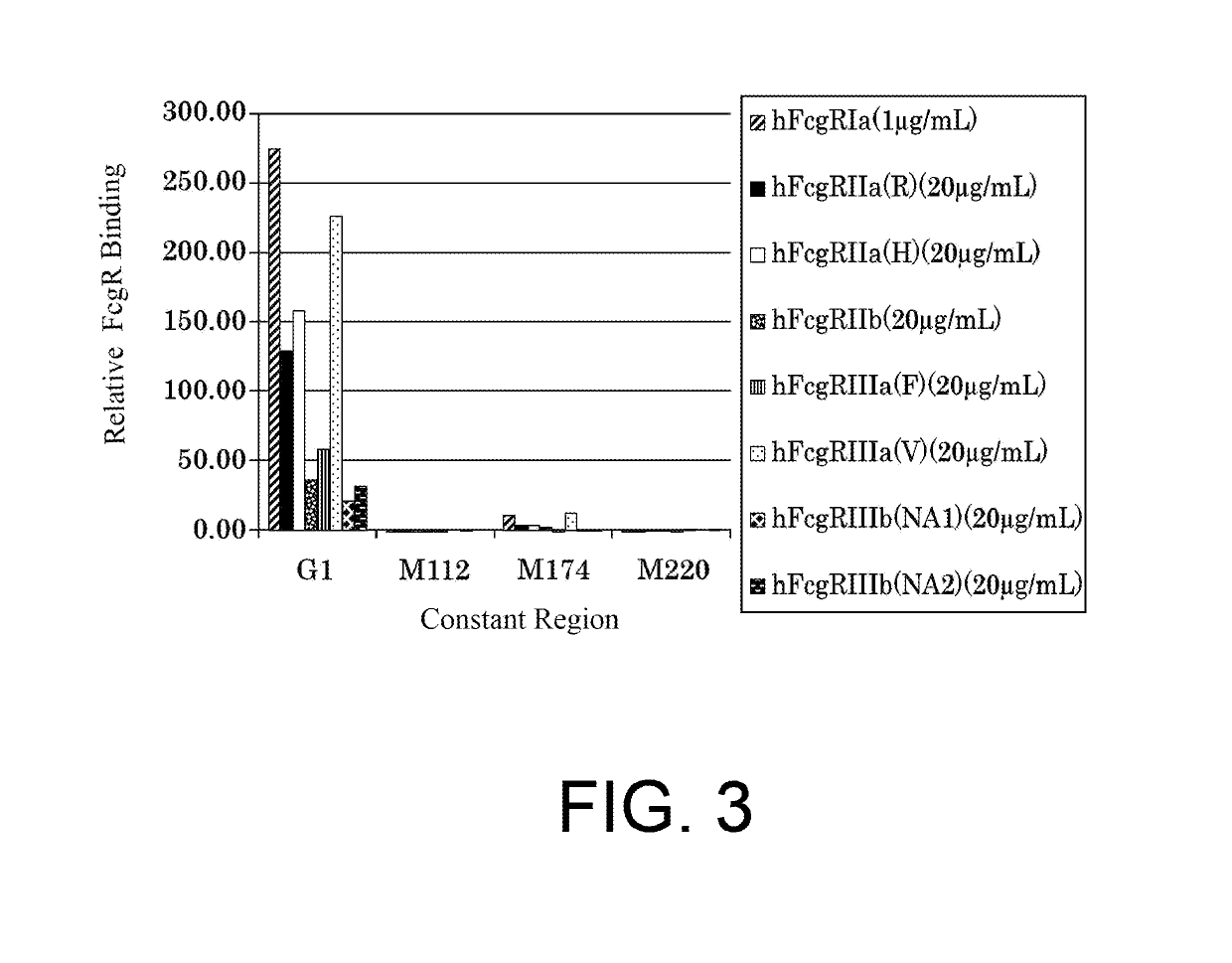
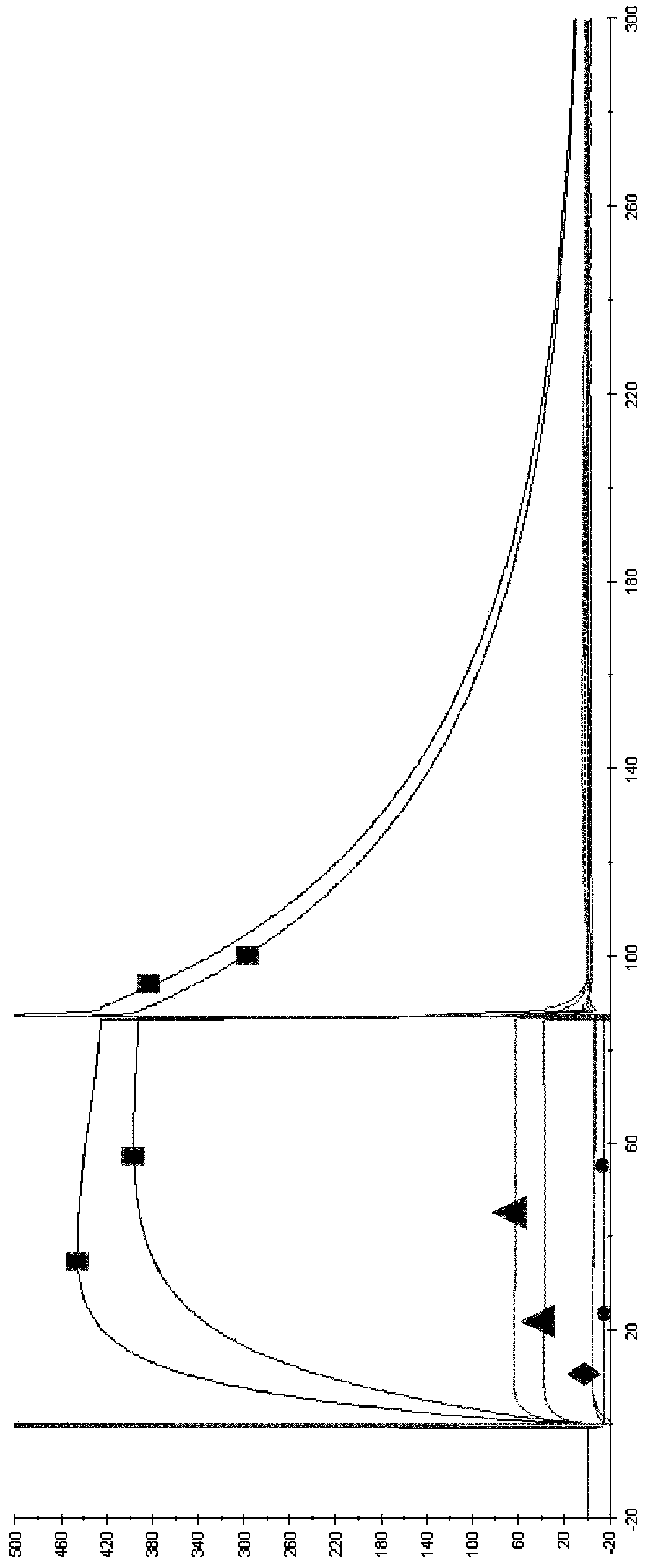
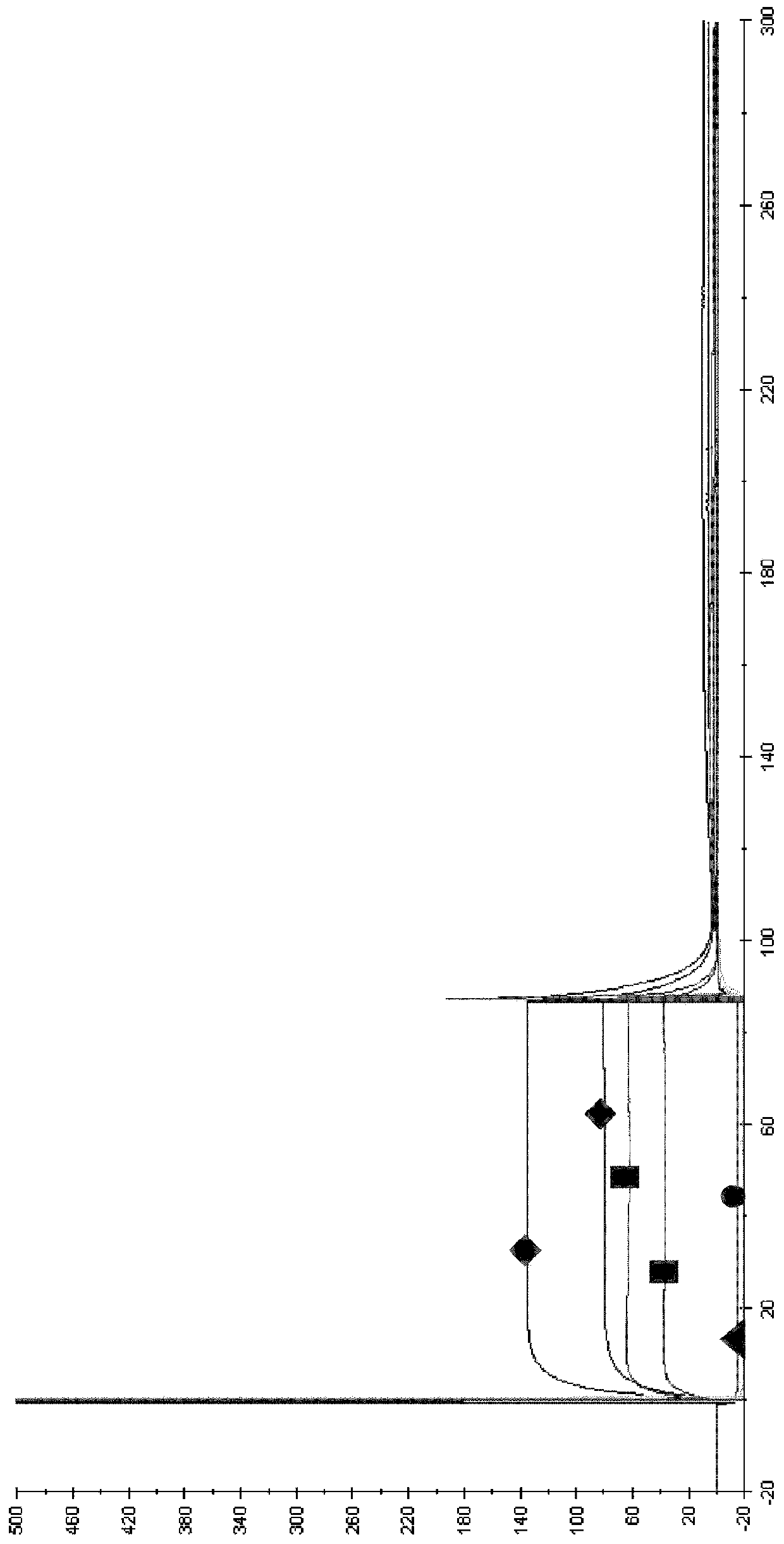
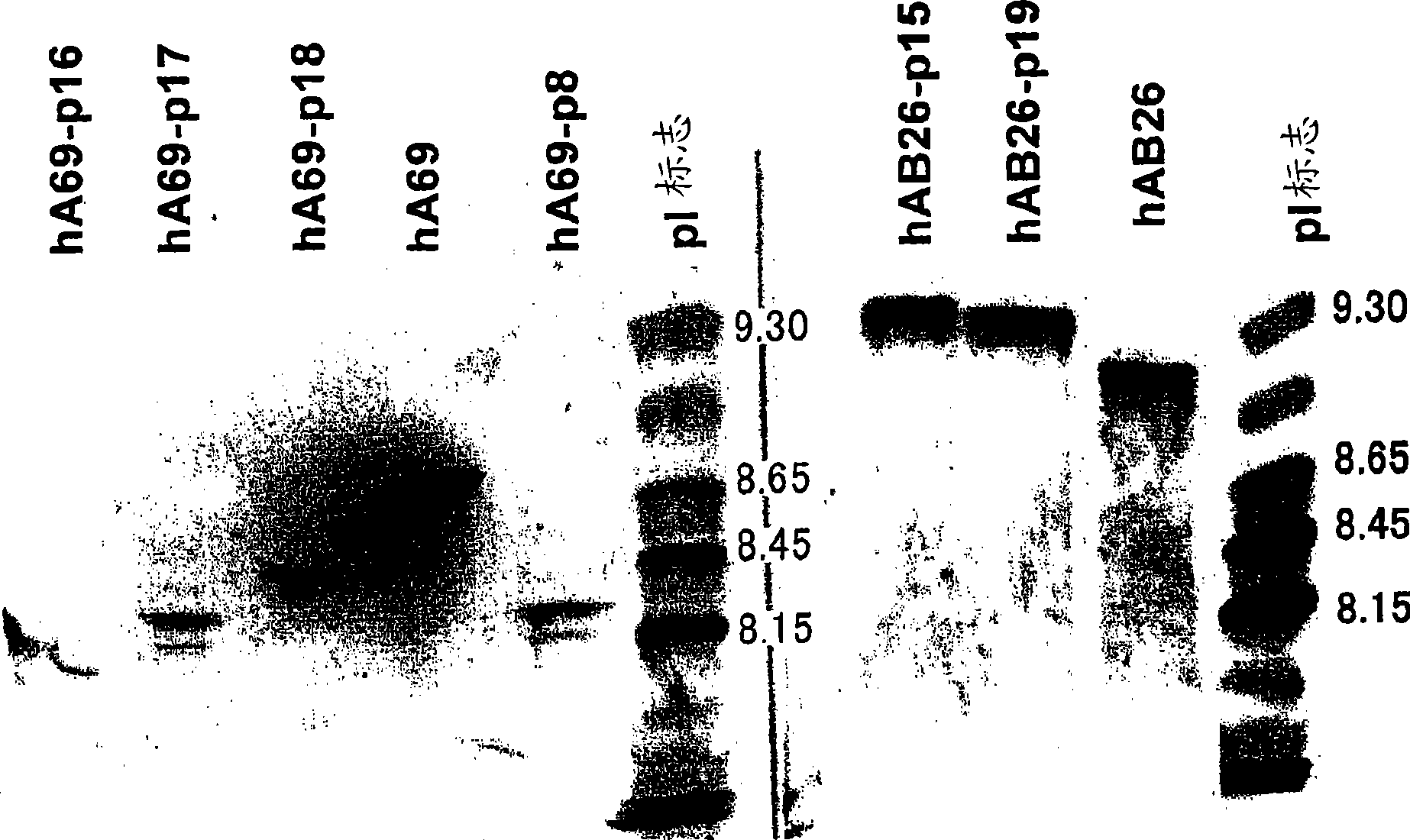
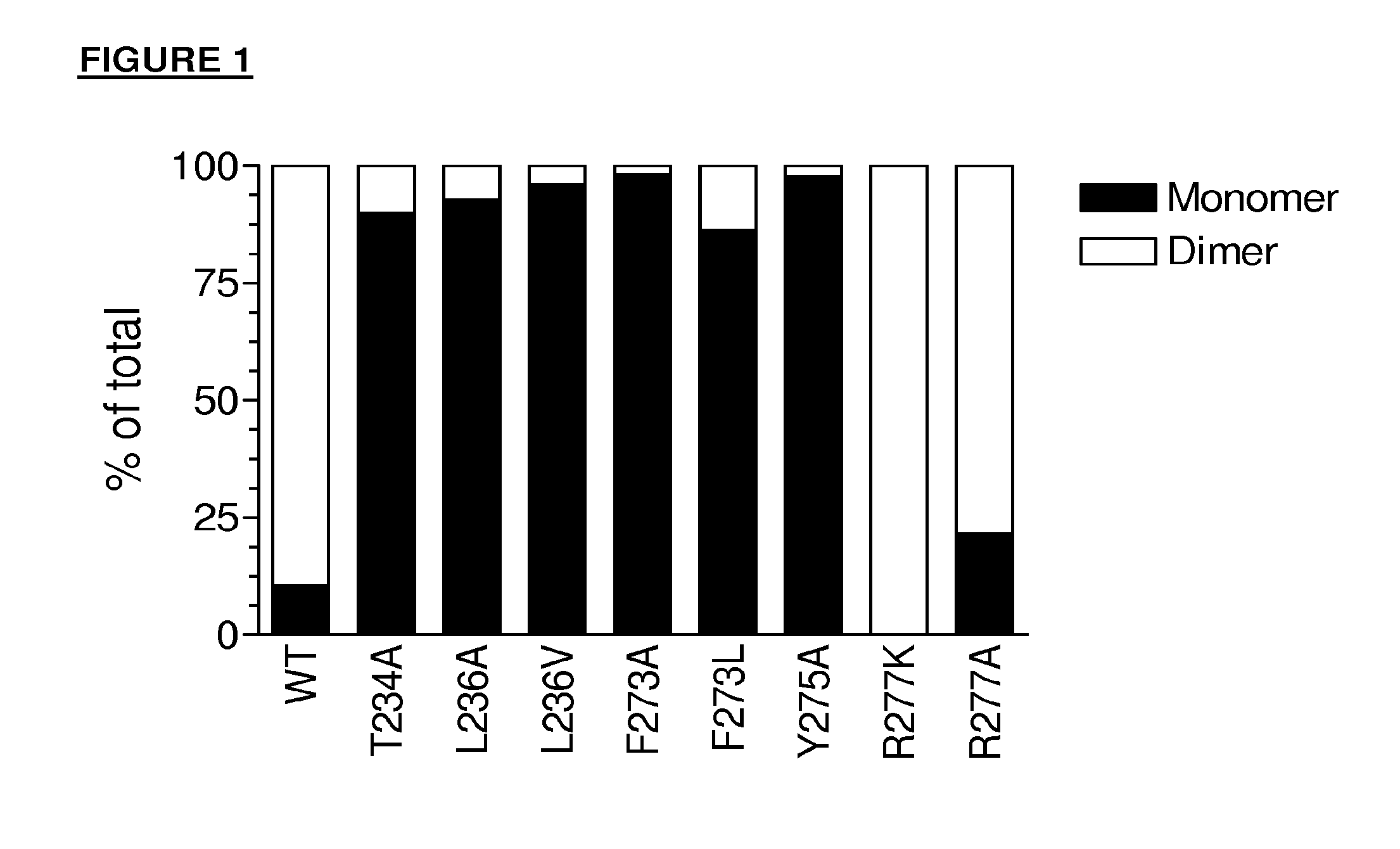
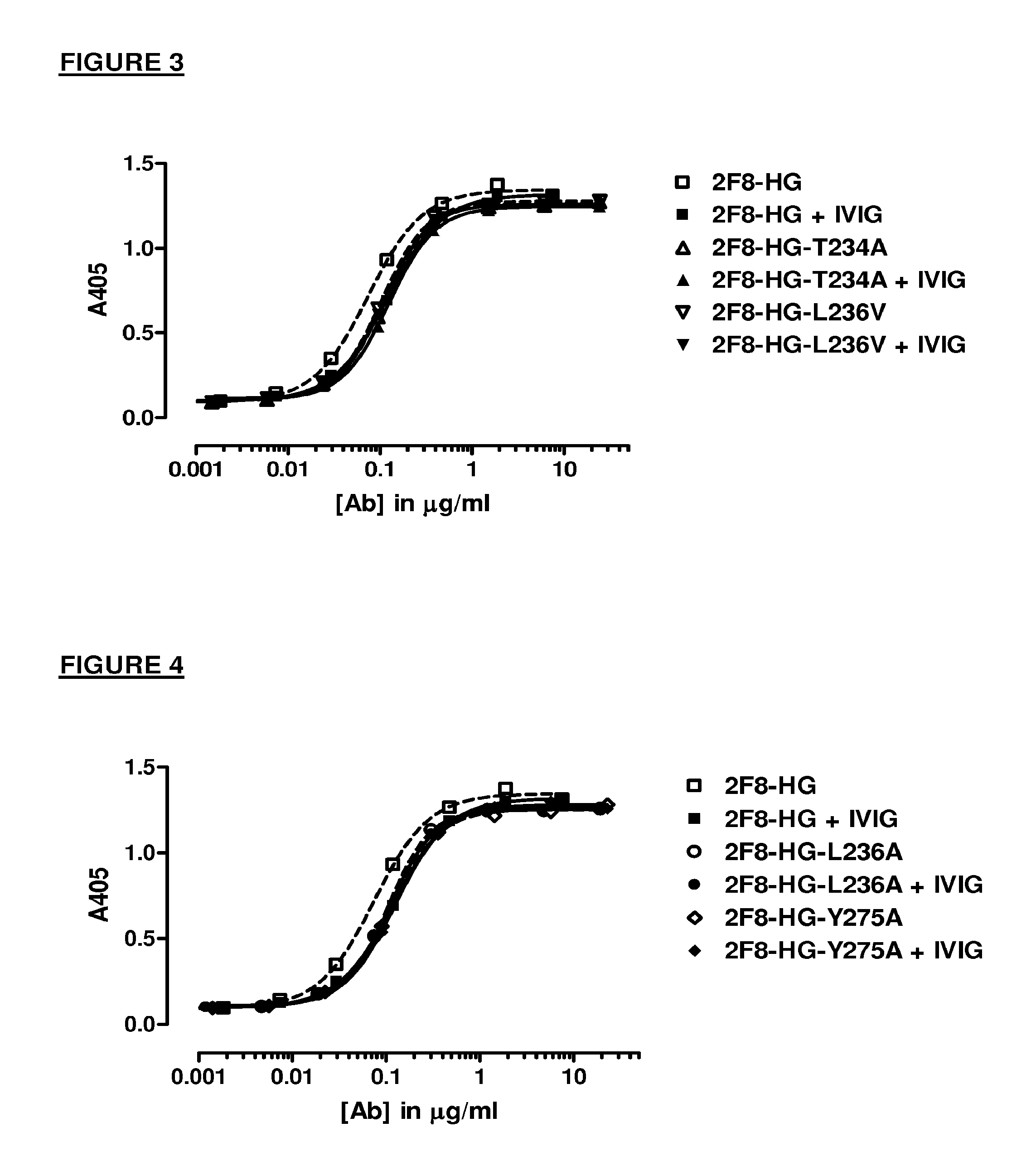
The present inventors succeeded in improving the antibody constant region to have increased stability under acid conditions, reduced heterogeneity originated from disulfide bonds in the hinge region, reduced heterogeneity originated from the H chain C terminus, and increased stability at high concentrations as well as in discovering novel constant region sequences having reduced Fcγ receptor-binding, while minimizing the generation of novel T-cell epitope peptides. As a result, the present inventors successfully discovered antibody constant regions with improved physicochemical properties (stability and homogeneity), immunogenicity, safety, and pharmacokinetics.
Owner:CHUGAI PHARMA CO LTD
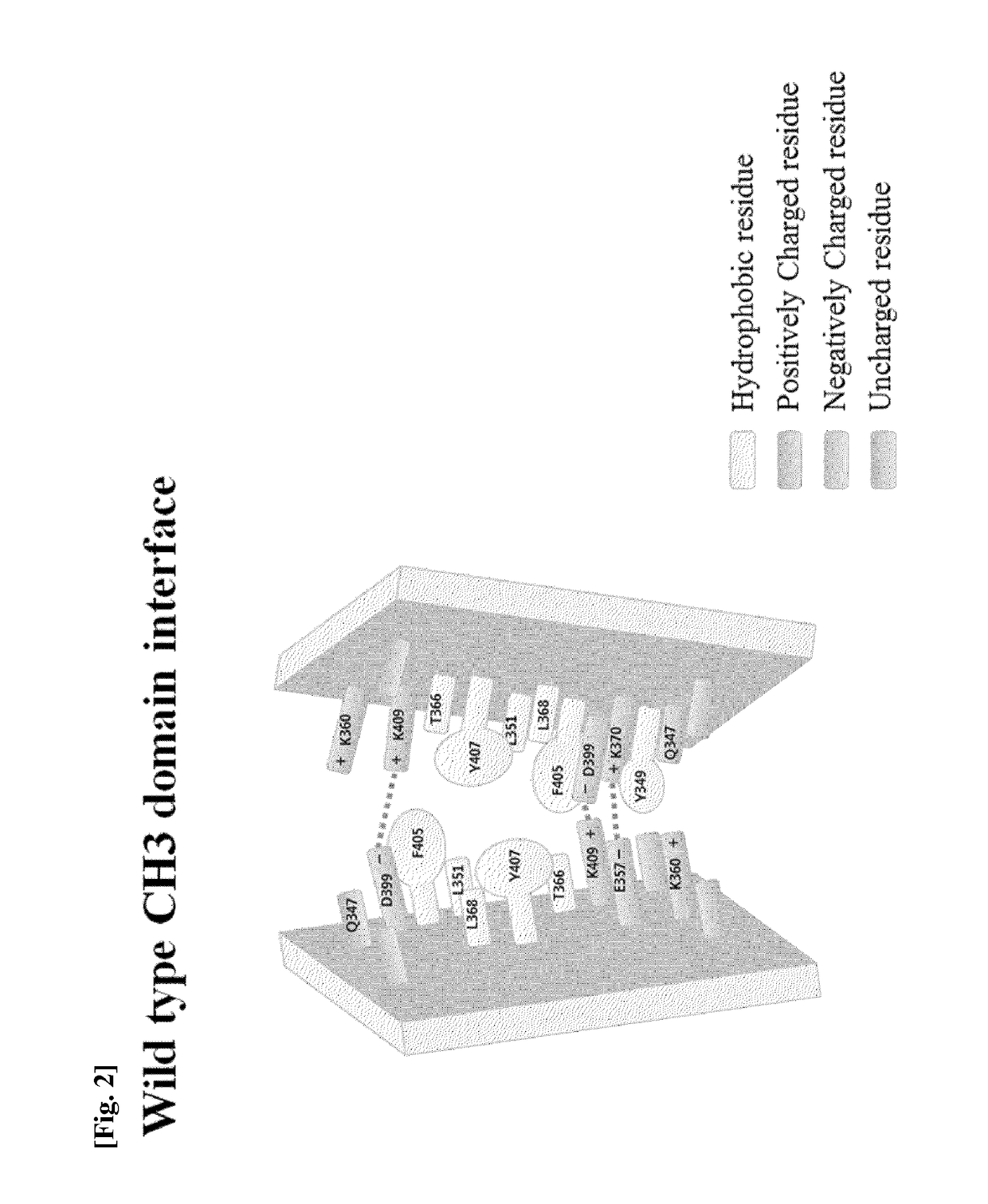
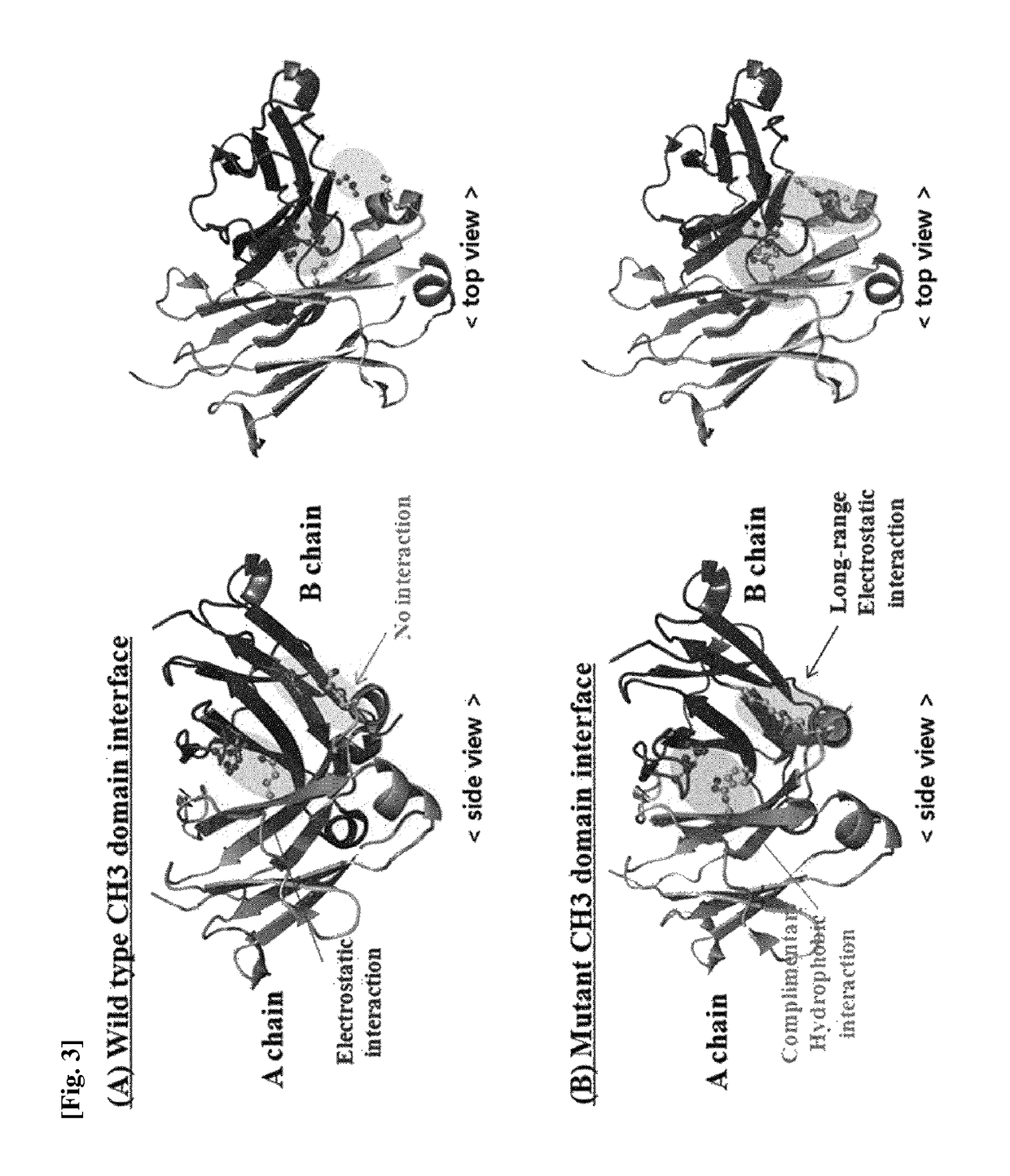
Ch3 domain variant pair inducing formation of heterodimer of heavy chain constant region of antibody at high efficiency, method for preparing same, and use thereof
ActiveUS20150307628A1High yieldMinimize formationHybrid immunoglobulinsAntibody mimetics/scaffoldsDiseaseProtein target
Disclosed are a CH3 domain variant pair of an antibody, a method for preparing same, and a use thereof. A mutation is induced in the CH3 domain so as to improve a yield of forming a heterodimer heavy chain constant region of an antibody. The CH3 domain heterodimer forms a heterodimer heavy chain constant region with a high efficiency of 90 to 95% or more and also has outstanding heat stability. A heterodimer heavy chain constant region including the CH3 domain heterodimer can construct a bispecific monoclonal antibody which simultaneously recognizes two kinds of antigens. The CH3 domain heterodimer and the bispecific antibody or fusion protein of an antibody constant region comprising same can be usefully applied to the treatment or prevention of a disease associated with a target antigen or a target protein.
Owner:AJOU UNIV IND ACADEMIC COOP FOUND
Antibody constant region variant
ActiveUS20130101581A1Improve propertiesIncreased riskImmunoglobulinsImmunological disordersAntibodyReceptor for activated C kinase 1
The present inventors carried out dedicated research to generate antibody constant regions with reduced Fcγ receptor-binding activity by altering amino acid sequences in the antibody constant region. As a result, the present inventors successfully identified novel constant region sequences with reduced Fcγ receptor-binding activity compared to conventional antibody constant regions.
Owner:CHUGAI PHARMA CO LTD
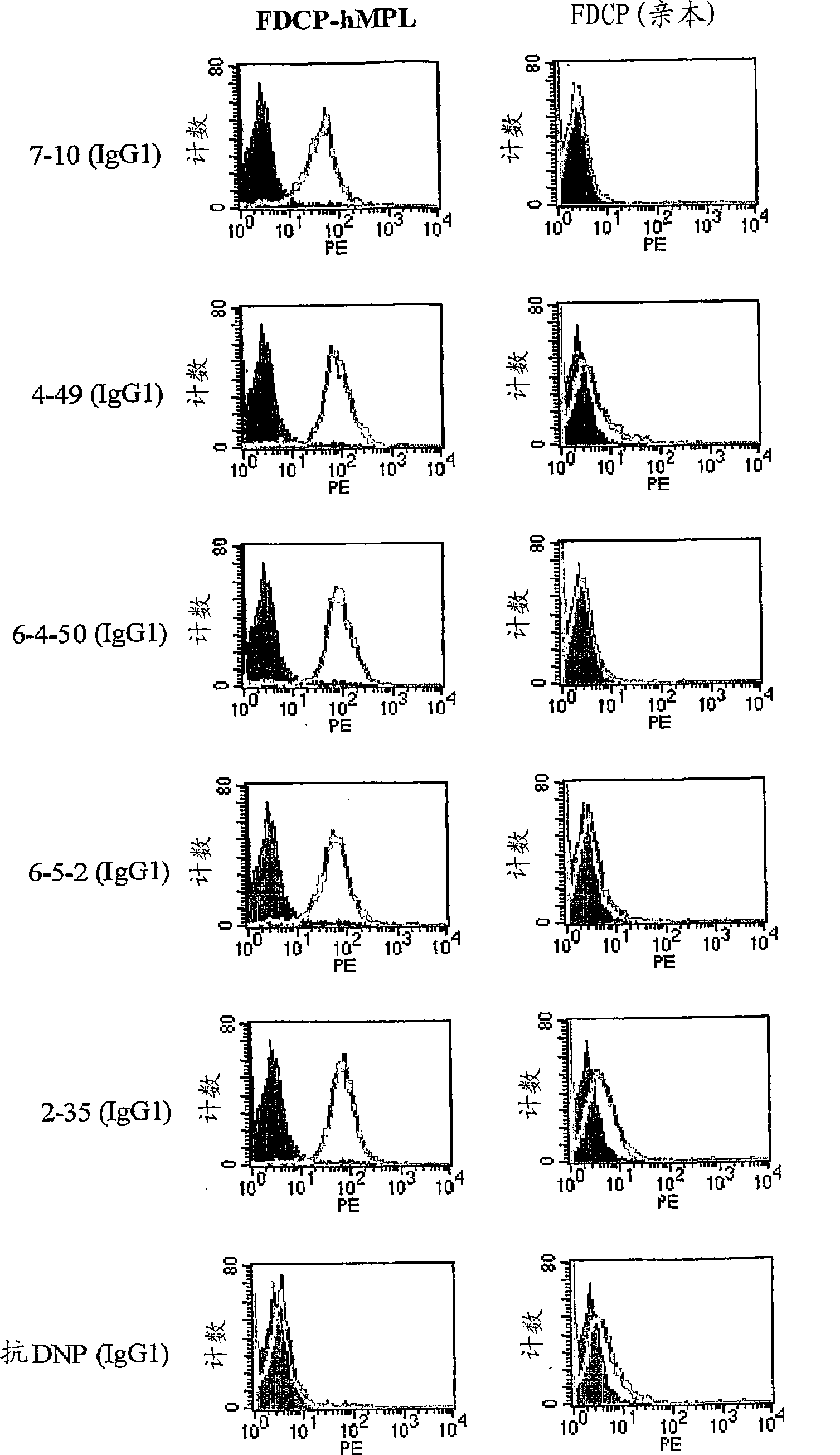
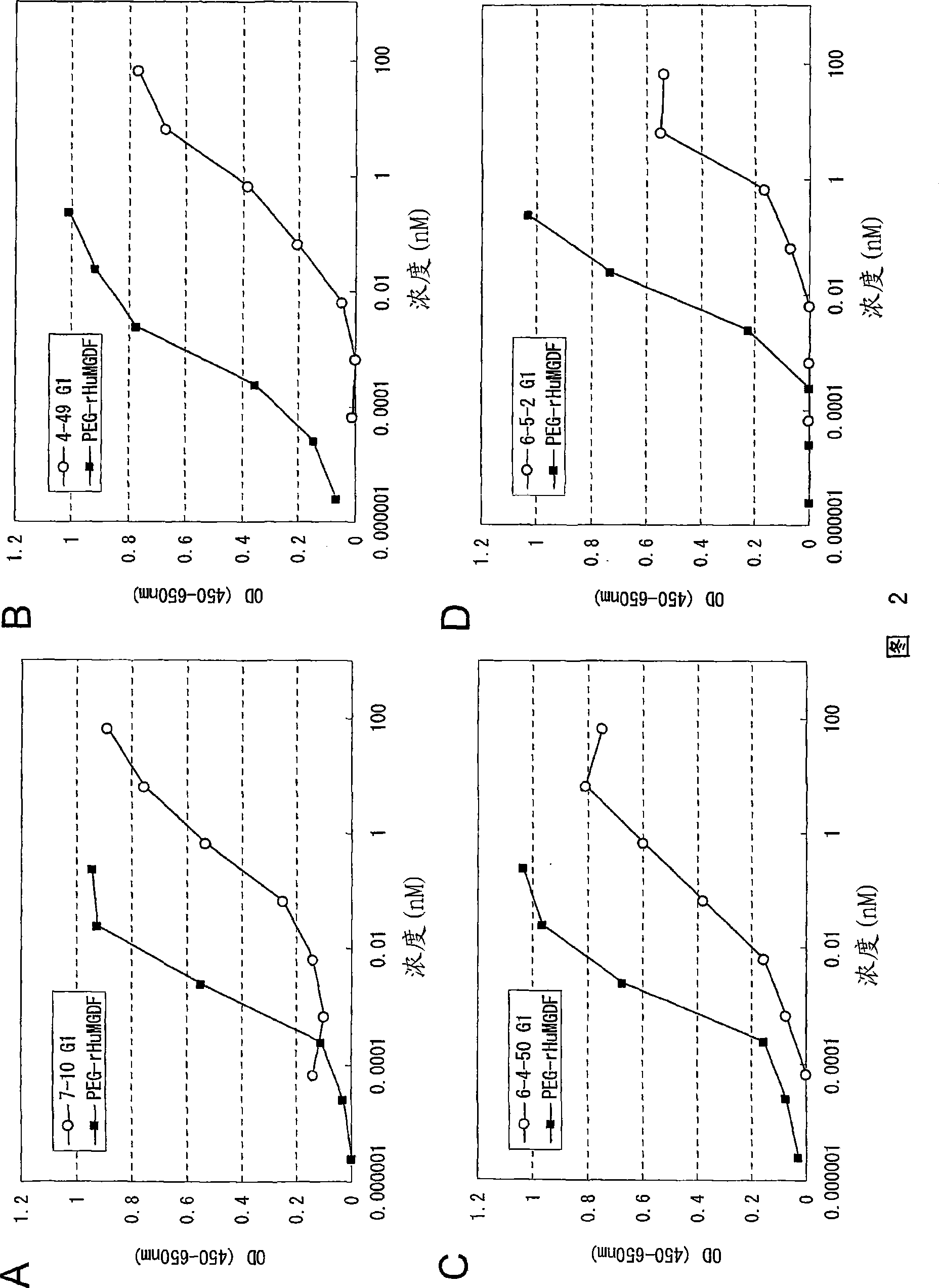
Agonist antibody to human thrombopoietin receptor
InactiveUS20100004429A1High activityLow antigenicityThrombopoietinHybrid immunoglobulinsHuman plateletUmbilical cord
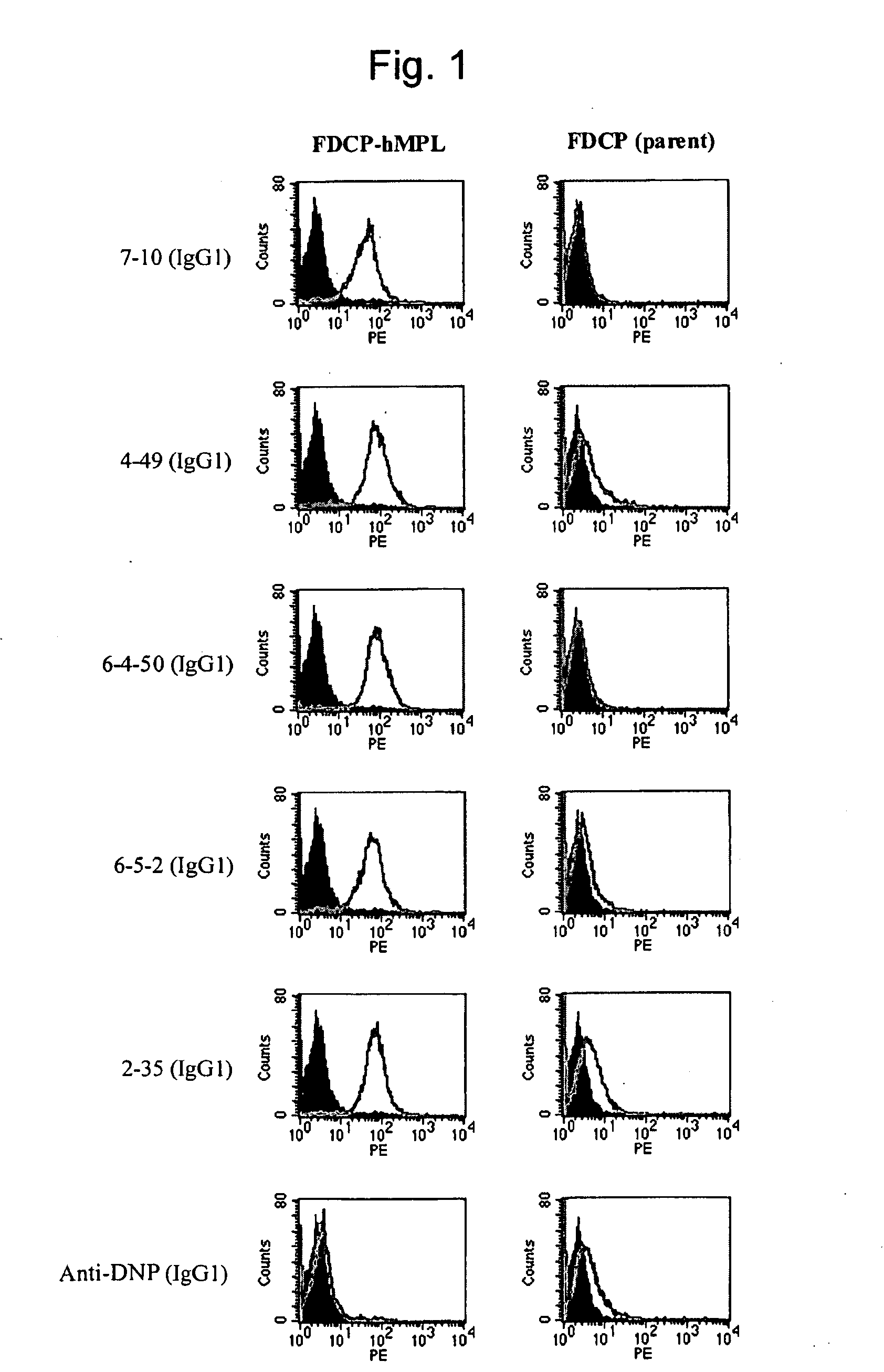
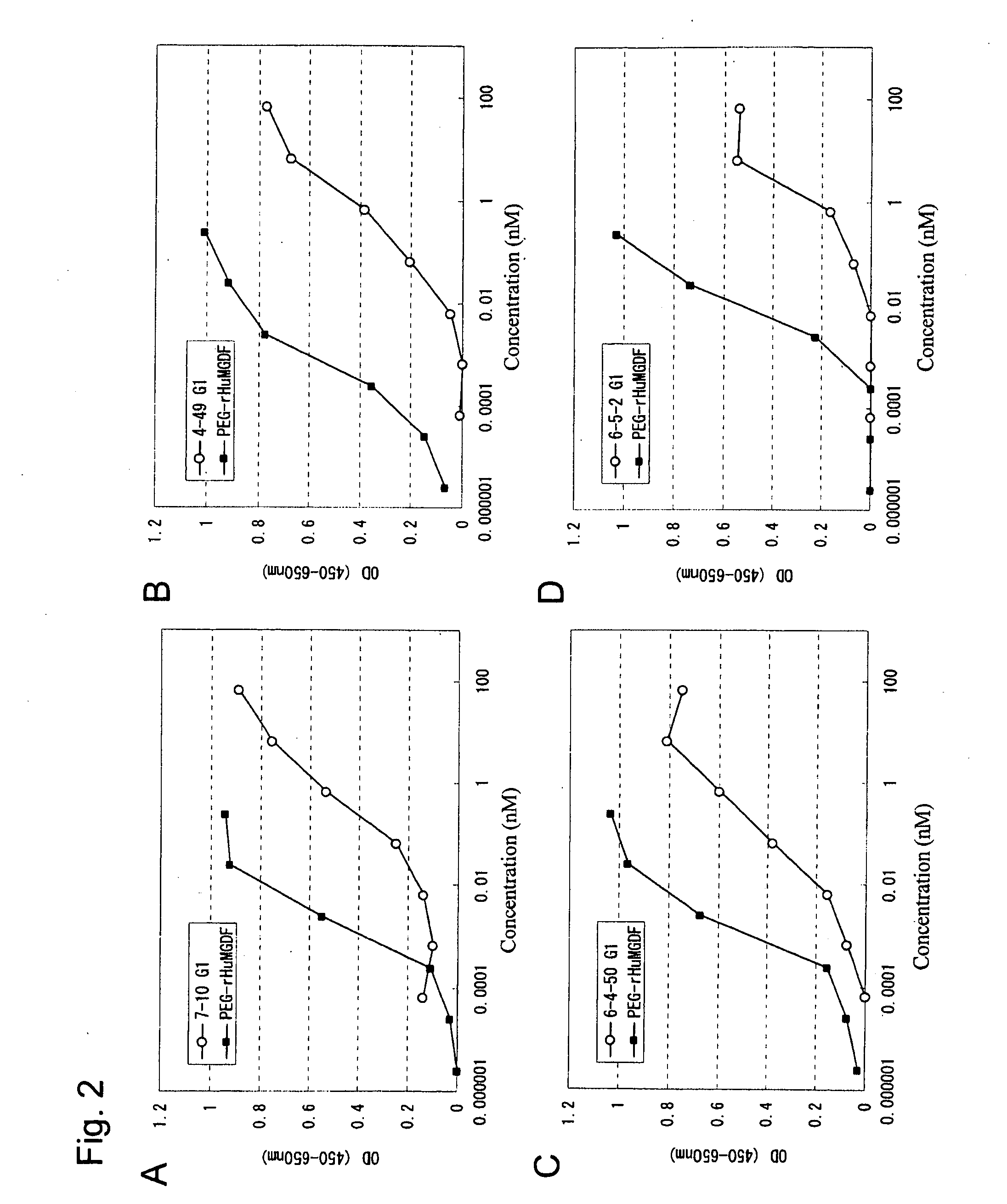
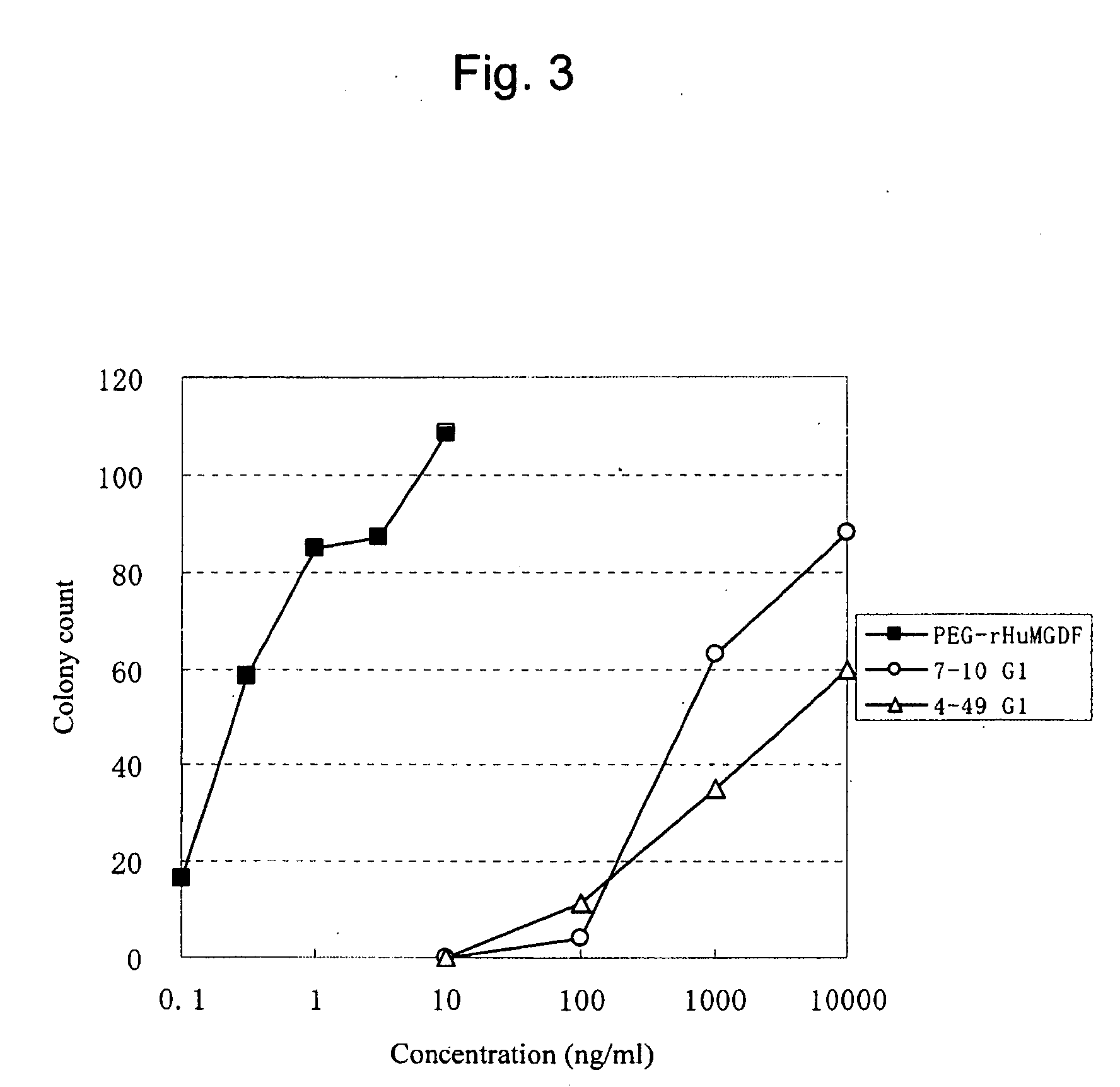
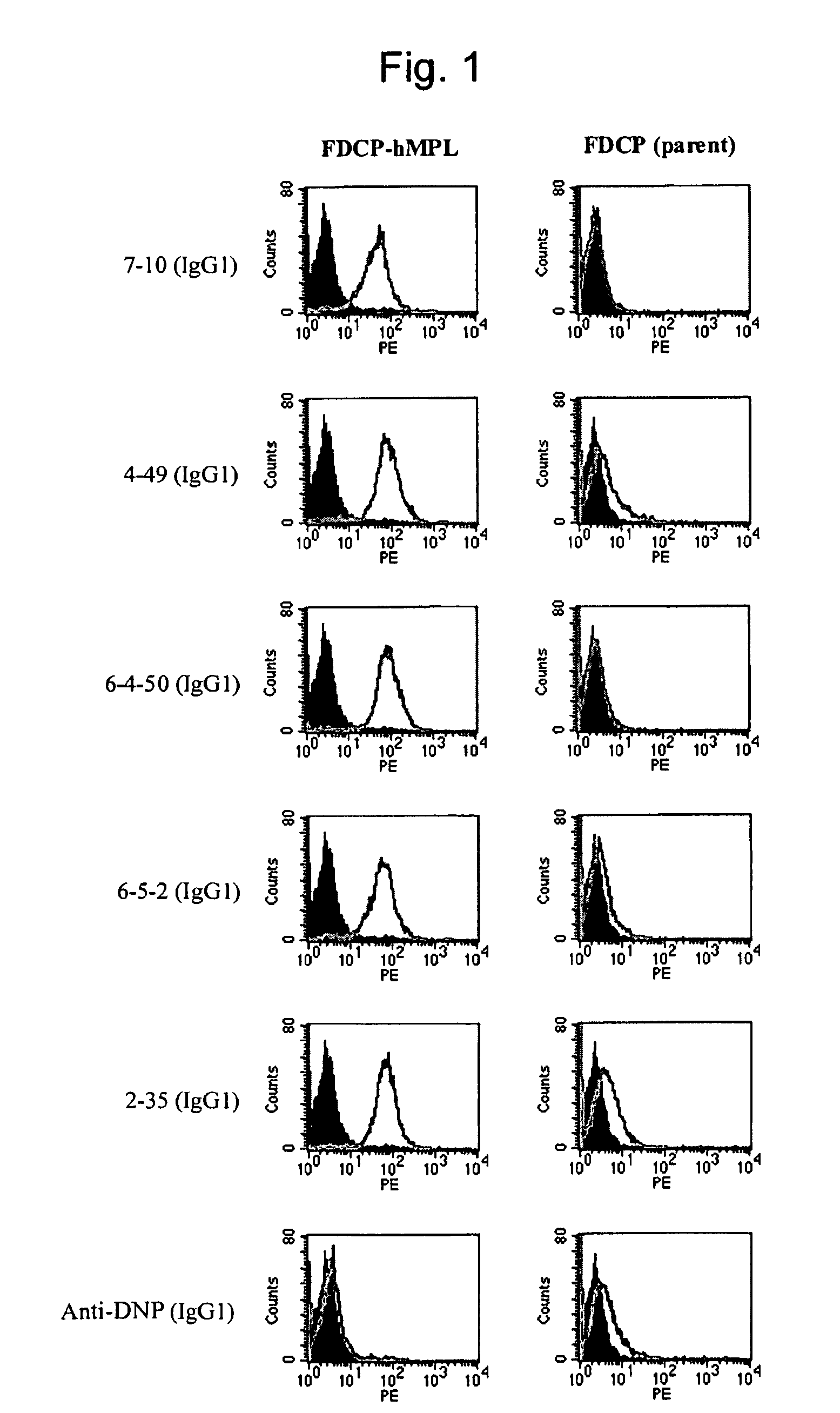
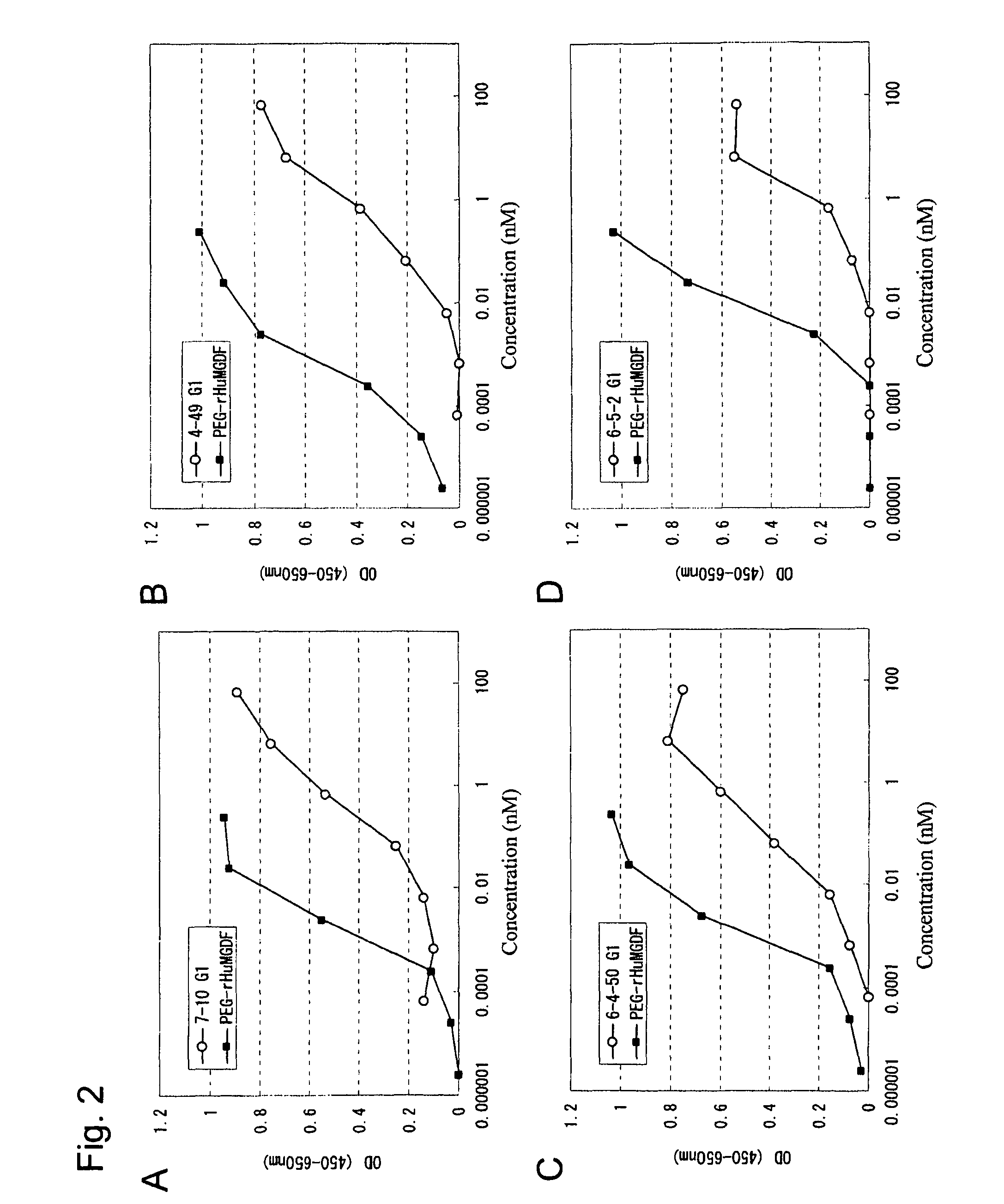
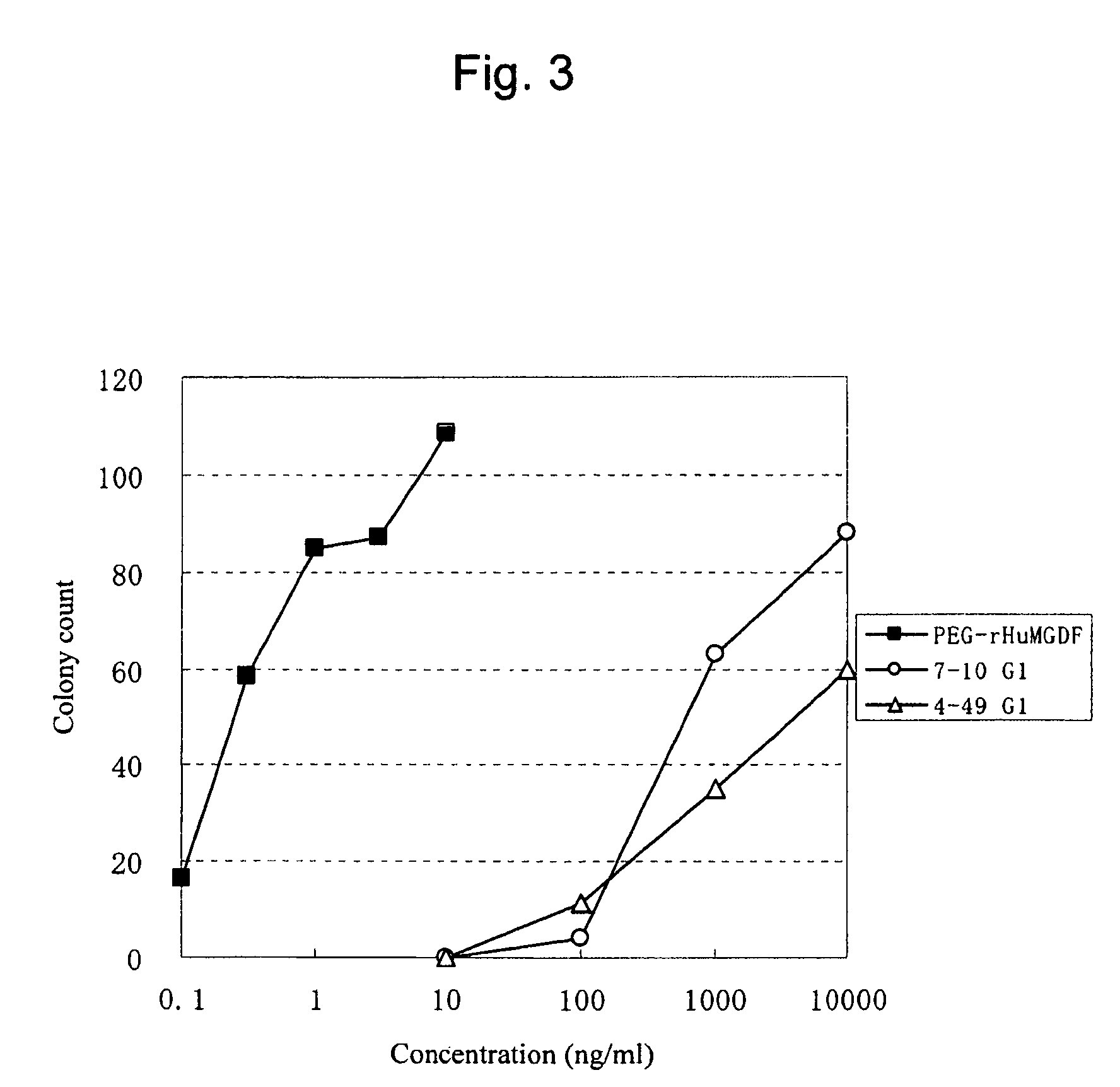
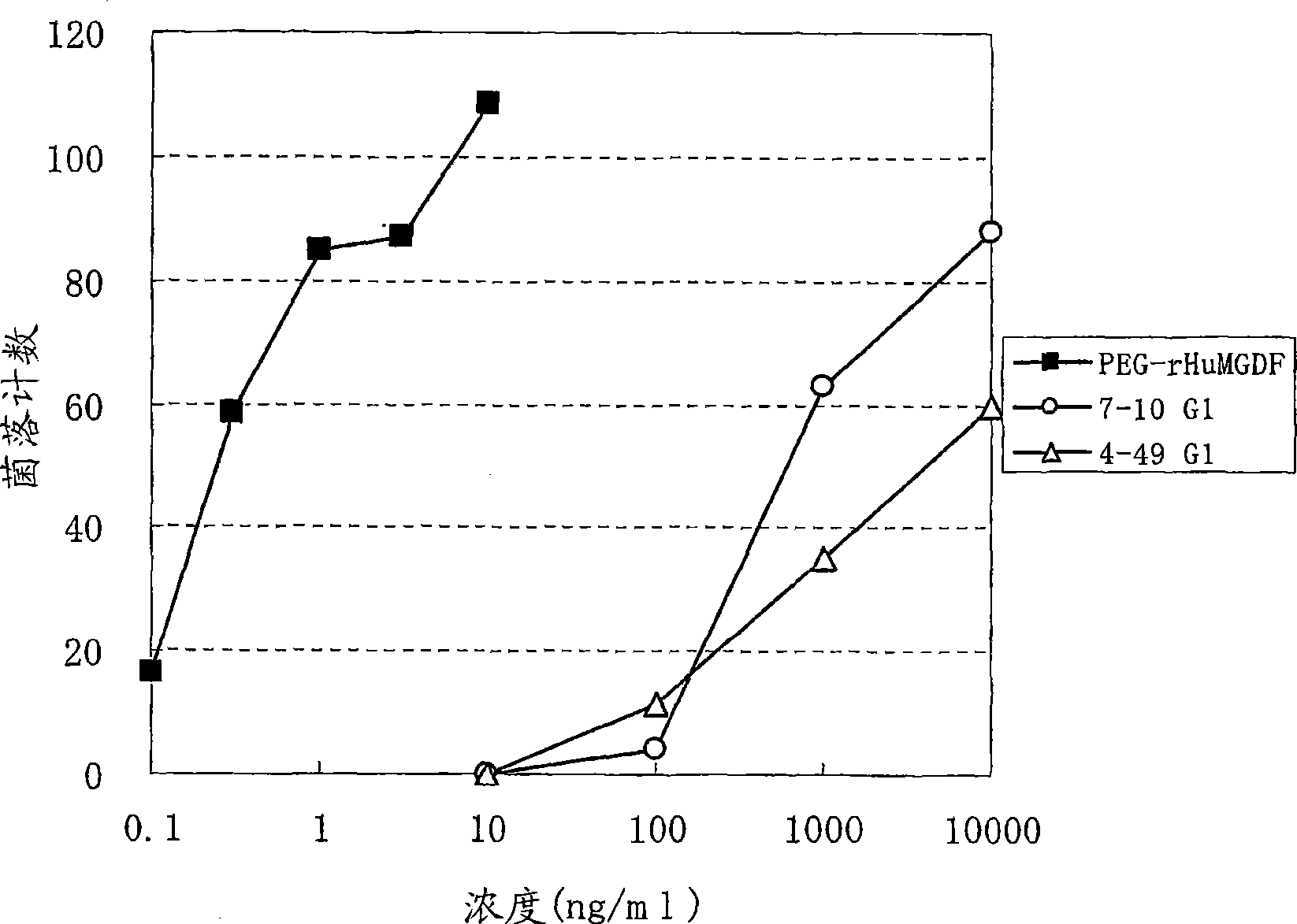
This invention provides an agonist antibody to a human thrombopoietin receptor (alias: human c-Mpl). More particularly, this invention provides an agonist antibody to a human thrombopoietin receptor, wherein the agonist antibody comprises: antibody constant regions comprising (1) amino acid sequences in a heavy chain constant region and a light chain constant region of a human antibody, (2) an amino acid sequence of a heavy chain constant region with a domain substituted between human antibody subclasses, and an amino acid sequence of a light chain constant region of a human antibody, or (3) amino acid sequences comprising a deletion(s), substitution(s), addition(s), or insertion(s) of one or several amino acid residues in the amino acid sequences of (1) or (2) above; and antibody variable regions capable of binding to and activating a human thrombopoietin receptor; and wherein the agonist antibody has the properties: (a) that the antibody induces colony formation at a concentration of 10,000 ng / ml or lower as determined by the CFU-MK colony formation assay using human umbilical-cord-blood-derived CD34+ cells; and (b) that the antibody has a maximal activity at least 50% higher than that of PEG-rHuMGDF and an 50% effective concentration (EC50) of 100 nM or less in the cell proliferation assay using UT7 / TPO cell. Also provided is a pharmaceutical composition for treating thrombocytopenia comprising said antibody.
Owner:KYOWA HAKKO KIRIN CO LTD
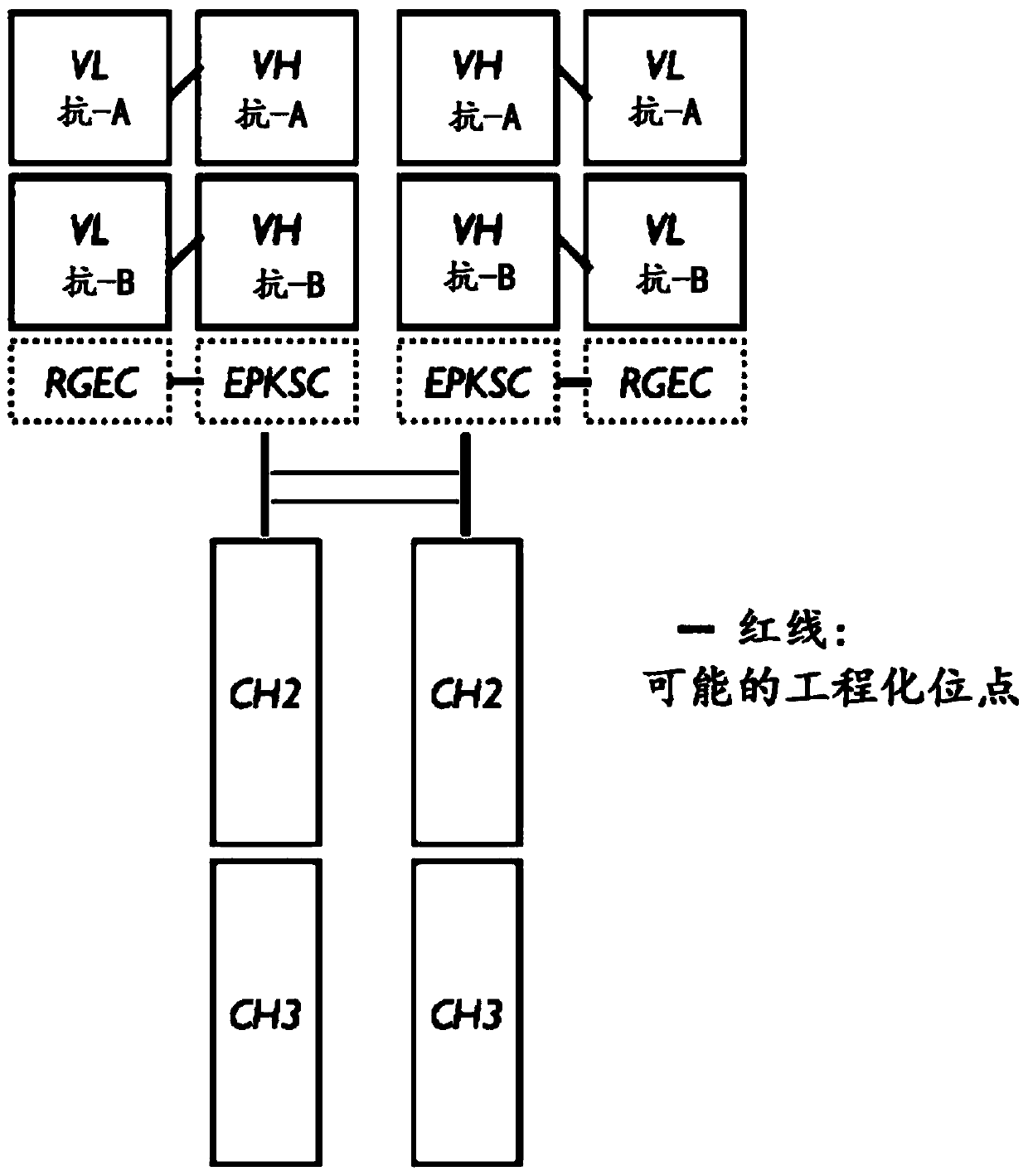
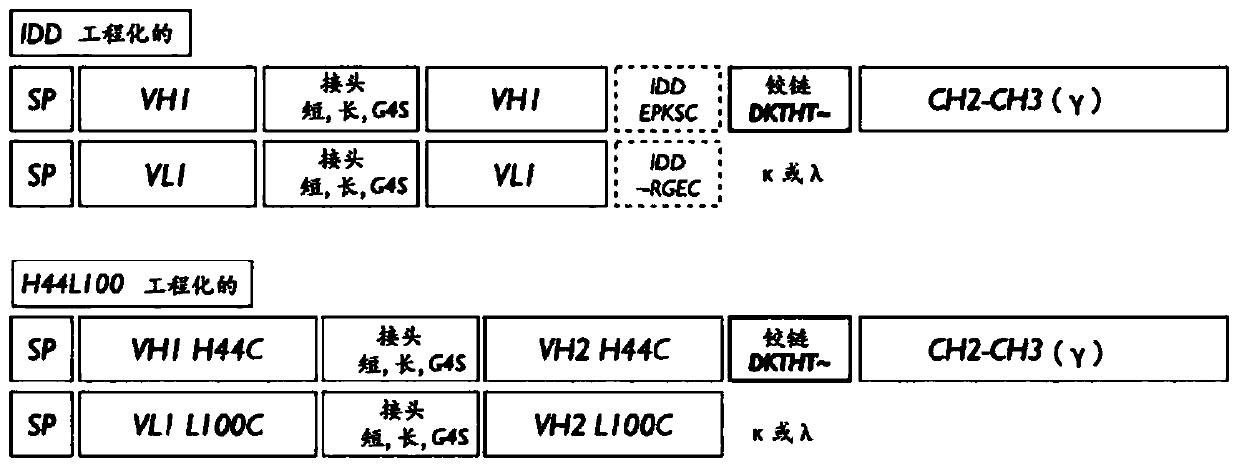
Engineered Antibody Constant Regions for Site-Specific Conjugation and Methods and Uses Therefor
The present invention is directed to antibodies, and antigen-binding portions thereof, engineered to introduce amino acids for site-specific conjugation. The invention relates to engineered antibody constant region (Fc, Cγ, Cκ, and Cλ) polypeptides, and portions thereof, and antibodies comprising the polypeptides. Further, the invention relates to Fc fusion proteins comprising an engineered Fc region. The invention also relates to methods and uses of the engineered antibodies and portions for, among other things, production of antibody-drug conjugate therapeutics.
Owner:PFIZER INC
Methods of increasing lean tissue mass using OB protein compositions
InactiveUS7208577B2Quality improvementLower Level RequirementsObesity gene productsPeptide/protein ingredientsTreatment useOb Protein
The present invention provides methods of creating and using OB protein compositions with an antibody constant region or portion thereof fused to an OB protein. The fusion protein is created by attaching the polyamino acids to the OB protein moiety. The fusion proteins can then be used for various therapeutic uses.
Owner:AMGEN INC
Anti-growth factor receptor avidin fusion proteins as universal vectors for drug delivery
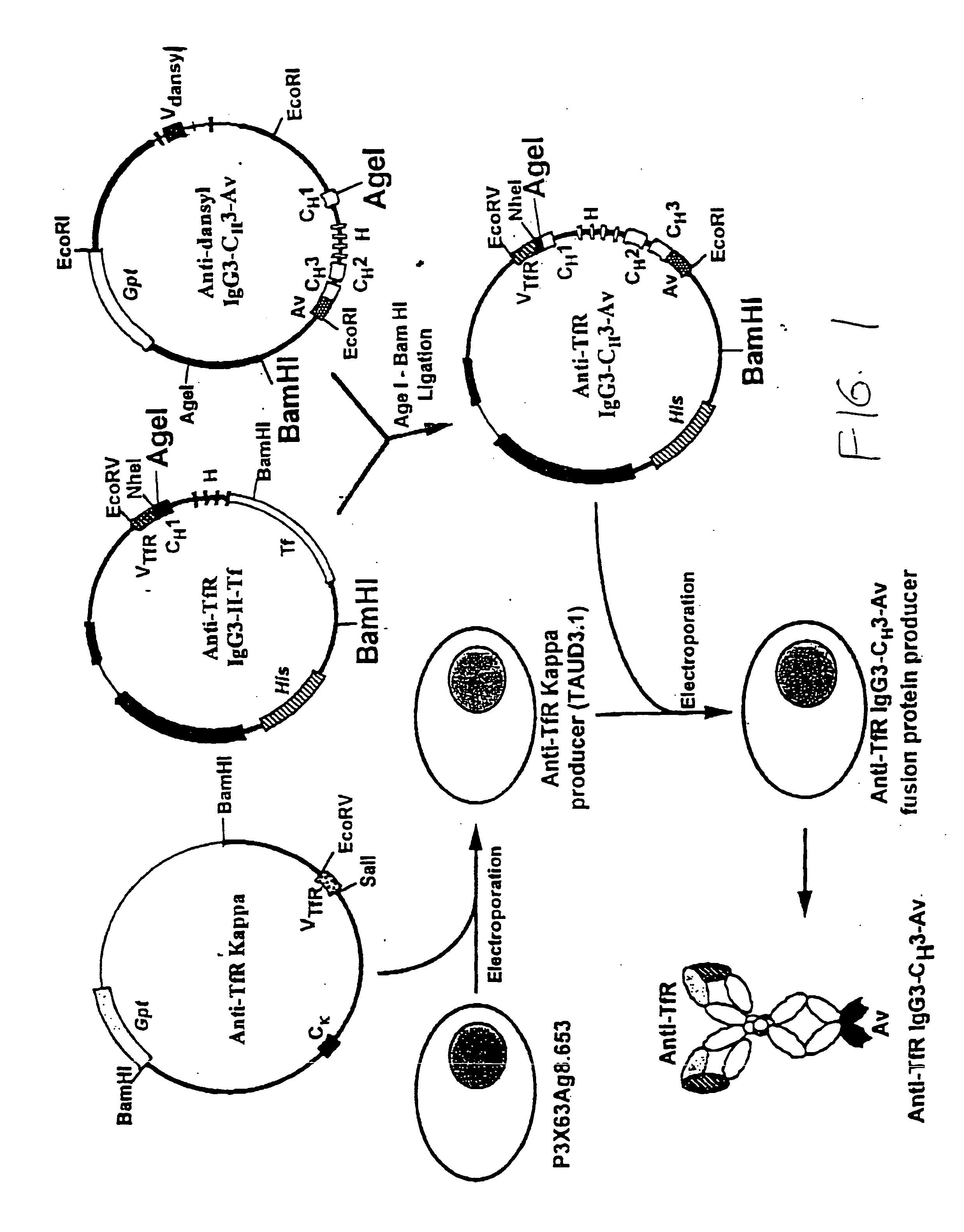
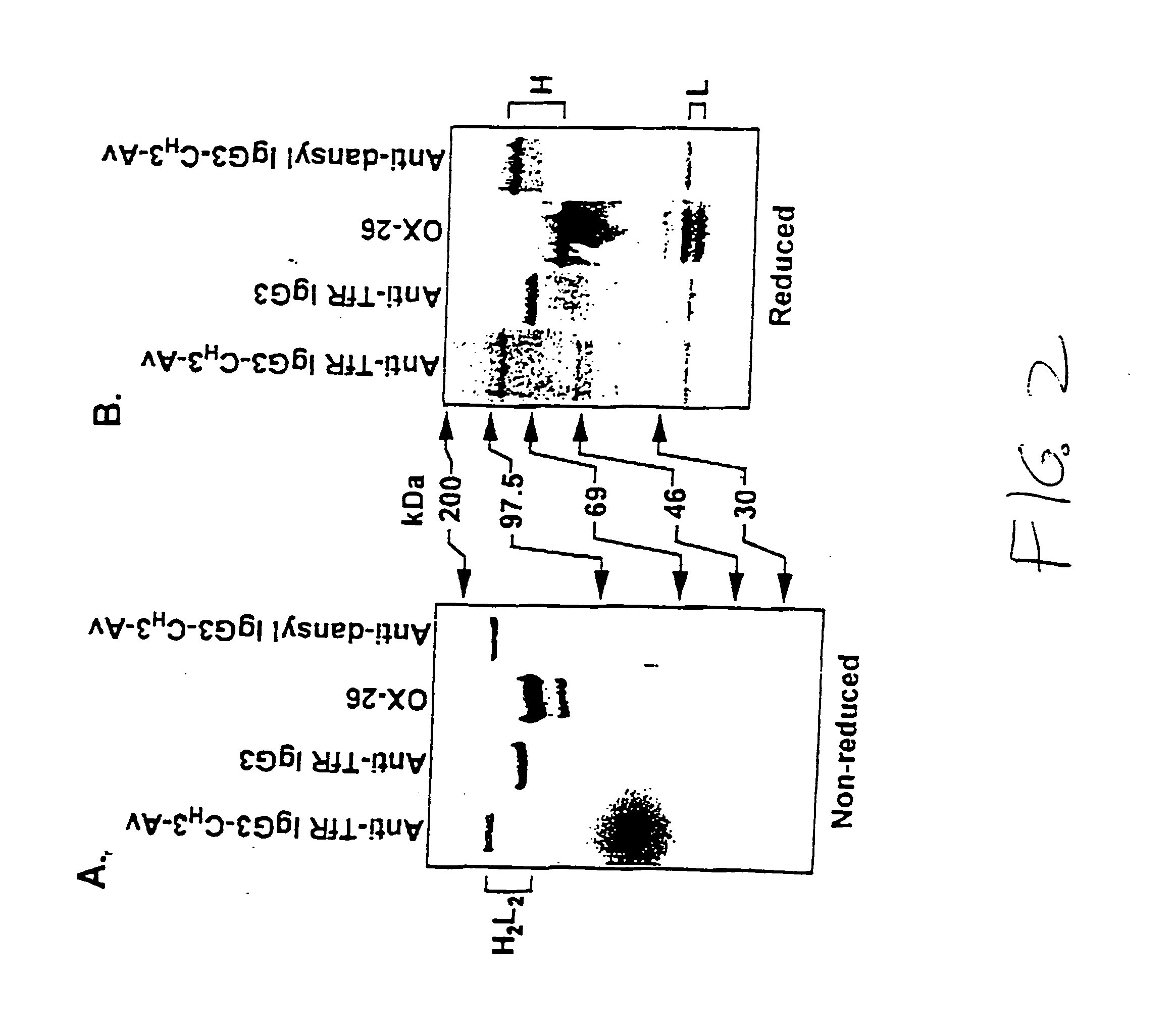
A fusion protein for delivery of a wide variety of agents to a cell via antibody-receptor-mediated endocytosis comprises a first segment and a second segment: the first segment comprising a variable region of an antibody that recognizes an antigen on the surface of a cell that after binding to the variable region of the antibody undergoes antibody-receptor-mediated endocytosis, and, optionally, further comprises at least one domain of a constant region of an antibody; and the second segment comprising a protein domain selected from the group consisting of avidin, an avidin mutein, a chemically modified avidin derivative, streptavidin, a streptavidin mutein, and a chemically modified streptavidin derivative. Typically, the antigen is a protein. Typically, the protein antigen on the surface of the cell is a receptor such as a transferrin receptor-or an insulin receptor. The invention also includes an antibody construct incorporating the fusion protein that is either a heavy chain or a light chain together with a complementary light chain or heavy chain to form an intact antibody molecule. The invention further includes targeting methods and screening methods.
Owner:RGT UNIV OF CALIFORNIA
Methods of modifying antibodies for purification of bispecific antibodies
ActiveUS9670269B2Efficient purificationFunction increaseImmunoglobulins against cytokines/lymphokines/interferonsImmunological disordersBinding siteBispecific antibody
The present inventors devised methods for efficiently purifying bispecific antibodies using a chromatography column based on the difference in isoelectric points between the H chains of two types of antibodies, wherein the difference is introduced by modifying the amino acids present on the surface of the antibody variable regions of two types of antibodies that constitute a bispecific antibody. Furthermore, the inventors devised methods for efficiently purifying bispecific antibodies using a chromatography column by linking respective antigen binding sites (heavy chain variable regions) to the antibody constant regions having different isoelectric points, and then coexpressing these antibodies.
Owner:CHUGAI PHARMA CO LTD
Modified antibody constant region
ActiveUS9688762B2Improving immunogenicityImprove propertiesAntipyreticAnalgesicsHigh concentrationHinge region
The present inventors succeeded in improving the antibody constant region to have increased stability under acid conditions, reduced heterogeneity originated from disulfide bonds in the hinge region, reduced heterogeneity originated from the H chain C terminus, and increased stability at high concentrations as well as in discovering novel constant region sequences having reduced Fcγ receptor-binding, while minimizing the generation of novel T-cell epitope peptides. As a result, the present inventors successfully discovered antibody constant regions with improved physicochemical properties (stability and homogeneity), immunogenicity, safety, and pharmacokinetics.
Owner:CHUGAI PHARMA CO LTD
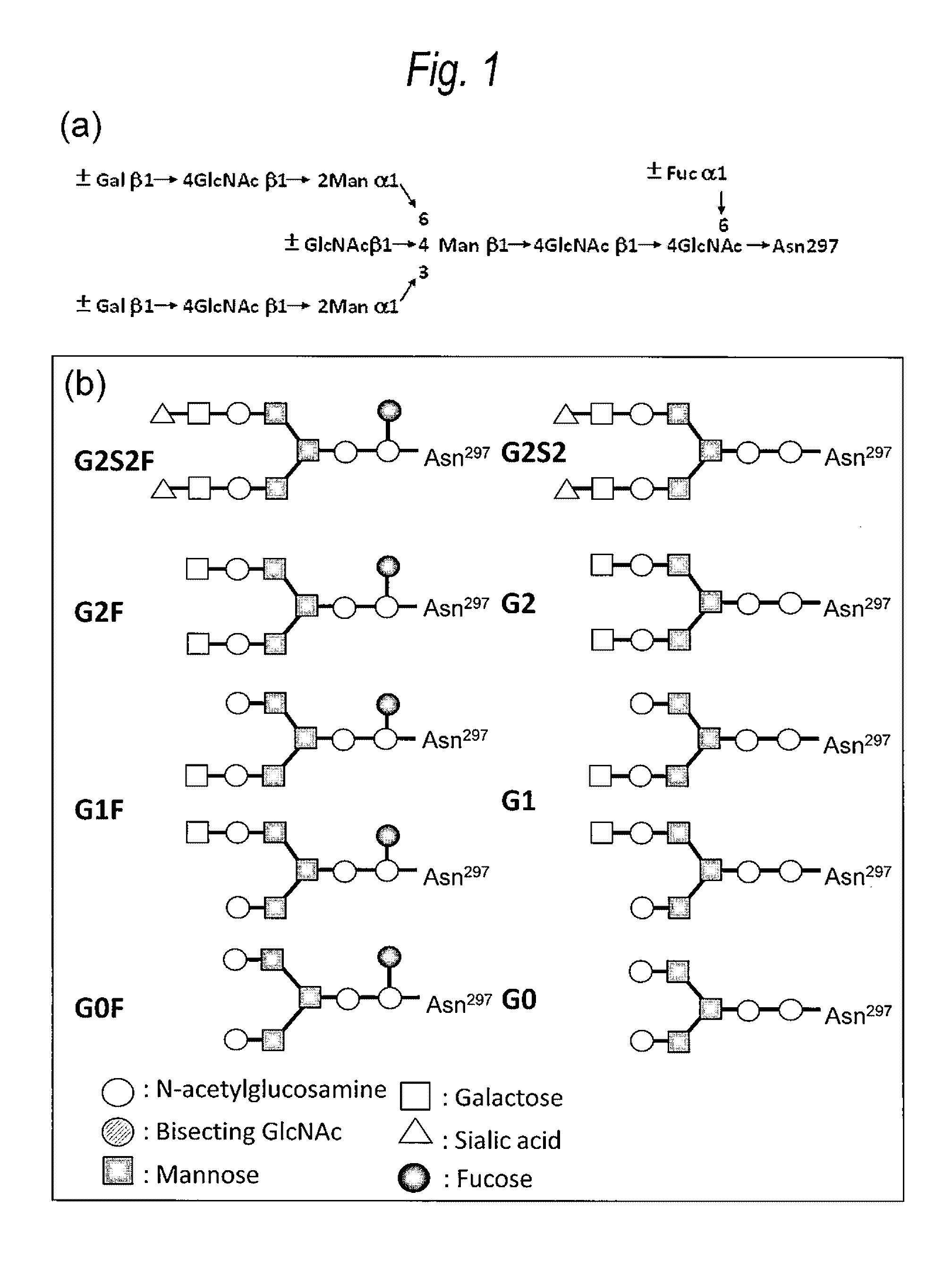
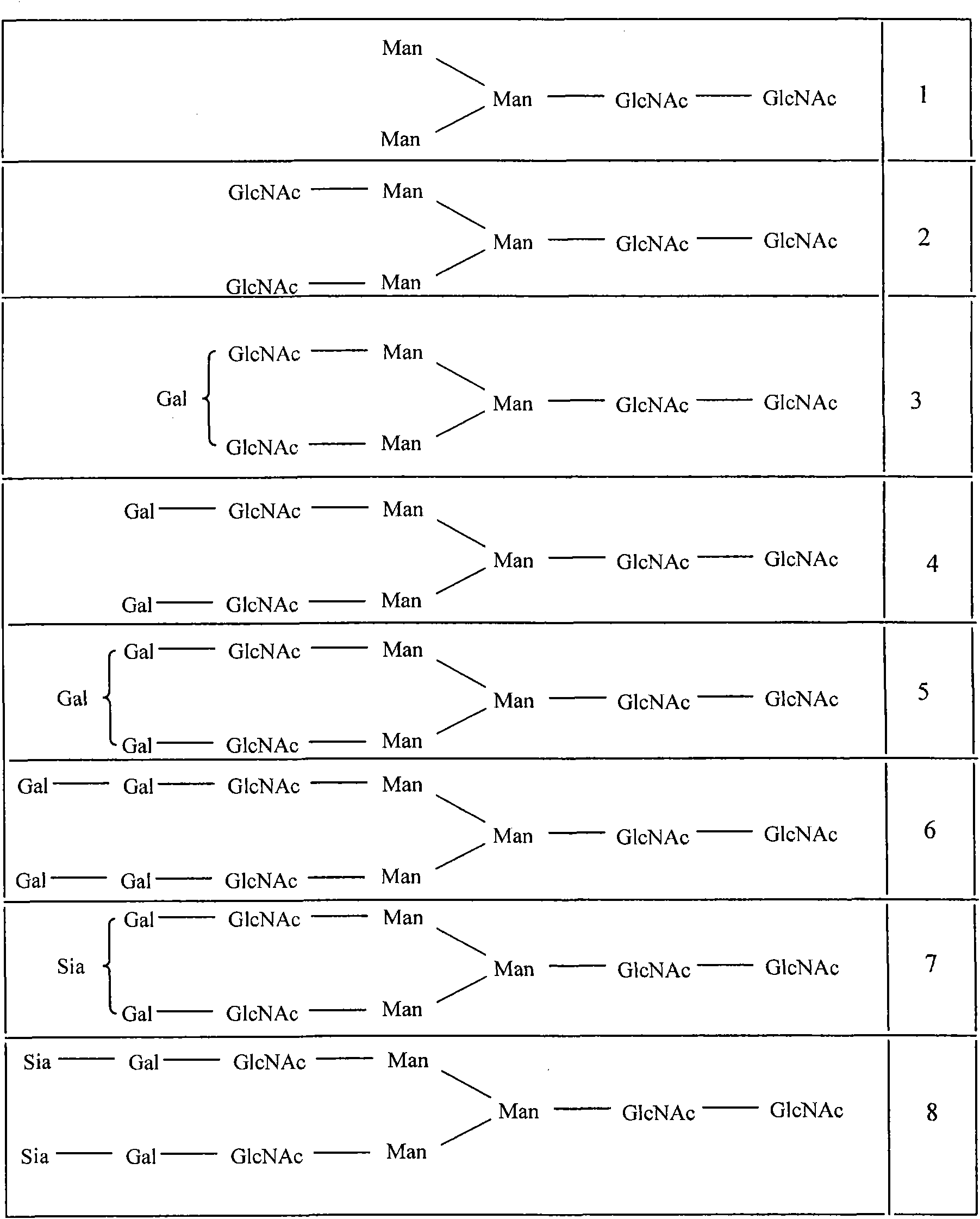
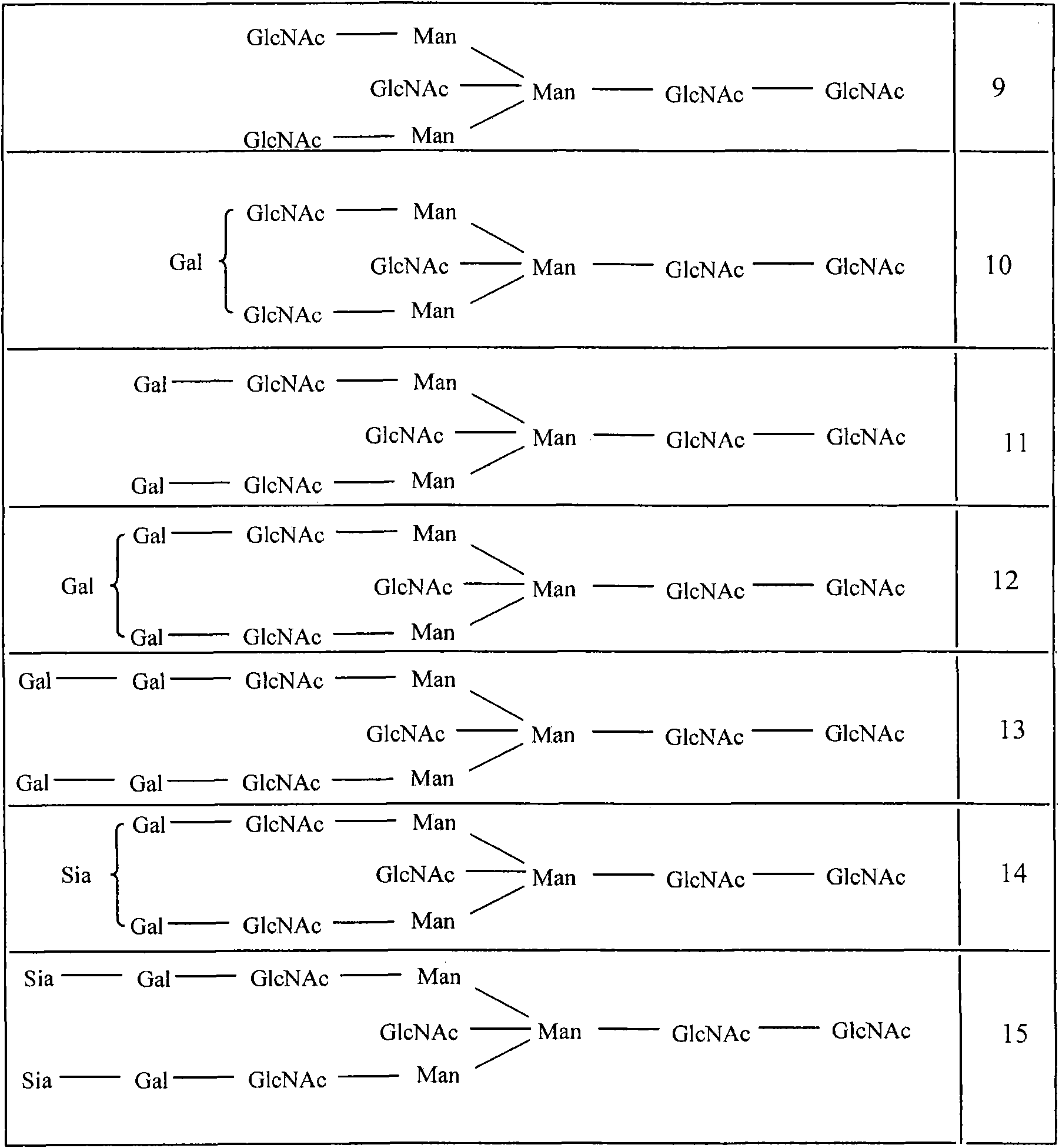
Antibody variants composition
Among N-glycoside-linked sugar chains which are bound to the Fc region of an antibody, sugar chains which are bound to Asn at position 297 relates to the activity and stability of the antibody in blood, but there is a possibility that extra sugar chains bound to the amino acid residues at positions other than 297 have influences upon the antibody constant region-mediated activity and a possibility of causing a problem of uniformity as a therapeutic antibody preparation. Accordingly, among N-glycoside-linked sugar chains which bind to the Fc region of the antibody, a method for controlling extra sugar chains which are bound to Asn residues at positions other than position 297 according to the EU index is required. The present invention provides an antibody variant composition, comprising amino acid residues of an Asn-X-Ser / Thr (X represents an amino acid residue other than Pro) sequence at positions other than positions 297 to 299 according to the EU index in an Fc region of a human IgG antibody, in which at least one amino acid substitution selected from an amino acid substitution of Asn to other amino acid residue, an amino acid substitution of X to Pro and an amino acid substitution of Ser / Thr to other amino acid residue is carried out, and a fragment of the antibody variant composition.
Owner:KYOWA HAKKO KIRIN CO LTD
Bispecific antibody
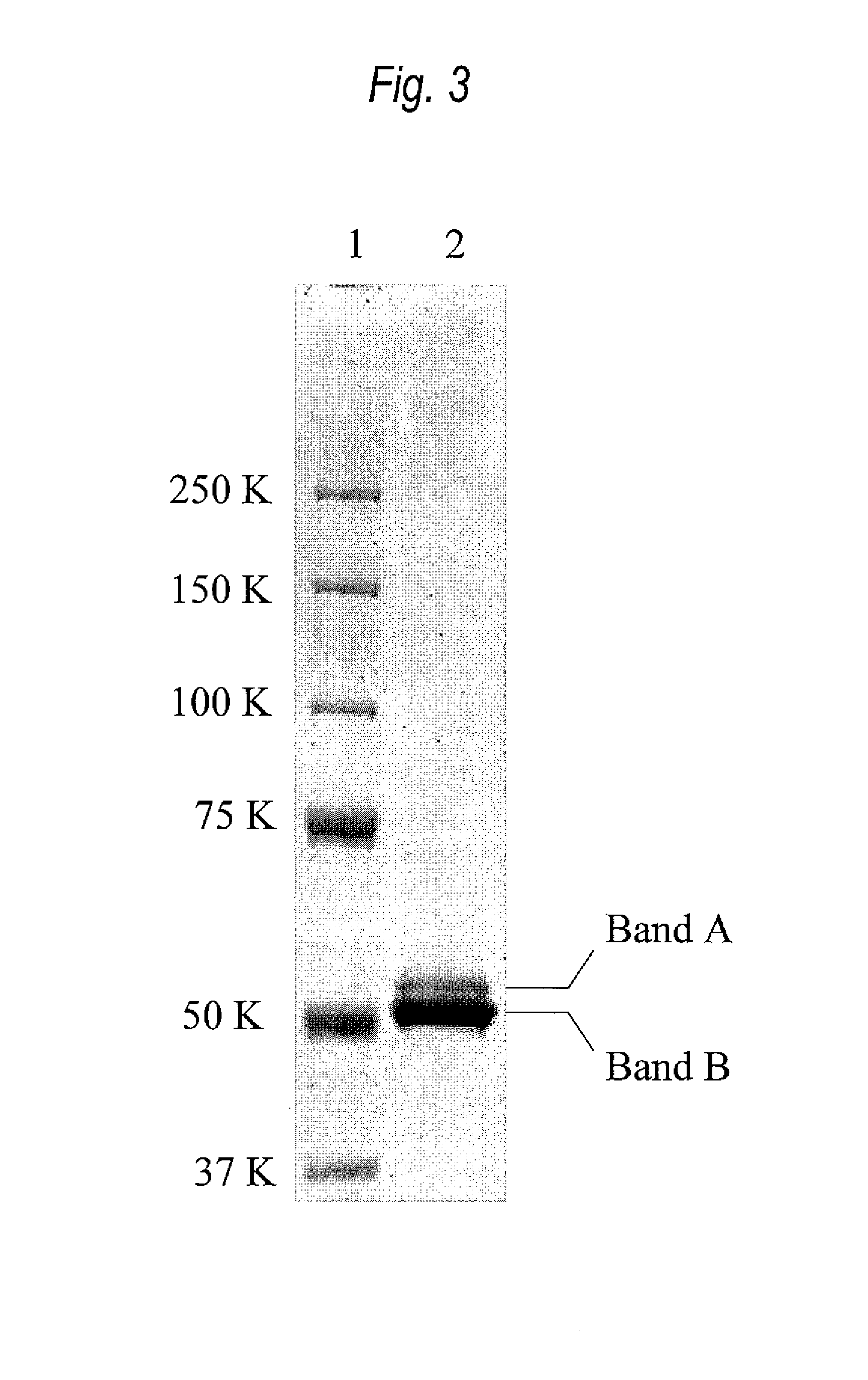
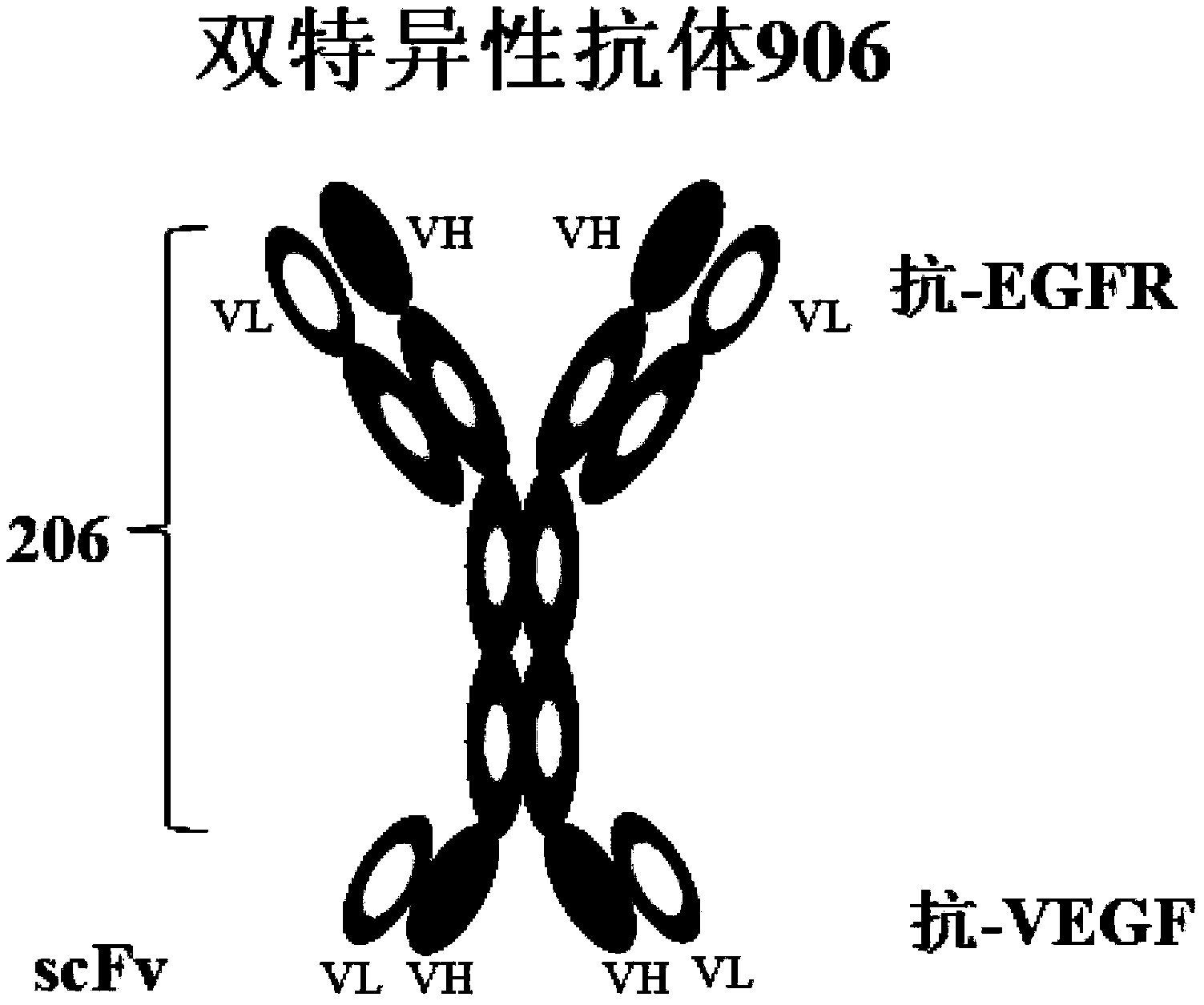
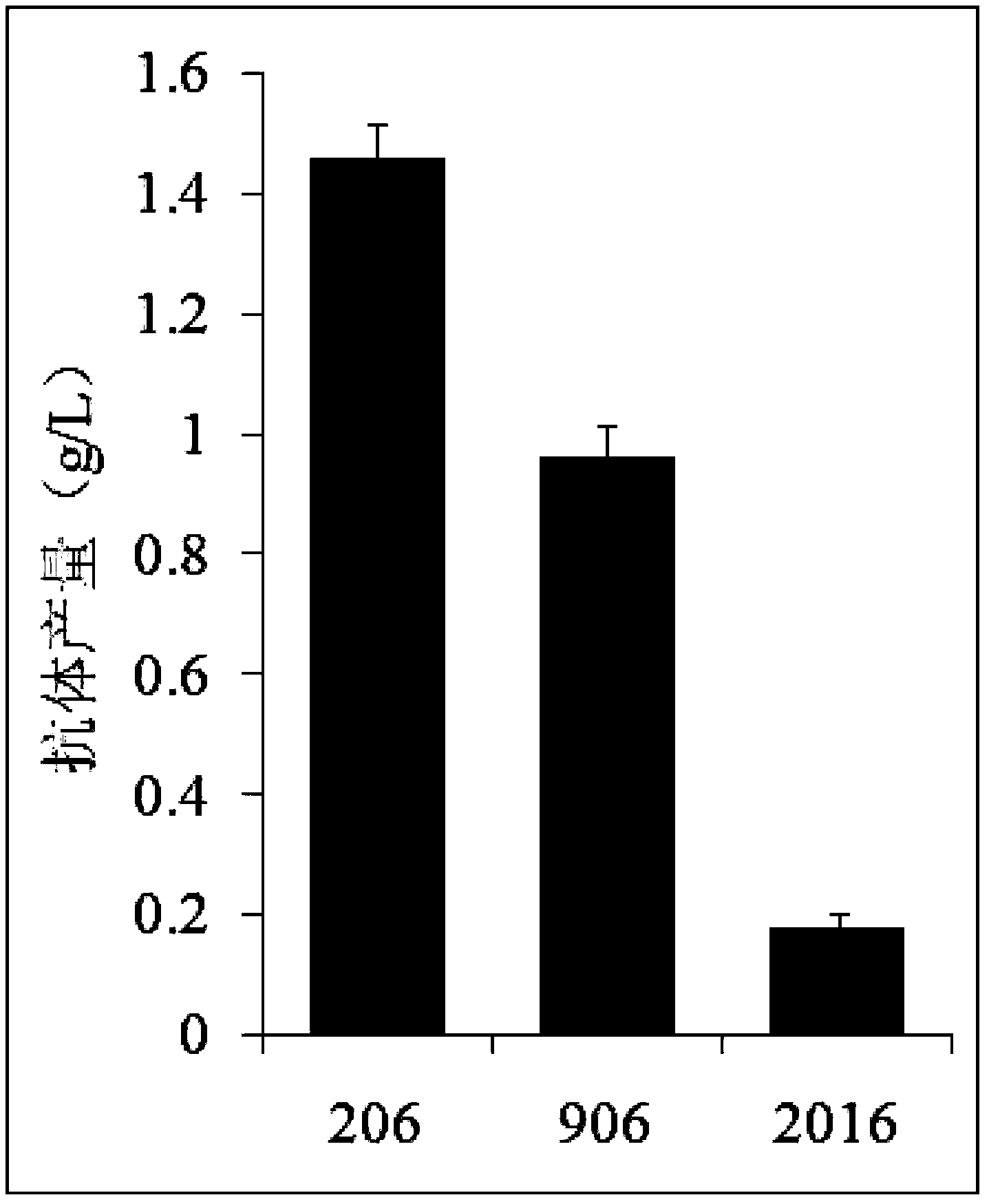
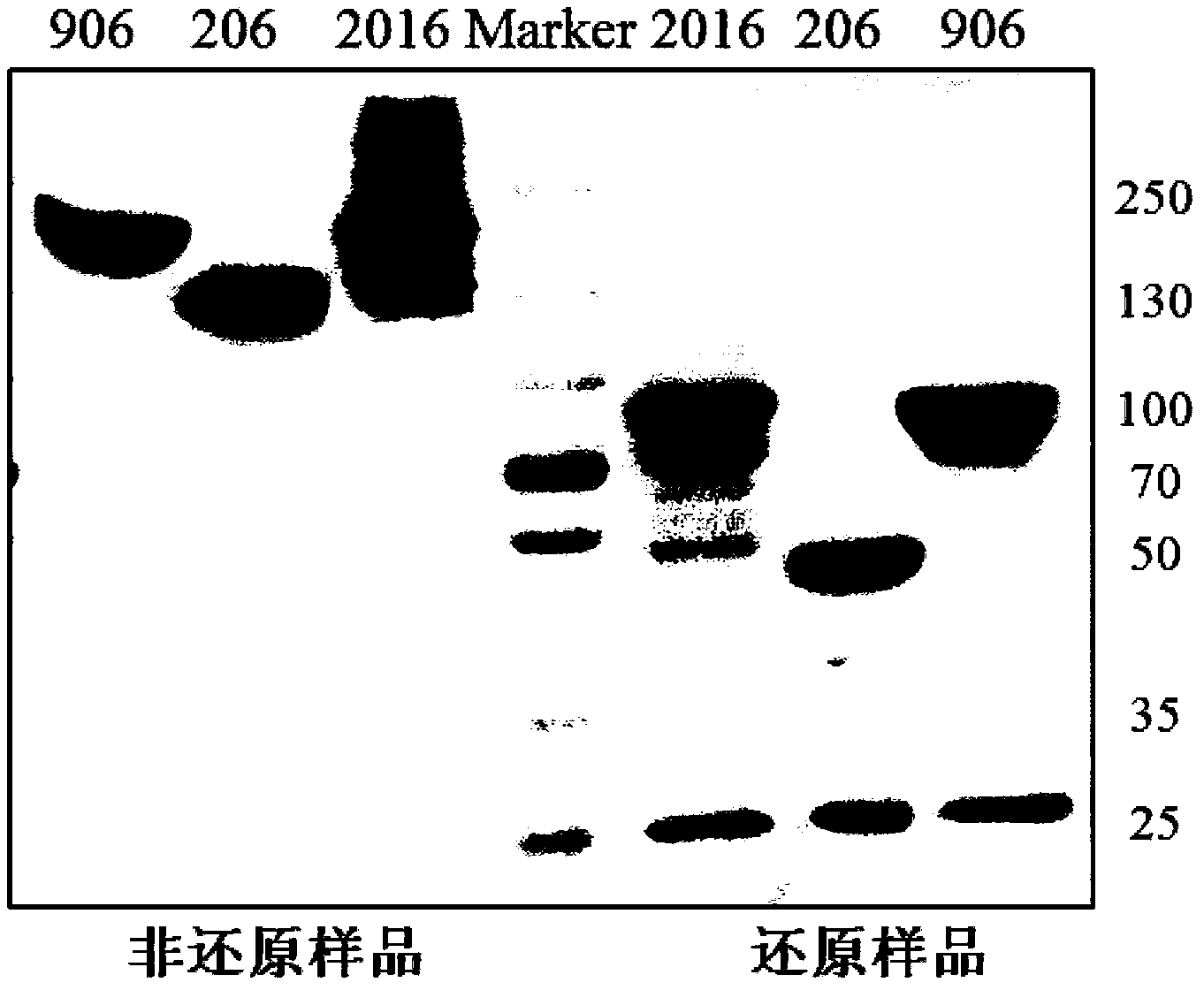
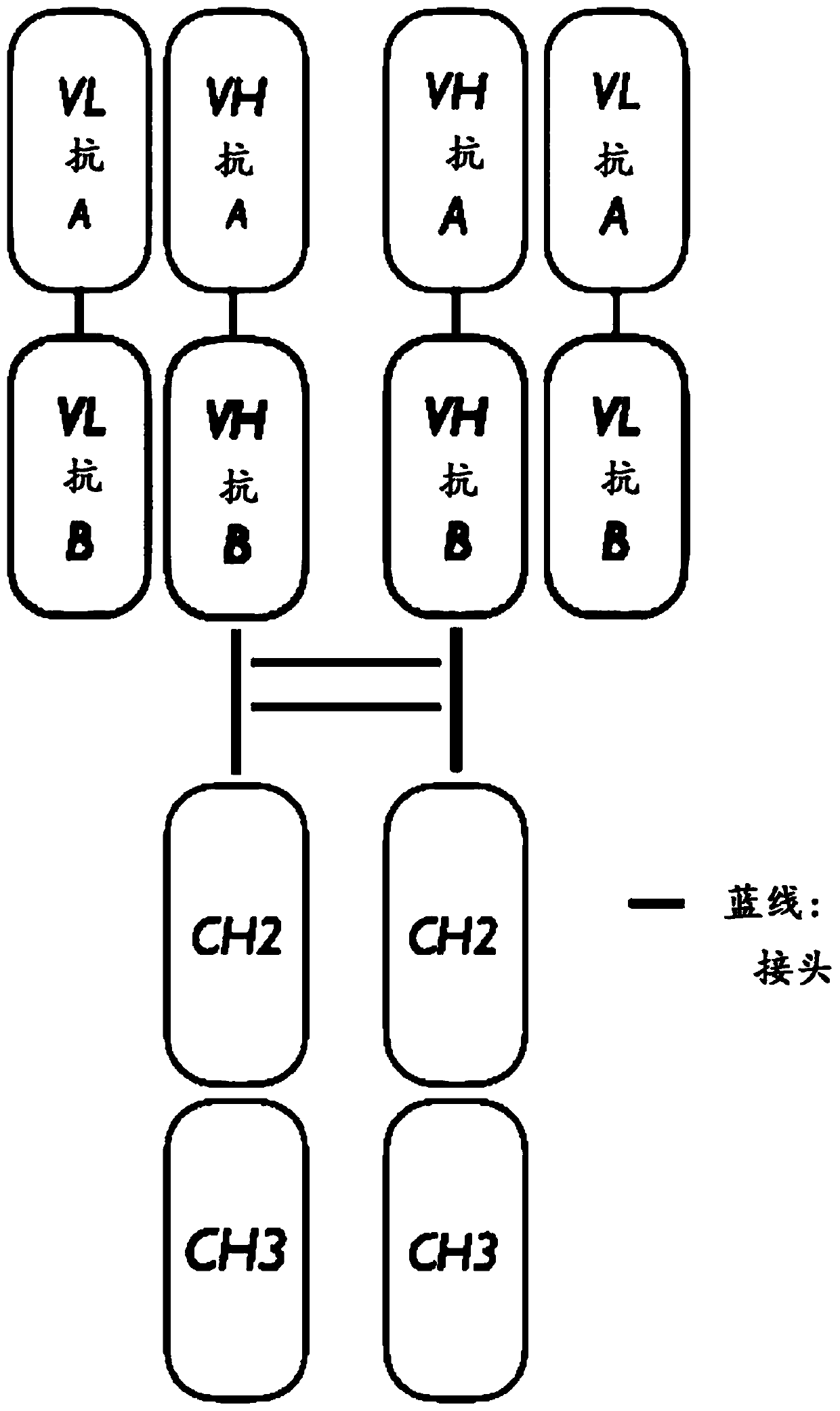
Provided are bispecific antibodies having a full-size antibody portion with two light chains and two heavy chains, wherein the two heavy chains each is fused to a single-chain variable fragment (scFv) portion. In certain embodiments, the full-size antibody has specificity to EGFR and the scFv has specificity to VEGF.
Owner:BIO THERA SOLUTIONS LTD
Agonist antibody to human thrombopoietin receptor
This invention provides an agonist antibody to a human thrombopoietin receptor (alias: human c-Mpl). More particularly, this invention provides an agonist antibody to a human thrombopoietin receptor, wherein the agonist antibody comprises: antibody constant regions comprising (1) amino acid sequences in a heavy chain constant region and a light chain constant region of a human antibody, (2) an amino acid sequence of a heavy chain constant region with a domain substituted between human antibody subclasses, and an amino acid sequence of a light chain constant region of a human antibody, or (3) amino acid sequences comprising a deletion(s), substitution(s), addition(s), or insertion(s) of one or several amino acid residues in the amino acid sequences of (1) or (2) above; and antibody variable regions capable of binding to and activating a human thrombopoietin receptor; and wherein the agonist antibody has the properties: (a) that the antibody induces colony formation at a concentration of 10,000 ng / ml or lower as determined by the CFU-MK colony formation assay using human umbilical-cord-blood-derived CD34+ cells; and (b) that the antibody has a maximal activity at least 50% higher than that of PEG-rHuMGDF and an 50% effective concentration (EC50) of 100 nM or less in the cell proliferation assay using UT7 / TPO cell. Also provided is a pharmaceutical composition for treating thrombocytopenia comprising said antibody.
Owner:KYOWA HAKKO KIRIN CO LTD
Superagonistic anti-CD28 antibodies
The present invention relates to one or more nucleic acid(s) encoding a binding molecule specifically binding to a human CD28 molecule, comprising (a) a nucleic acid sequence encoding a VH region and a nucleic acid sequence encoding a VL region comprising CDRs in a human immunoglobulin framework, wherein (i) the CDRs of the VH region (CDR-H) comprise the amino acid sequences of SEQ ID NOS: 2 or 18 (CDR-H3), 4 or 20 (CDR-H2) and 6 or 22 (CDR-H1) or are encoded by the nucleic acid sequences of SEQ ID NOS: 1 or 17 (CDR-H3), 3 or 19 (CDR-H2) and 5 or 21 (CDR-H1); and (ii) the CDRs of the VL region (CDR-L) comprise the amino acid sequences of SEQ ID NOS: 8 or 24 (CDR-L3), 10 or 26 (CDR-L2) and 12 or 28 (CDR-L1) or are encoded by the nucleic acid sequences of SEQ ID NOS: 7 or 23 (CDR-L3), 9 or 25 (CDR-L2) and 11 or 27 (CDR-L1); and (b) a nucleic acid sequence encoding the constant region of a human IgG1 or IgG4 antibody.
Owner:THERAMAB
Methods of increasing lean tissue mass using OB protein compositions
InactiveUS20050176107A1Increase of lean tissue massQuality improvementObesity gene productsPeptide/protein ingredientsOb ProteinAntibody constant region
The present invention provides methods of creating and using OB protein compositions with an antibody constant region or portion thereof fused to an OB protein. The fusion protein is created by attaching the polyamino acids to the OB protein moiety. The fusion proteins can then be used for various therapeutic uses.
Owner:AMGEN INC
Antibody constant region variants with reduced Fcgammar binding
ActiveUS10435458B2Immunoglobulins against cell receptors/antigens/surface-determinantsImmunological disordersAntibodyAntibody constant region
The present inventors carried out dedicated research to generate antibody constant regions with reduced Fcγ receptor-binding activity by altering amino acid sequences in the antibody constant region. As a result, the present inventors successfully identified novel constant region sequences with reduced Fcγ receptor-binding activity compared to conventional antibody constant regions.
Owner:CHUGAI PHARMA CO LTD
Modified antibody constant region
InactiveCN110114369AAntibody mimetics/scaffoldsAntibody ingredientsAntiendomysial antibodiesFc domain
The present invention relates to a canine IgG Fc domain, the amino acid sequence of which comprising at least one mutation selected among: the substitution of amino acid 15.1 of CH2 domain according to the IMGT numbering system for C-domain with tyrosine; the substitution of amino acid 16 of CH2 domain according to the IMGT numbering system for C-domain with threonine; and the substitution of amino acid 18 of CH2 domain according to the IMGT numbering system for C-domain with glutamic acid. The present invention also relates to an Fc-fusion protein, comprising the canine IgG Fc domain, that isgenetically linked to a peptide or a protein or an engineered ligand-binding proteins or a VHH domain, and to an antibody comprising the canine IgG Fc domain.
Owner:威隆股份公司
Antibody constant region variant
InactiveUS20190211081A1Desirable propertyImprove propertiesImmunoglobulins against cell receptors/antigens/surface-determinantsBiologyDisulfide bond
By altering amino acid sequences, the present inventors successfully produced constant regions that can confer antibodies with particularly favorable properties for pharmaceutical agents. When used to produce antibodies, the altered constant regions produced according to the present invention significantly reduce heterogeneity. Specifically, the antibody homogeneity can be achieved by using antibody heavy chain and light chain constant regions introduced with alterations provided by the present invention. More specifically, the alterations can prevent the loss of homogeneity of antibody molecules due to disulfide bond differences in the heavy chain. Furthermore, in a preferred embodiment, the present invention can improve antibody pharmacokinetics as well as prevent the loss of homogeneity due to C-terminal deletion in antibody constant region.
Owner:CHUGAI PHARMA CO LTD
Antibody variants composition
Among N-glycoside-linked sugar chains which are bound to the Fc region of an antibody, sugar chains which are bound to Asn at position 297 relates to the activity and stability of the antibody in blood, but there is a possibility that extra sugar chains bound to the amino acid residues at positions other than 297 have influences upon the antibody constant region-mediated activity and a possibility of causing a problem of uniformity as a therapeutic antibody preparation. Accordingly, among N-glycoside-linked sugar chains which bind to the Fc region of the antibody, a method for controlling extra sugar chains which are bound to Asn residues at positions other than position 297 according to the EU index is required. The present invention provides an antibody variant composition, comprising amino acid residues of an Asn-X-Ser / Thr (X represents an amino acid residue other than Pro) sequence at positions other than positions 297 to 299 according to the EU index in an Fc region of a human IgG antibody, in which at least one amino acid substitution selected from an amino acid substitution of Asn to other amino acid residue, an amino acid substitution of X to Pro and an amino acid substitution of Ser / Thr to other amino acid residue is carried out, and a fragment of the antibody variant composition.
Owner:KYOWA HAKKO KIRIN CO LTD
Antibody modification method for purifying bispecific antibody
Owner:CHUGAI PHARMA CO LTD
High throughput screening method and use thereof to identify a production platform for a multifunctional binding protein
InactiveUS20110111977A1Improve throughputLow immunogenicityLibrary screeningMicroorganism librariesBiological bodyHigh-Throughput Screening Methods
Methods of identifying and expressing an antibody variant are disclosed wherein the method comprises identifying a binding region in an antibody, fusing the binding region to a plurality of scaffolds of antibody constant regions to obtain antibody fragment variants, expressing the antibody fragment variants in organisms to form constructs and expressing the constructs carried by the organisms to form induced cultures, wherein the organisms are expressed in HTP mode.
Owner:PFENEX
IgG-like long-acting immunological fusion protein and applications thereof
ActiveCN108623691AProlong biological half-lifeHigh affinityPeptide/protein ingredientsMetabolism disorderDiseaseAutoimmune disease
The invention discloses an IgG-like long-acting immunological fusion protein and applications thereof. The IgG-like long-acting immunological fusion protein comprises an effector molecule and an IgG antibody constant region, wherein the effector molecule is linked to the IgG antibody constant region through a linker peptide, the effector molecule is a protein capable of exerting physiological functions in vivo, and the IgG antibody constant region is a structure obtained by removing two heavy chain variable regions and two light chain variable regions from an IgG antibody. According to the present invention, the IgG-like immunological fusion protein can effectively prolong the biological half-life of the protein drug (effector molecule) under the premise of the ensuring of the high affinity to the targeting molecule and the good in vivo activity, is far better than the similar Fc immunological fusion protein, and can be used for the treatment of diabetes, tumors, autoimmune diseases, endocrine and various diseases.
Owner:BEIJING BIYANG BIOTECH
CH3 domain variant pair inducing formation of heterodimer of heavy chain constant region of antibody at high efficiency, method for preparing same, and use thereof
ActiveUS9951145B2High yieldMinimize formationHybrid immunoglobulinsAntibody mimetics/scaffoldsDiseaseProtein target
Disclosed are a CH3 domain variant pair of an antibody, a method for preparing same, and a use thereof. A mutation is induced in the CH3 domain so as to improve a yield of forming a heterodimer heavy chain constant region of an antibody. The CH3 domain heterodimer forms a heterodimer heavy chain constant region with a high efficiency of 90 to 95% or more and also has outstanding heat stability. A heterodimer heavy chain constant region including the CH3 domain heterodimer can construct a bispecific monoclonal antibody which simultaneously recognizes two kinds of antigens. The CH3 domain heterodimer and the bispecific antibody or fusion protein of an antibody constant region comprising same can be usefully applied to the treatment or prevention of a disease associated with a target antigen or a target protein.
Owner:AJOU UNIV IND ACADEMIC COOP FOUND
Methods of modifying antibodies for purification of bispecific antibodies
ActiveUS20170283483A1Efficient purificationFunction increaseImmunoglobulins against cytokines/lymphokines/interferonsImmunological disordersBinding siteBispecific antibody
The present inventors devised methods for efficiently purifying bispecific antibodies using a chromatography column based on the difference in isoelectric points between the H chains of two types of antibodies, wherein the difference is introduced by modifying the amino acids present on the surface of the antibody variable regions of two types of antibodies that constitute a bispecific antibody. Furthermore, the inventors devised methods for efficiently purifying bispecific antibodies using a chromatography column by linking respective antigen binding sites (heavy chain variable regions) to the antibody constant regions having different isoelectric points, and then coexpressing these antibodies.
Owner:CHUGAI PHARMA CO LTD
Novel bispecific antibody and purpose thereof
PendingCN106831995AHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsBispecific antibodyHinge region
The invention provides a novel bispecific human IgG1 antibody, which comprises two kinds of Fc fragments with the same hinge regions, wherein the amino acid sequence of the hinge region is SEQ ID NO:1 or SEQ ID NO:28; the 216th to 230th site sequences in a natural human IgG1 antibody constant region are replaced. The amino acid position of the antibody constant region is determined according to EU numbering. In addition, the invention also provides a purpose of the bispecific antibody.
Owner:BEIJING WISDOMAB BIOTECHNOLOGY CO LTD
Antibody variants having modifications in the constant region
InactiveUS20150368345A1Trend downHighly undesiredIn-vivo radioactive preparationsAntipyreticAntiendomysial antibodiesMedicine
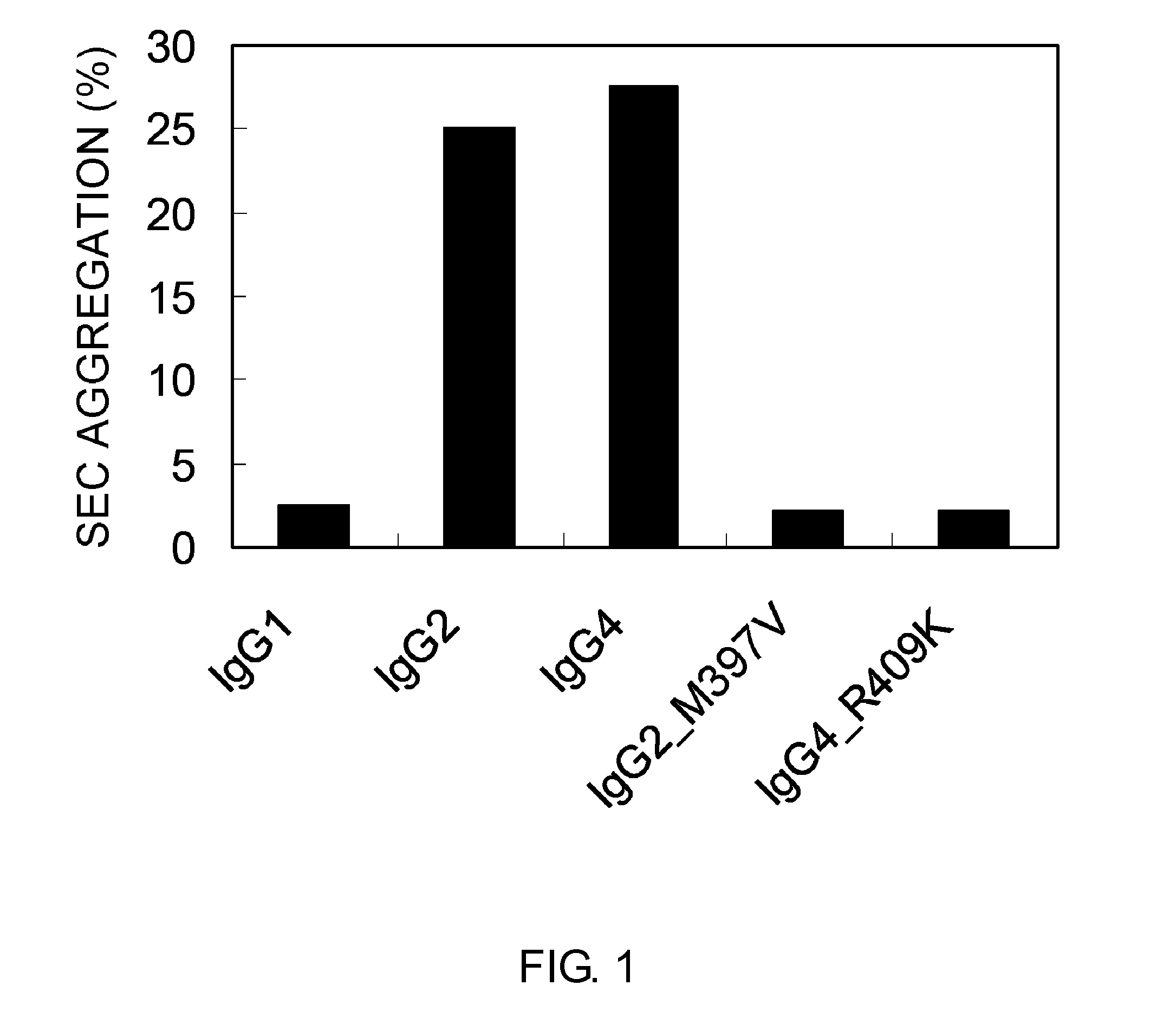
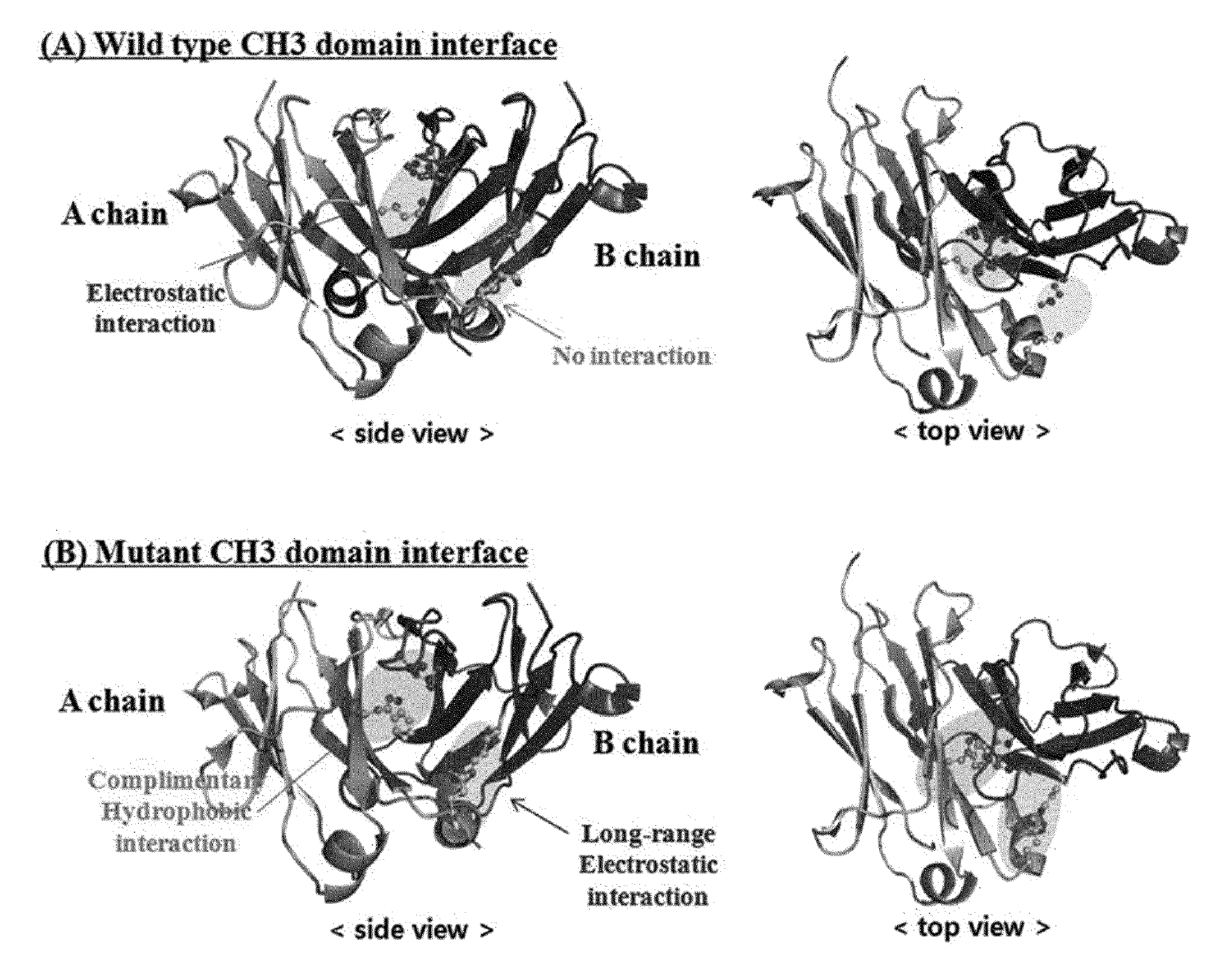
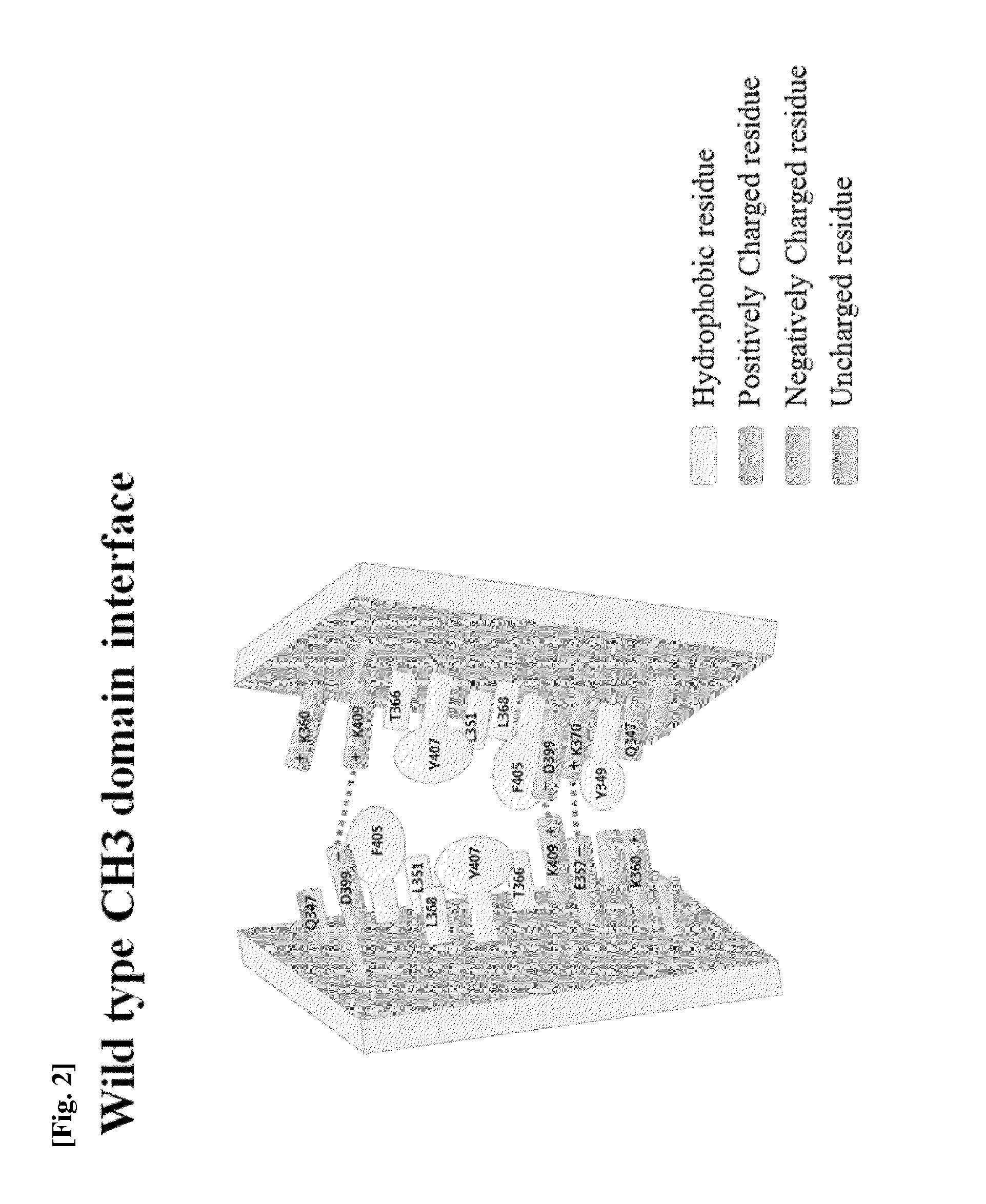
The present invention relates to positions in the constant region of antibodies, in particular the CH3 region of lgG4, which affect the strength of CH3-CH3 interactions. Mutations that either stabilize or destabilize this interaction are disclosed.
Owner:GENMAB AS
IgG1 Fc monomer and application thereof
The invention belongs to the technical field of biology, and particularly relates to an IgG1Fc monomer and a preparation method and application thereof. In accordance with the sequence of a novel IgG1Fc monomer reformed in an antibody IgG1 constant region, through an antibody engineering technique, a human antibody IgG1 constant region Fc is reformed, so that an Fc dipolymer becomes an Fc monomer,an FcRn combination function is maintained, and an extremely low irrelevant protein non-specific combination characteristic is achieved; main features of the Fc monomer lie in that the Fc monomer hasmutation at positions of T366, L368, P395 and K409 in a CH3 region of the antibody constant region, has high-efficiency expression in prokaryotic cells, can use combination of a pH dependent specialcombination mode with FcRn, and has extremely low nonspecific combination characteristics. Through the novel Fc monomer, the IgG1Fc monomer can fuse or couple with various protein, polypeptide, micromolecules, nucleic acid and the like for different target points, so that fused or coupled molecules have the characteristic of combining pH dependence with FcRn.
Owner:SUZHOU FORLONG BIOTECHNOLOGY CO LTD
Host cell lines for production of antibody constant region with enhanced effector function
InactiveCN101627111AReduce or prevent growthHigh activityAntibody ingredientsTissue cultureSerum free mediaChemical composition
Host cell lines for biopharmaceutical production of antibodies, antibody fragments or antibody-derived fusion proteins are selected as having the capability of inducing improved cellular effector functions, e.g., Fc-medicated effector functions. The host cells are derived from the rat myeloma cell line YB2 / 0 and are adapted to growth in chemically-defined medium.
Owner:CENTOCOR
Agonistic antibody directed against human thrombopoietin receptor
The present invention discloses an agonistic antibody directed against human thrombopoietin receptor (also referred to as 'human c-Mpl). Specifically, the antibody has a constant region having a set of amino acid sequences selected from the following items (1) to (3): (1) amino acid sequences for a heavy-chain constant region and a light-chain constant region of a human antibody; (2) an amino acid sequence for a human antibody heavy-chain constant region in which the domain is replaced by one of other human antibody subclass and an amino acid sequence for a human antibody light-chain constant region; and (3) a set of amino acid sequences having the deletion, substitution, addition or insertion of one or several amino acid residues in each of the amino acid sequences shown in (1) and (2), and the antibody has a variable region capable of binding to a human thrombopoietin receptor to activate the receptor. The antibody also has the following properties: (a) the antibody can induce the formation of a colony at a concentration of 10,000 ng / mL or less in the CFU-MK colony formation assay using a human umbilical cord blood CD34+ cell; and (b) the antibody has the maximum activity higher than that of PEG-rHuMGDF by 50% or more and a 50% effective concentration (EC50) of 100 nM or less in the cell growth assay using an UT7 / TPO cell. Also disclosed is a pharmaceutical composition for the treatment of thrombocytopenia, which comprises the antibody.
Owner:KIRIN PHARMA
Novel multi-specific binding proteins
InactiveCN110382549AHybrid immunoglobulinsAntibody mimetics/scaffoldsCell biologyAntibody constant region
The present disclosure relates to a novel multi-specific binding protein, specifically, a novel multi-specific binding protein prepared by supplementing drawbacks of various conventionally disclosed multi-specific binding proteins, for example, a bispecific binding protein, more specifically, a novel multi-specific binding protein including a polypeptide, wherein heavy chain CH1 domain and CL domain of an antibody constant region are not included but a heavy chain variable region and / or a light chain variable region are consecutively linked in the polypeptide.
Owner:Y BIOLOGICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com