Modified antibody constant region
An antibody and domain technology, applied in the direction of antibodies, antibody medical components, antibody mimetics/scaffolds, etc., can solve the problem of difficult identification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] Example 1: Preparation of Mutant Canine IgG Fc Domains
[0128] gene synthesis
[0129] Anti-HIV-1 gp120 neutralizing antibody (Zhou T et al.: "Structural definition of a conserved neutralization epitope on HIV-1 gp120" ( "Structural definition of a conserved neutralizing epitope on HIV-1 gp120"), Nature.2007 Feb 15;445(7129):732-7([10])) for the design of codon-optimized DNA for mammalian expression sequence. For both heavy and light chains, a unique NotI restriction site followed by a consensus Kozak sequence (GCCGCCACC) followed by a signal peptide (MGVPTQLGLLLWLTDARC (SEQ ID NO: 5) for the light chain and DNA of strand MEWSWVFLFFLSVTTGVHS (SEQ ID NO: 6)), B12 variable regions (VH and VL) and unique restriction sites (NheI for VH and BsiWI for VL). The VH and VL constructs delivered in the shuttle vector were digested with restriction enzymes (Not I and NheI for VH; NotI and BsiWI for VL) and ligated into which canine IgG2 (isotype B = Chain B) CH1+hinge+CH2+CH...
Embodiment 2
[0141] Example 2: Measurement of canine mAb-FcRn interaction in vitro
[0142] Canine IgG Fc mutant YTE, comprising substitution of amino acid 15.1 of the CH2 domain with tyrosine, substitution of amino acid 16 of the CH2 domain with threonine, and substitution of amino acid 18 of the CH2 domain with glutamic acid (“YTE variant body"), prepared as in Example 1 above.
[0143] In addition, mutant N114A, which corresponds to the canine IgG Fc domain, whose amino acid sequence comprises substitution of amino acid 114 of the CH3 domain with alanine ("NA mutation"), and mutant N90A N40A N114A, which corresponds to canine An IgG Fc domain of a family whose amino acid sequence comprises a substitution of amino acid 90 of the CH2 domain with alanine, substitution of amino acid 40 of the CH3 domain with alanine, and substitution of amino acid 114 of the CH3 domain with alanine ("AAA" mutation), prepared as in Example 1.
[0144] In the first case the interaction between canine IgG2...
Embodiment 3
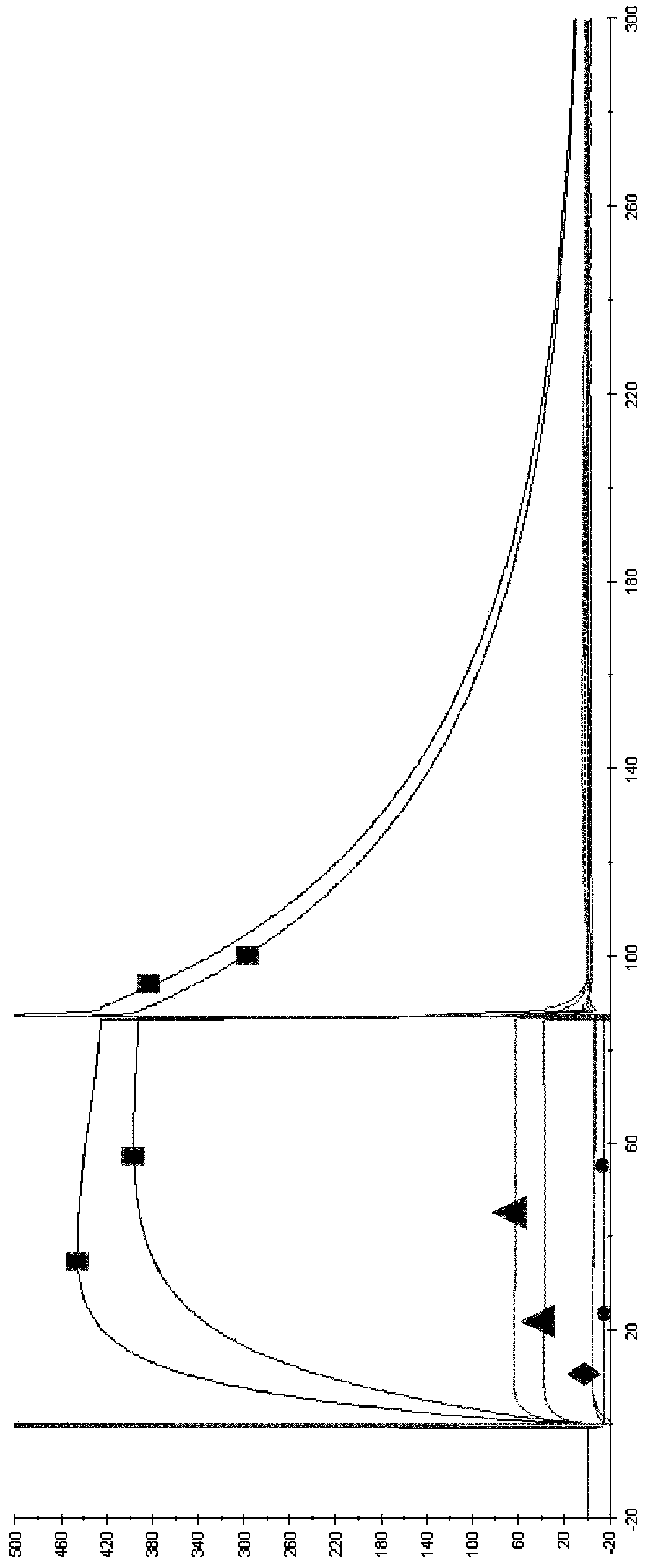
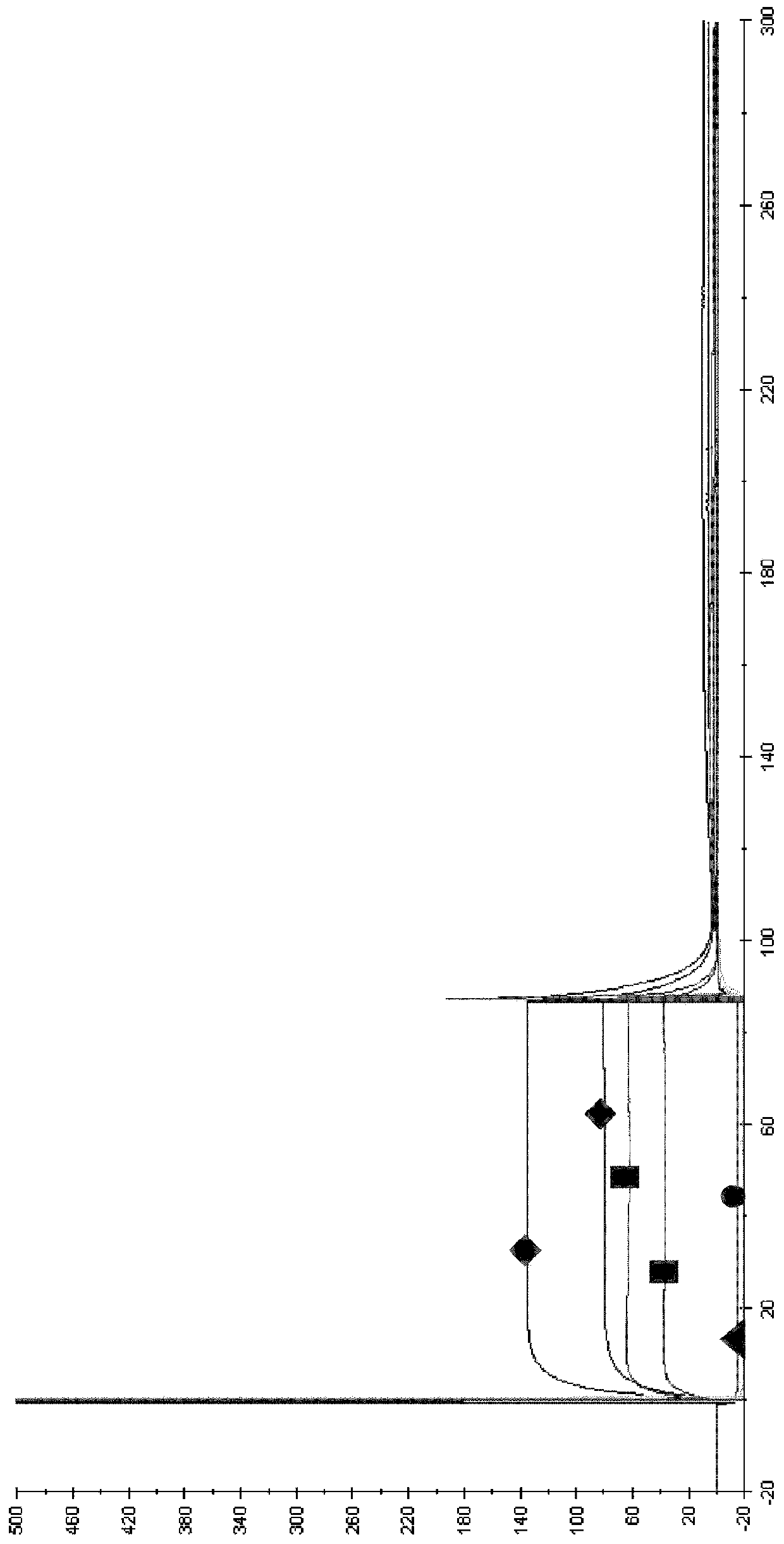
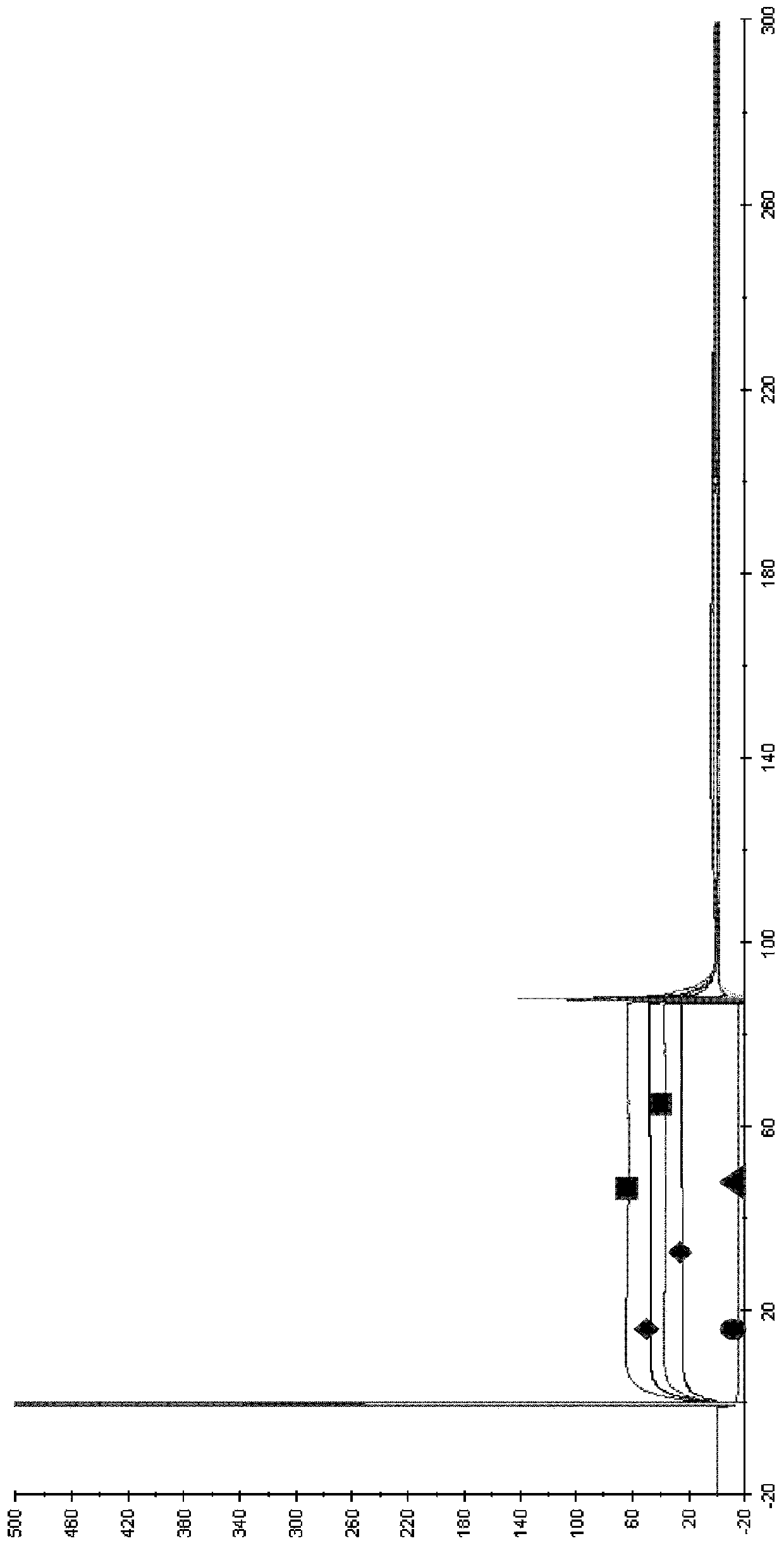
[0156] Example 3: Determination of Canine WT in Dogs Following Intravenous Administration at a Dose of 0.2 mg / kg b12-IgGB and canine Pharmacokinetic Parameters of YTE-b12-igGB Variants in Animals
[0157] The aim of this study was to verify that the elimination half-life of immunoglobulin (Ig) containing a modified Fc fragment is greater than that of wild-type Ig in the family Canidae.
[0158] For this purpose, the plasma pharmacokinetic parameters of these 2 canine IgGs were compared in dogs following intravenous administration at a dose of 0.2 mg / kg:
[0159] Immunoglobulin A: wild type
[0160] Immunoglobulin B: YTE variant
[0161] animal
[0162] Nine male and / or female Beagle dogs weighing 8.0 kg to 17.5 kg at baseline participated in the study. Breed, body weight, sex, date of birth and source of animal are listed in Table 1 below
[0163] Table 1: Characteristics of the animals used in the study
[0164]
[0165] Measurement conditions
[0166] Immu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com