Antibody modification method for purifying bispecific antibody
A bispecific antibody, multispecific antibody technology, applied in chemical instruments and methods, antibodies, cells modified by introducing foreign genetic material, etc., can solve problems such as difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0105] First, the present invention provides antibody modification methods for preparing multispecific antibodies. The preferred version of the preparation method of the present invention comprises modifying both or one of the nucleic acid encoding the amino acid residues of the first polypeptide and the nucleic acid encoding the amino acid residues of the second polypeptide, so that the isoelectric points of the first polypeptide and the second polypeptide method of making a difference. That is, by changing the charge of the amino acid residues of the first polypeptide and the second polypeptide, a difference in isoelectric point (pI) can be introduced into the polypeptides, and a multispecific antibody can be prepared using the difference in isoelectric point. Specifically, it is a preparation method comprising the steps of (a)-(c) below.
[0106] (a) modifying both or one of the nucleic acid encoding the amino acid residues of the first polypeptide and the nucleic acid enc...
Embodiment 1
[0237] [Example 1] Humanization of bispecific antibody with hybrid L chain
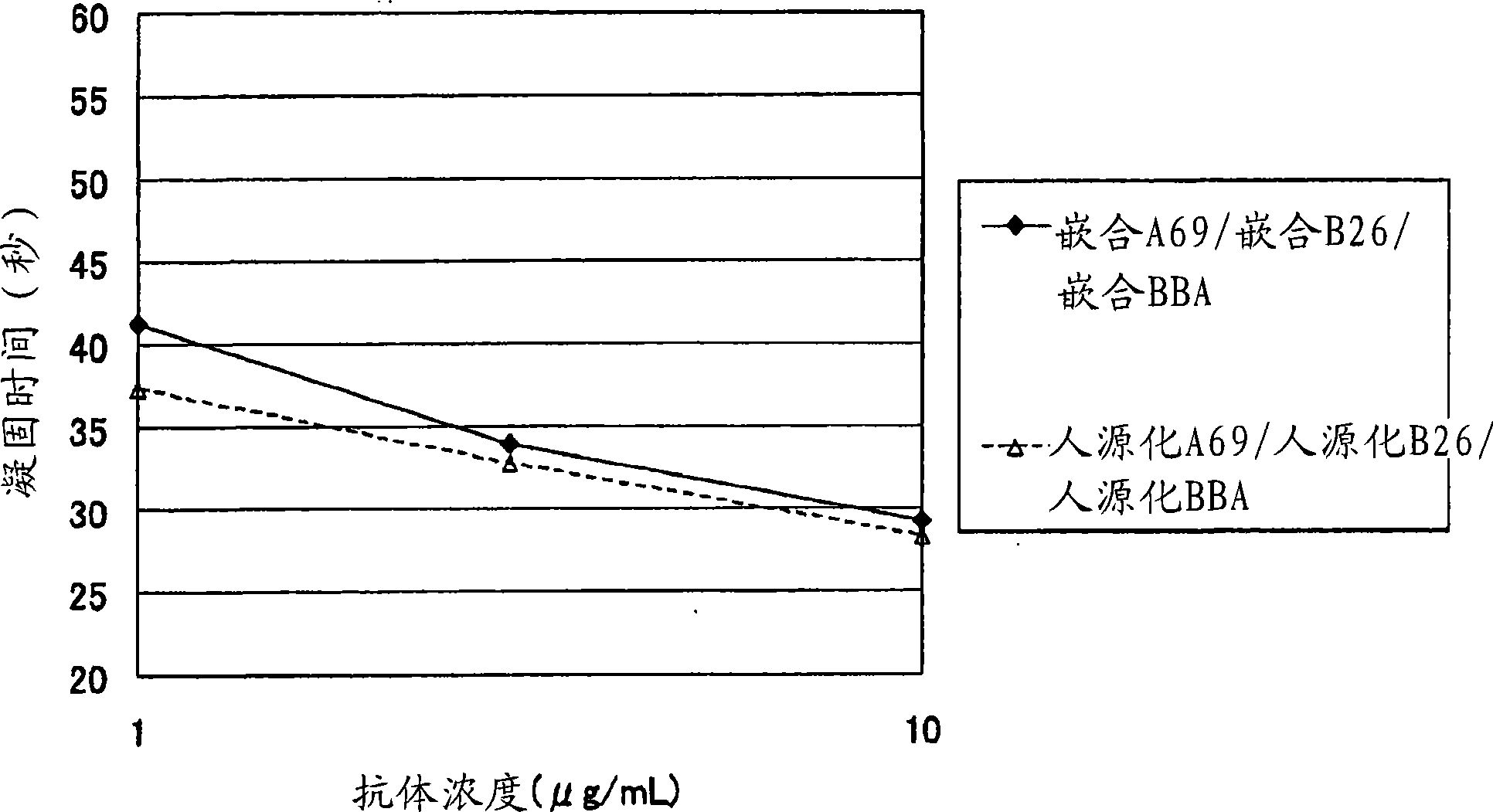
[0238] In Japanese Patent Application No. 2005-112514, a bispecific antibody containing a combination of anti-FactorIXa antibody A69-VH, anti-FactorX antibody B26-VH, and hybrid L chain (BBA) with the highest coagulation time shortening effect was humanized as follows .
[0239] 1-1 Homology search of human antibody
[0240] Human antibody amino acid sequence data were obtained from the generally published Kabat database (ftp: / / ftp.ebi.ac.uk / pub / databases / kabat / ) and the IMGT database (http: / / imgt.cines.fr / ), using the construct The database is divided into mouse A69-H chain variable region (amino acid sequence: SEQ ID NO.19), mouse B26-H chain variable region (amino acid sequence: SEQ ID NO.20), mouse BBA-L chain The variable region (amino acid sequence: SEQ ID NO.21) was searched for homology. As a result, it was confirmed that it has a high homology with the human antibody sequence shown below, ...
Embodiment 2
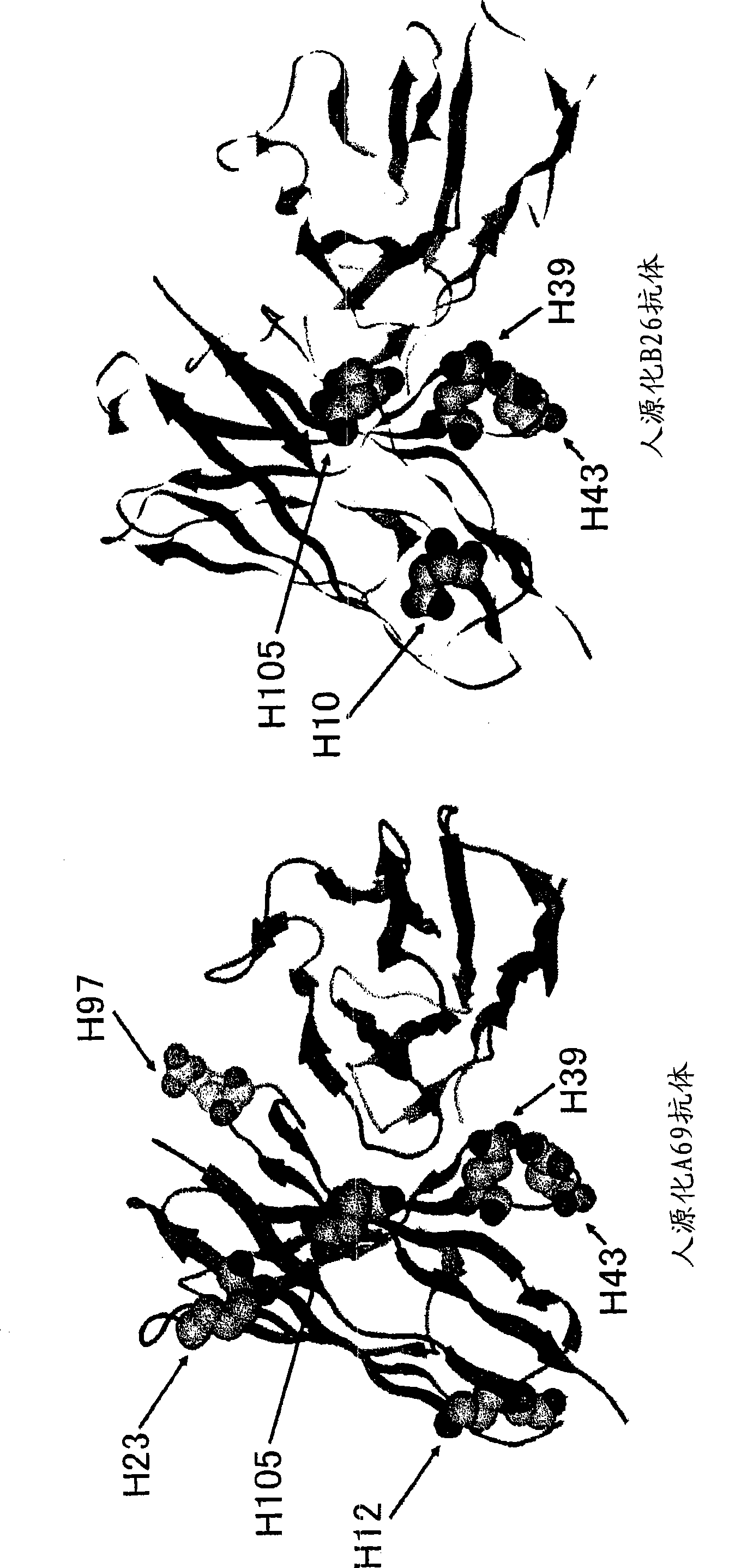
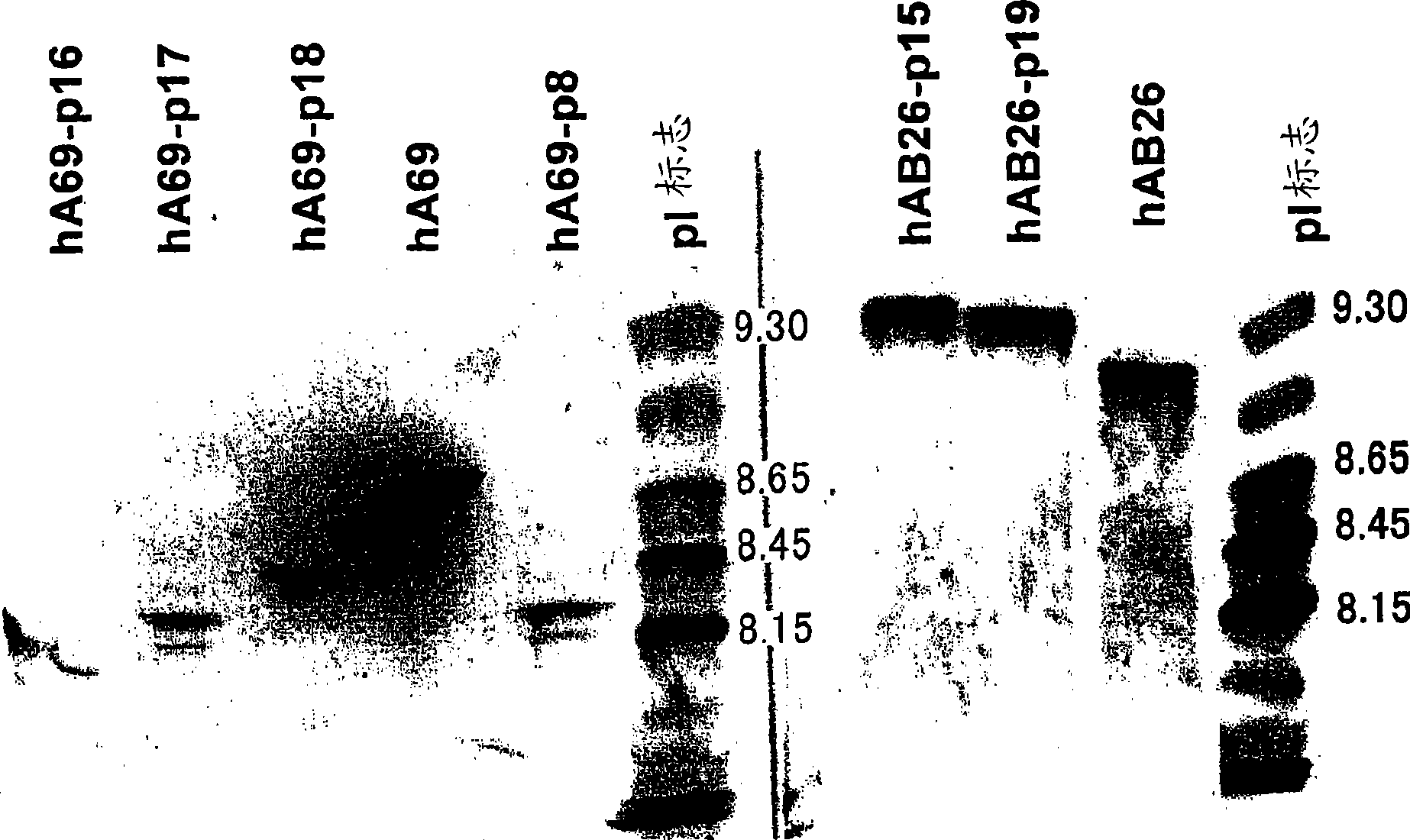
[0273] [Example 2] Determination of amino acid modification positions in the variable region for the isolation of bispecific antibodies In the expression of bispecific antibodies, two types of H chains and one type of L chain were used to humanize the A69-H chain and humanized BBA-L chain homodimer, humanized B26-H chain and humanized BBA-L chain homodimer, humanized A69-H chain and humanized B26-H chain Three antibodies were expressed as heterodimers with humanized BBA-L chains. These three antibodies were separated, and the isoelectric point of the humanized A69H chain variable region was lowered, and the isoelectric point of the humanized B26H chain variable region was raised, and amino acid modifications were performed in order to purify only the bispecific antibody.
[0274] First, in order to confirm the amino acid residues exposed on the surface of the variable regions of the humanized A69 antibody and the humanized B26 antibody, the humanized A69 antibody was prepared ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com