Antibody constant region variant
a constant region and antibody technology, applied in the field of constant region antibodies, can solve the problems of unfavorable binding to the fc receptor, unfavorable method, and posing immunogenicity risks, and achieve the effects of low heterogeneity, high heterogeneity, and reduced antibody heterogeneity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
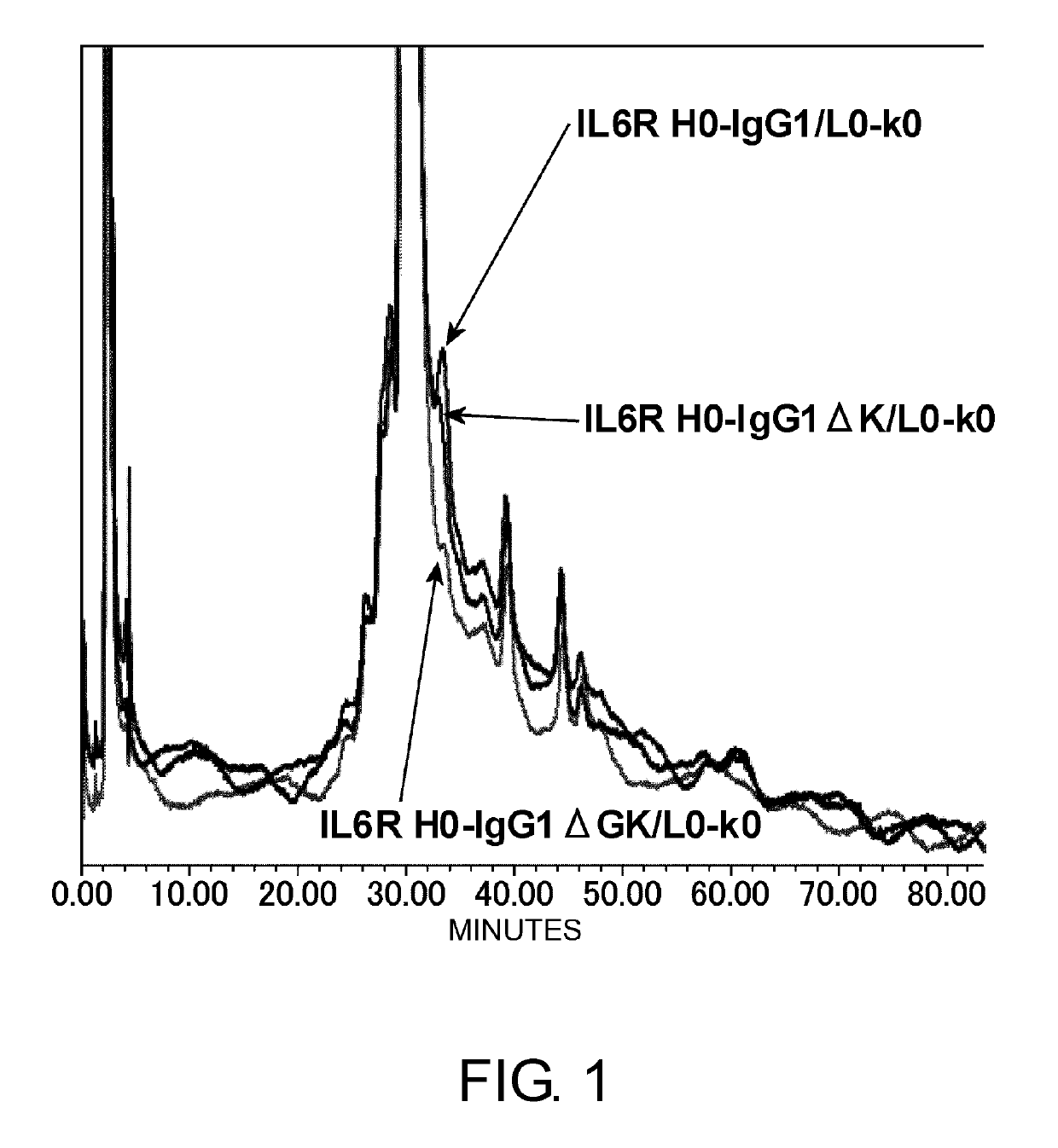
[Example 1] Improvement of C-Terminal Heterogeneities of IgG Molecules
[0366]Construction of an expression vector for H-chain C-terminal ΔGK antibody
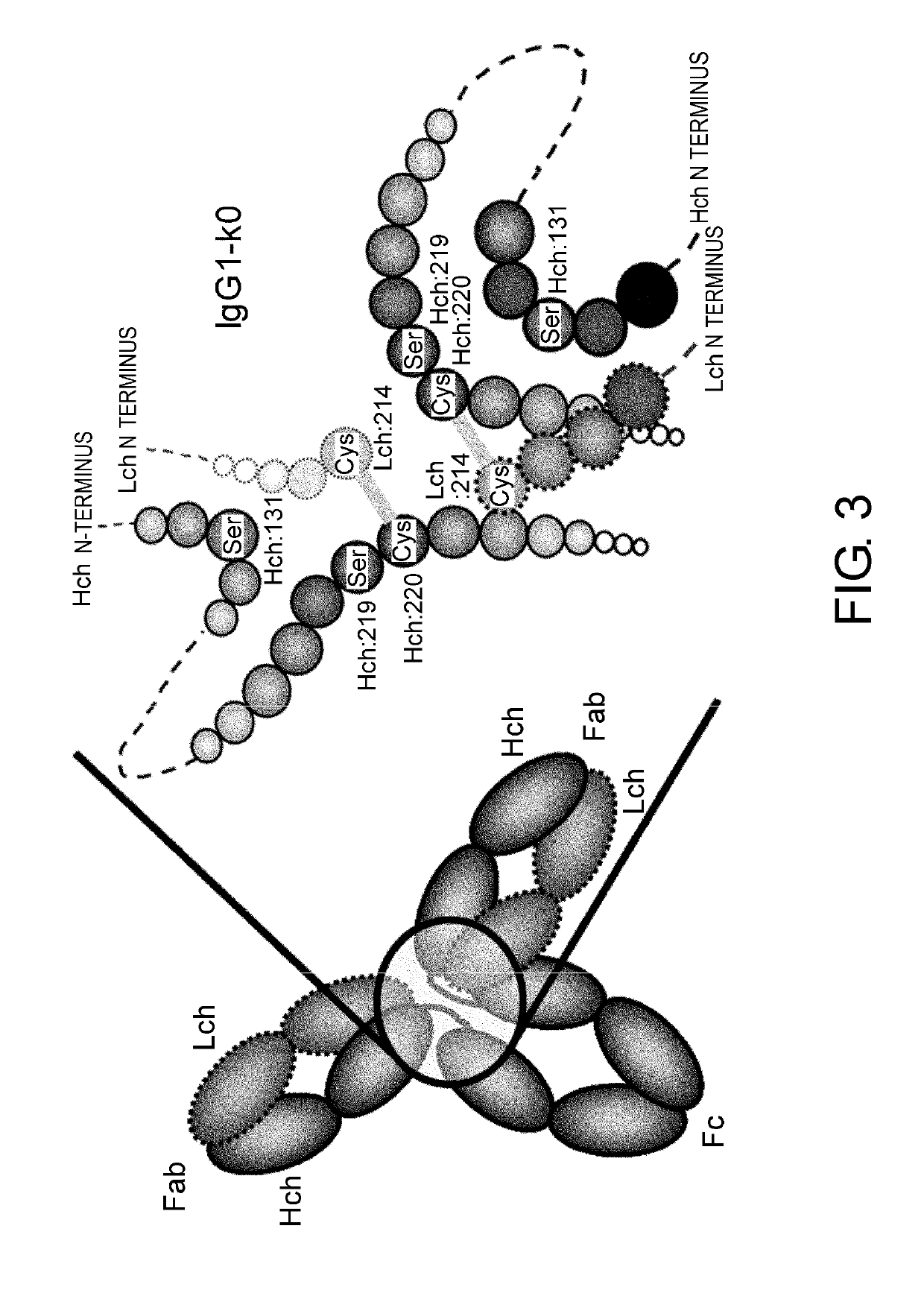
[0367]Heterogeneities of the C-terminal sequence of the IgG antibody H chain that have been reported are deletion of the C-terminal amino acid lysine residue, and amidation of the C-terminal carboxyl group due to deletion of both of the two C-terminal amino acids, glycine and lysine residues (Anal Biochem. 2007 Jan. 1; 360(1): 75-83). In TOCILIZUMAB which is an anti-IL-6 receptor antibody, the main component is a sequence in which the C-terminal amino acid lysine present on the nucleotide sequence is deleted by post-translational modification, but an accessory component with remnant lysine and an accessory component with an amidated C-terminal carboxyl group produced by deletion of both glycine and lysine are also present as heterogeneities. It is not easy to manufacture such an antibody as a pharmaceutical in a large scale, while mainta...
example 2
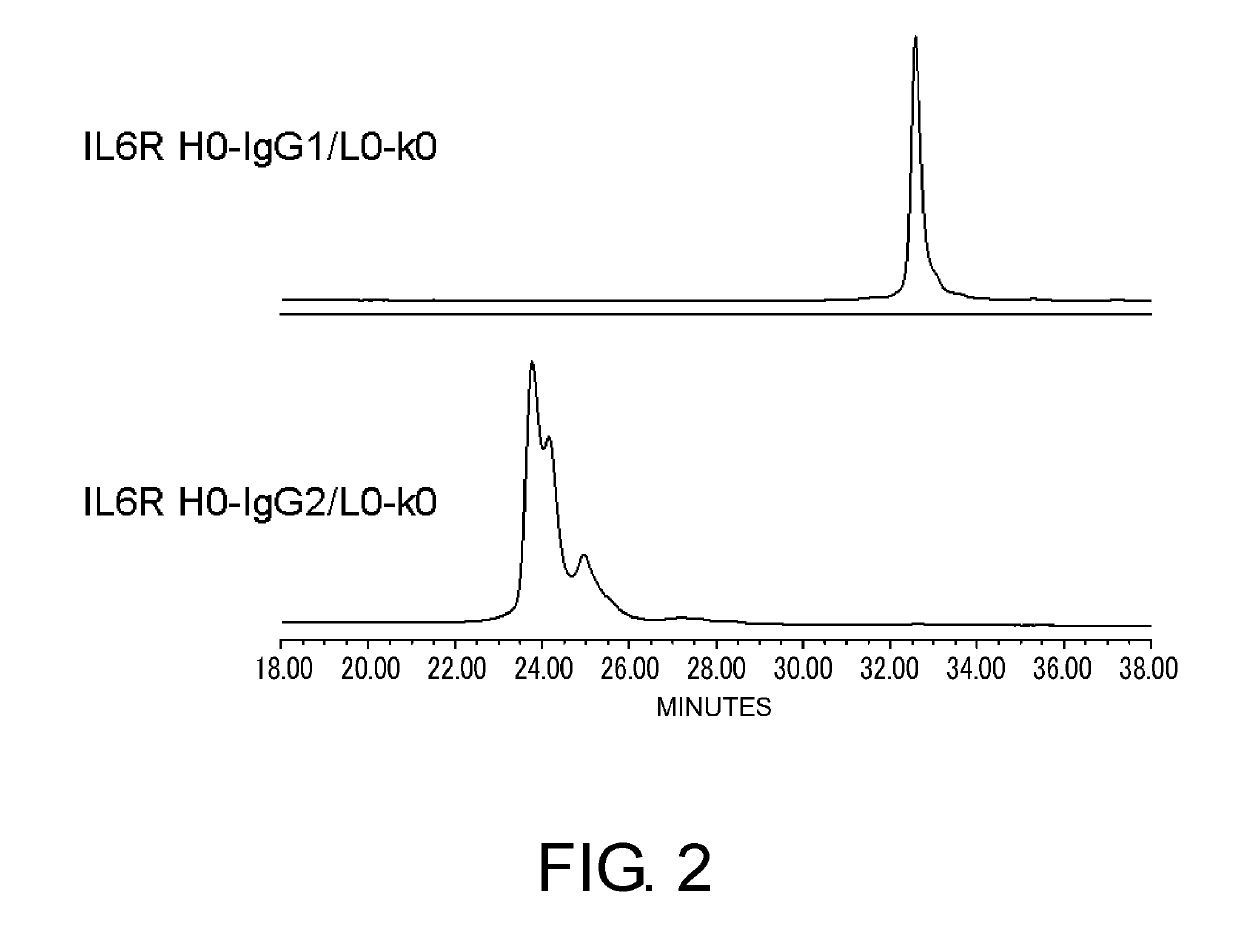
[Example 2] Novel Constant Regions with Reduced Heterogeneity, which Retain the Stability of Natural IgG2
Heterogeneity of Natural IgG1 and Natural IgG2
[0373]For antibody pharmaceuticals against cancer such as those that kill target cells with effector functions and such, IgG1 constant region (isotype) having effector function is preferred. However, for antibody pharmaceuticals that neutralize the functions of a target antigen or antibody pharmaceuticals that bind to target cells but do not kill them, binding to Fcγ receptors is not preferred.
[0374]As methods for decreasing the binding to Fcγ receptors, the method of changing the IgG antibody isotype from IgG1 to IgG2 or IgG4 has been considered (Ann Hematol. 1998 June; 76(6): 231-48), and from the viewpoint of binding to Fcγ receptor I and pharmacokinetics of each isotype, IgG2 was considered to be more desirable than IgG4 (Nat Biotechnol. 2007 December; 25(12): 1369-72). On the other hand, when developing antibodies into pharmaceut...
example 3
[Example 3] Pharmacokinetics-Improving Effect of Novel Constant Region M58-k0 Pharmacokinetics of IgG-Type Antibodies
[0394]The prolonged retention (slow elimination) of IgG molecule in plasma is known to be due to the function of FcRn which is known as a salvage receptor of IgG molecule (Nat. Rev. Immunol. 2007 September; 7(9): 715-25). When taken up into endosomes via pinocytosis, IgG molecules bind to FcRn expressed in endosomes under the acidic conditions within the endosomes (approx. pH 6.0). While IgG molecules that do not bind to FcRn are transferred and degraded in lysosomes, those bound to FcRn are translocated to the cell surface and then released from FcRn back into plasma again under the neutral conditions in the plasma (approx. pH 7.4).
[0395]Known IgG-type antibodies include the IgG1, IgG2, IgG3, and IgG4 isotypes. The plasma half-lives of these isotypes in human are reported to be about 36 days for IgG1 and IgG2; about 29 days for IgG3; and 16 days for IgG4 (Nat. Biotec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com