Anti-PD-1 humanized monoclonal antibody and application thereof
A monoclonal antibody, PD-1 technology, applied in the field of molecular immunology, can solve the problem of low affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1PD-1 antigen
[0033] The cDNA of human PD-1 was synthesized from Nanjing GenScript Company, the GeneID is 5133, and the cDNAID is NM_005018.2. Human IgG1Fc tag was added after the synthetic extracellular region PD-1 gene, and two restriction enzyme sites of XbaI and BamHI were introduced at both ends to connect to the pTT5 expression plasmid, which was verified to be correct by sequencing. The sequenced plasmid was transfected into Trans10 (purchased from Beijing Quanshijin Biotechnology Co., Ltd.), and a single clone was picked and inoculated into 1 liter of LB liquid medium until OD 600 When the temperature is 1, the bacteria are collected by centrifugation, and the plasmid is extracted with a large plasmid extraction kit (purchased from Qiagen).
[0034] The correct expression vector identified by sequencing was transfected into 293F cells (purchased from Invitrogen), cultured at 37°C, 5% CO2, and 130 rpm / min for 7 days, and the super...
Embodiment 2
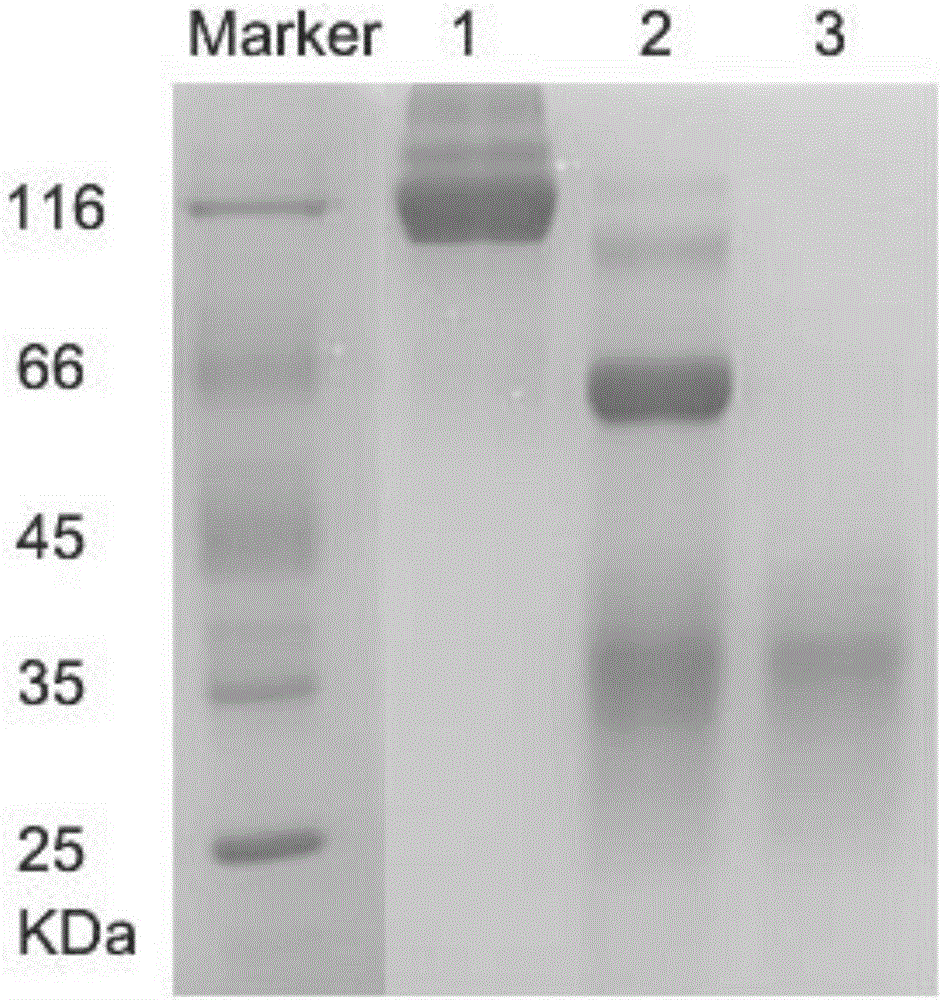
[0035] Example 2 Expression and purification of PD-1 antibody AbB7 / AbB8
[0036] The cDNA of the light chain of the artificially synthesized antibody AbB7 (its sequence is shown in SEQ ID NO.1), the cDNA of the artificially synthesized antibody AbB7 heavy chain (its sequence is shown in SEQ ID NO.3), the cDNA of the artificially synthesized antibody AbB8 heavy chain (its sequence As shown in SEQ ID NO.5), the synthesized cDNAs were respectively cloned into pTT5 plasmids, and the correct construction of the plasmids was confirmed by sequencing.
[0037] Co-transfect the correct AbB7 / AbB8 heavy chain expression vector and light chain expression vector (1:1) identified by sequencing into 293F cells at 37 degrees, 5% CO 2After culturing at 130rpm / min for 7 days, the supernatant was collected by centrifugation. Centrifuge the supernatant at 4000rpm for 10min, and filter it with a 0.45μm filter membrane to collect the filtrate; add 400mM NaCl to the filtrate; adjust the pH to 8.0. ...
Embodiment 3
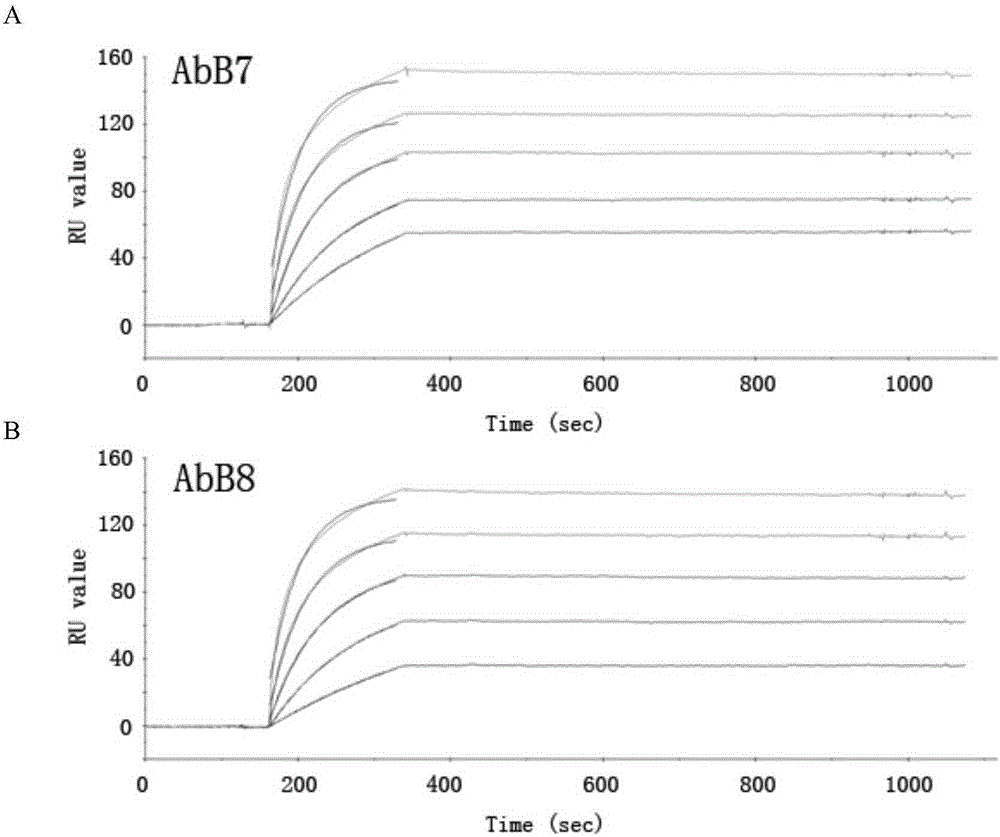
[0038] Example 3 Determination of affinity Kd of PD-1 humanized antibody AbB7 / AbB8 by SPR method
[0039] The characterization affinity and binding kinetics of anti-PD-1 antibodies AbB7 and / AbB8 were analyzed by Biacore3000 instrument (purchased from GE). The recombinant human PD1 extracellular region protein was covalently coupled with biotin using a biotin-labeling kit (Pierce), and then flowed through an avidin-labeled SA chip (purchased from GE), so that the reaction value RU reached about 450 . Binding was measured by flowing antibodies in PBS buffer at concentrations of 0.0133, 0.0266, 0.0532, 0.1064, 0.2128 μM and a flow rate of 50 μl / min. Antigen-antibody binding kinetics were followed for 3 minutes and dissociation kinetics were followed for 10 minutes. Association and dissociation curves were fitted to a 1:1 Langmuir binding model using BIAevaluation software. In order to minimize the role of avidity in estimating the binding constant, only the initial data segmen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com