Combination of Anti-PD-1 Antibodies and Anti-CD20/Anti-CD3 Antibodies to Treat Cancer
a technology of anti-cd20/anti-cd3 and anti-pd-1, which is applied in the field of cancer treatment, can solve the problems of less than 50% chance of relapse-free survival, poor prognosis of aggressive lymphomas, and not all patients respond, so as to inhibit the growth of cancer, improve the treatment effect, and improve the symptom or indication of at least one symptom
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
fficacy of Anti-PD-1 Antibody in Combination with Anti-CD20×CD3 Bispecific Antibody Against B16_CD20 Tumors
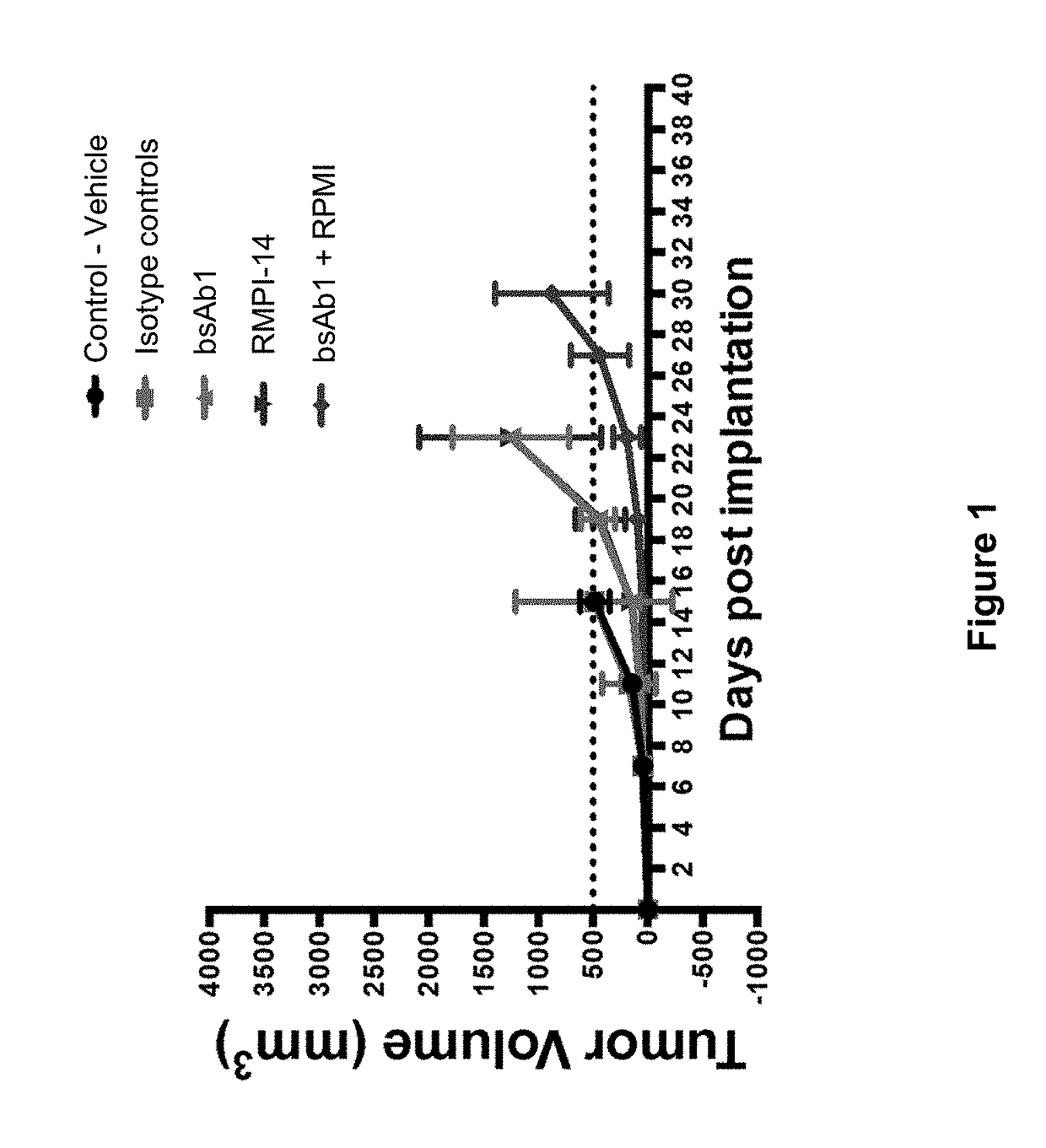
[0098]In this Example, the effect of PD-1 blockade in combination with CD20-targeted immunotherapy was examined against established B16_CD20 tumors in mice humanized for CD20 and CD3 using anti-mouse PD-1 and anti-human CD20×CD3 bispecific antibodies.
[0099]The exemplary bispecific anti-CD20 / anti-CD3 antibody used in the Examples herein is “bsAb1” (also known as “Antibody 1” as disclosed in US20150266966), a fully human bispecific monoclonal antibody against CD20 and CD3 wherein the antibody comprises an anti-CD20 binding arm comprising a first heavy chain variable region (A-HCVR) comprising the amino acid sequence of SEQ ID NO: 11 and a light chain variable region (LCVR) comprising the amino acid sequence of SEQ ID NO: 12; and an anti-CD3 binding arm comprising a second heavy chain variable region (B-HCVR) comprising the amino acid sequence of SEQ ID NO: 13 and a LCVR comprisin...
example 2
fficacy of Anti-PD-1 Antibody and Anti-CD20×CD3 Bispecific Antibody Using a Sequential Dosing Regimen
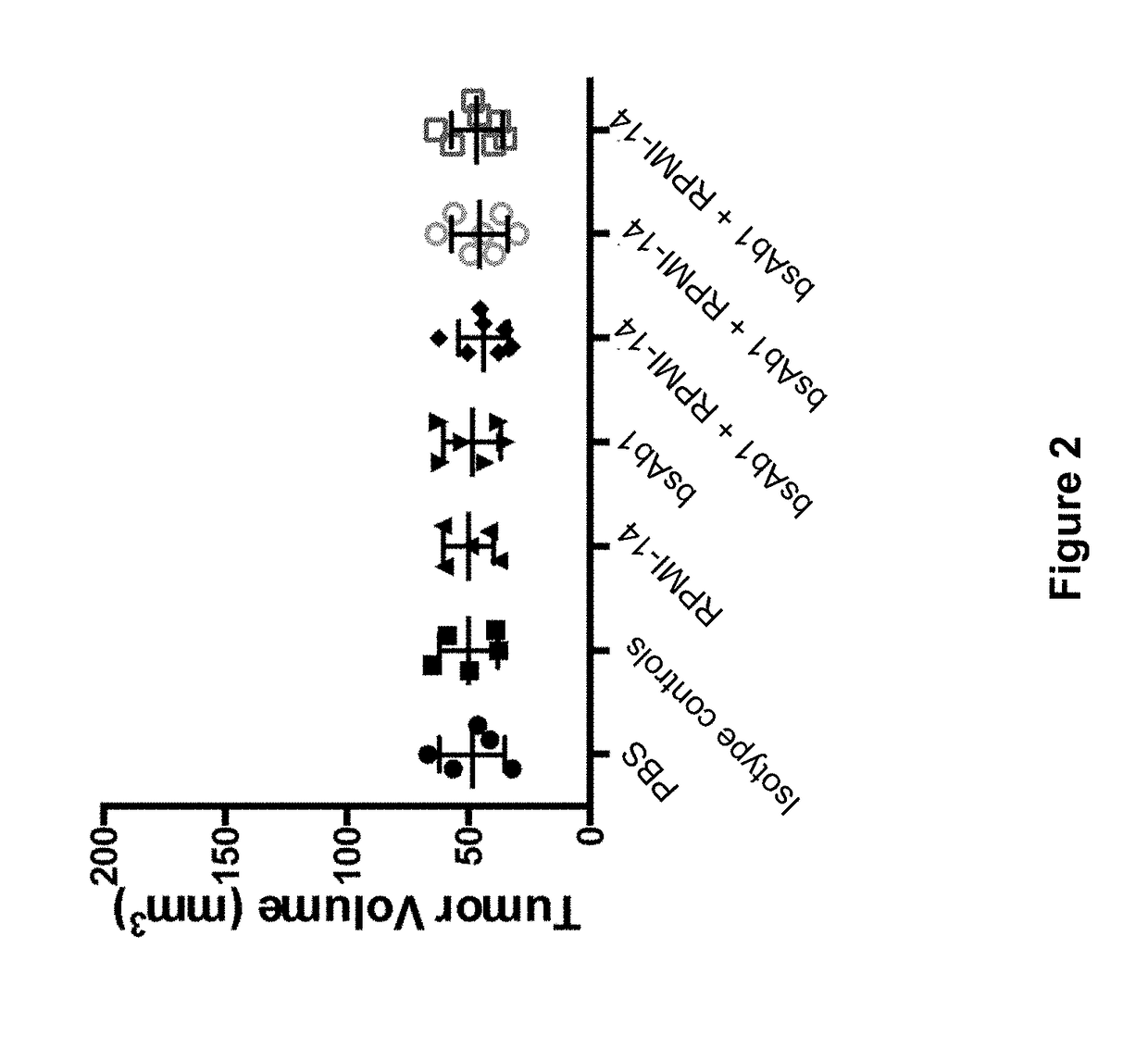
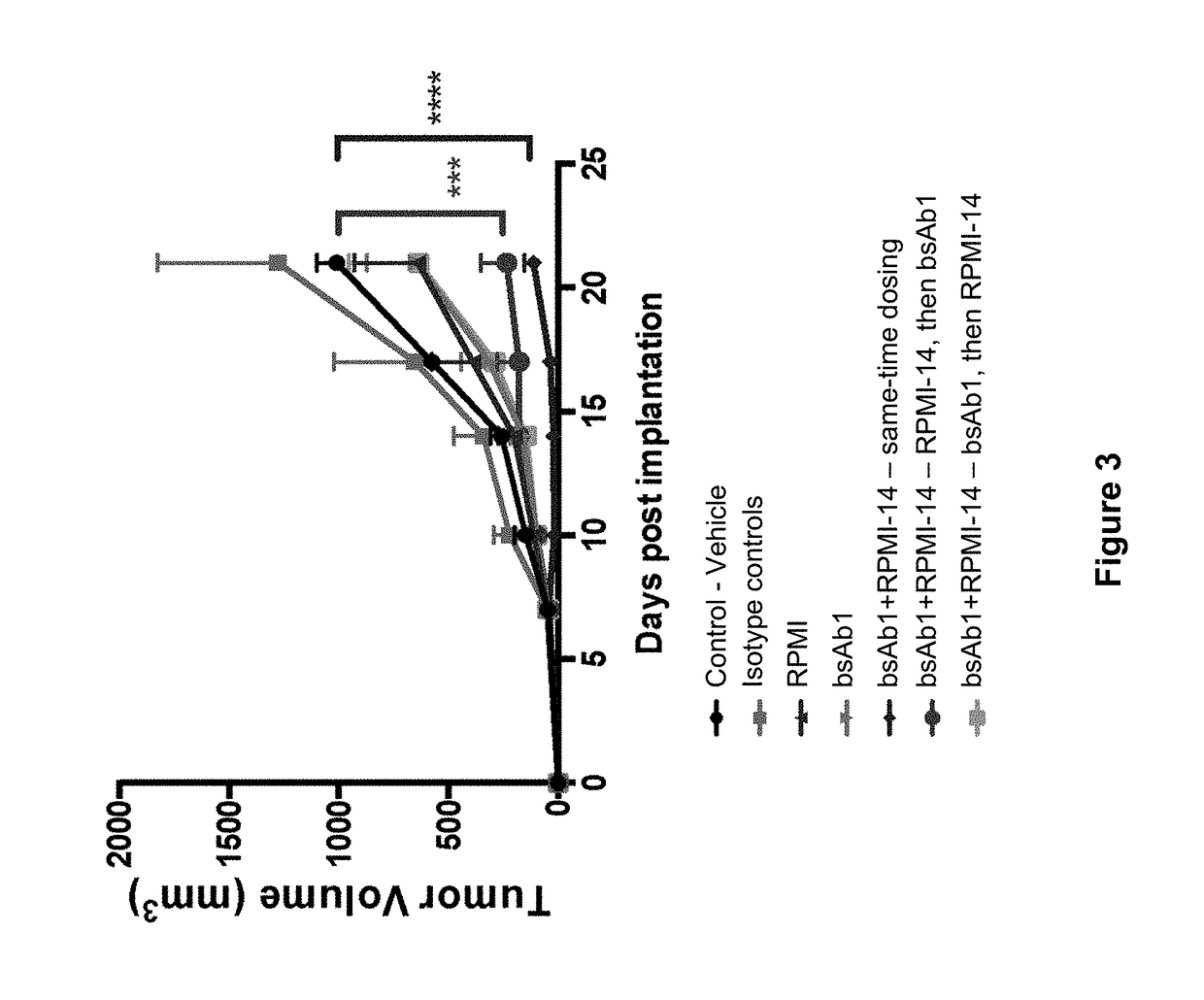
[0105]In this Example, the anti-tumor effect of anti-mouse PD-1 antibody in combination with anti-human CD20×CD3 bispecific antibody was examined using a sequential dosing regimen.
[0106]Double humanized CD20 CD3 mice were engineered using VelociGene® technology (Valenzuela et al 2003, Nat. Biotechnol. 21: 652-659; U.S. patent application Ser. No. 14 / 949,834, filed on Nov. 23, 2015).
[0107]Mice were subcutaneously implanted with 2.5×105 B16_CD20 cells on day −7. On day 0, thirty-five mice with tumor volumes between 30 and 65 mm3 were selected and randomized into seven treatment groups (FIG. 2). Tumor growth was measured by calipers and body weights recorded twice a week for the entire length of the study. Mice were treated twice a week intraperitoneally with antibodies as follows: Group 1: control (PBS); Group 2: Isotype controls (a bivalent human antibody against an irrelevant antigen...
example 3
Trial of Anti-PD-1 Antibody and Anti-CD20×CD3 Antibody in Patients with B-Cell Malignancies
[0109]This study is an open-label, multicenter, dose escalation study with multiple dose escalation and expansion arms to investigate the efficacy, safety, and tolerability of anti-PD-1 antibody and anti-CD20 / anti-CD3 bispecific antibody, alone and in combination, in adult patients with B-cell malignancies (including B-cell non-Hodgkin lymphoma, Hodgkin's lymphoma, and acute lymphoblastic leukemia).
[0110]The exemplary anti-PD-1 antibody used in this Example is REGN2810 (also known as H4H7798N as disclosed in US20150203579), a fully human monoclonal anti-PD-1 antibody comprising a heavy chain comprising the amino acid sequence of SEQ ID NO: 9 and a light chain comprising the amino acid sequence of SEQ ID NO: 10; an HCVR / LCVR amino acid sequence pair comprising SEQ ID NOs: 1 / 2; and heavy and light chain CDR sequences comprising SEQ ID NOs: 3-8.
[0111]The exemplary bispecific anti-CD20 / anti-CD3 an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com