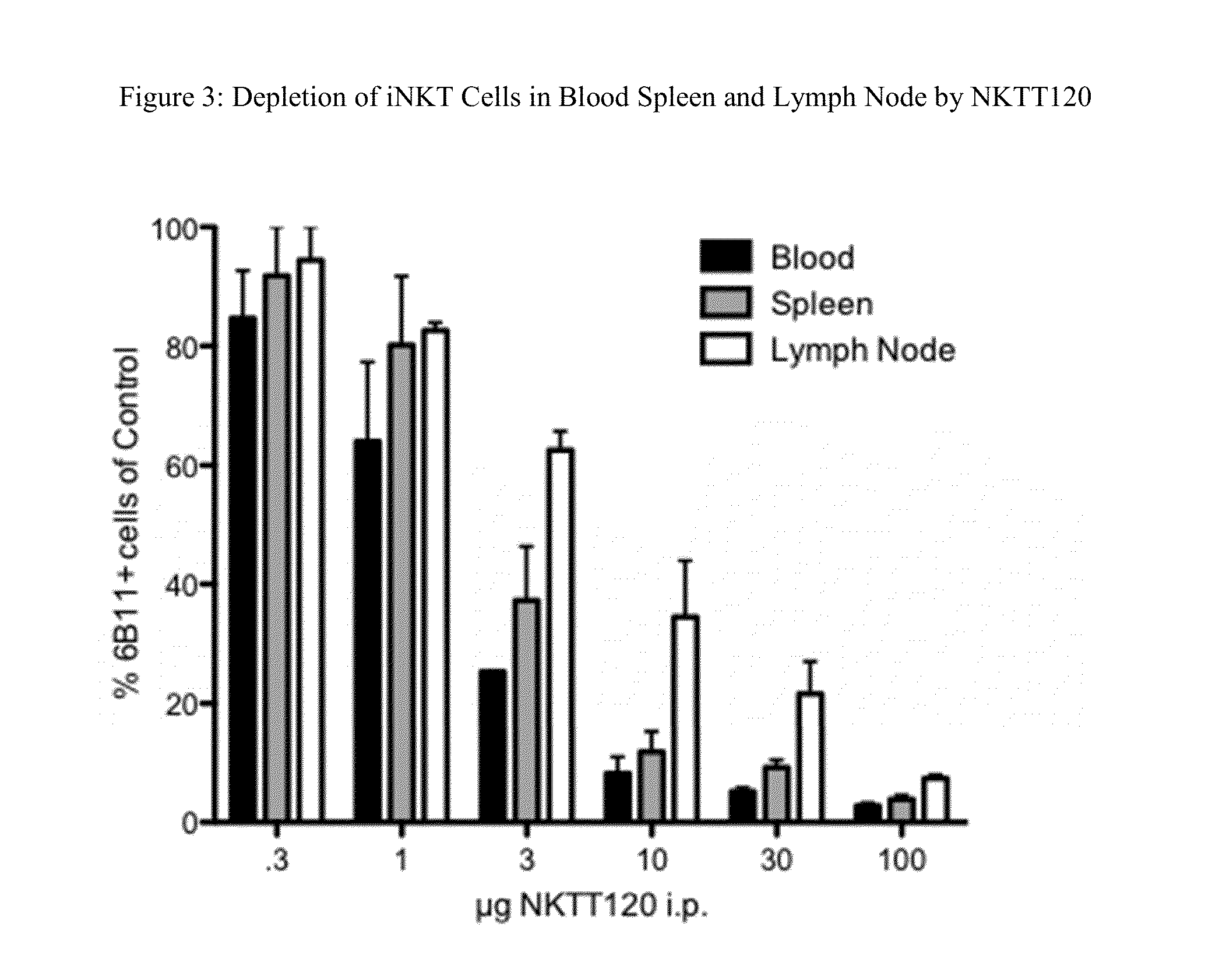
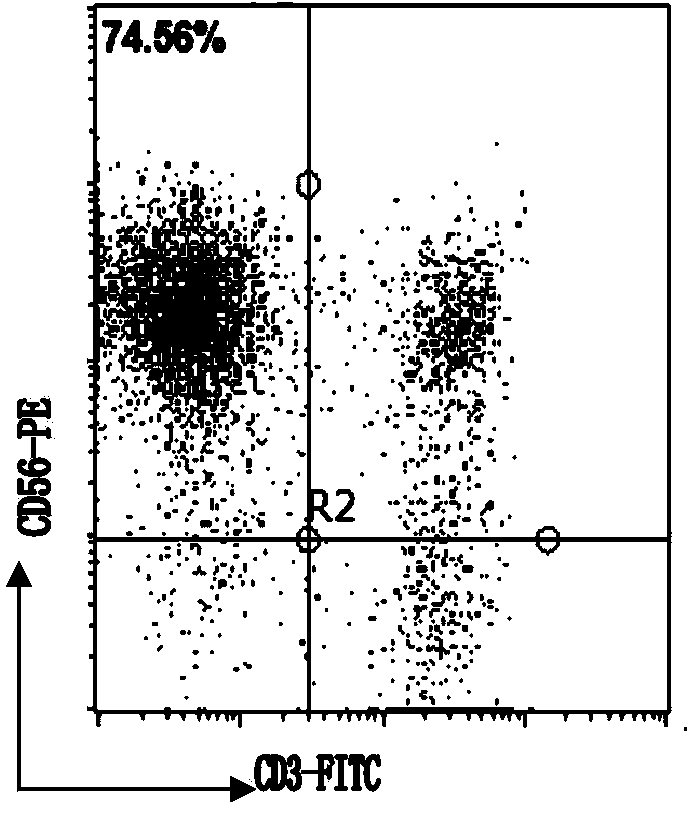
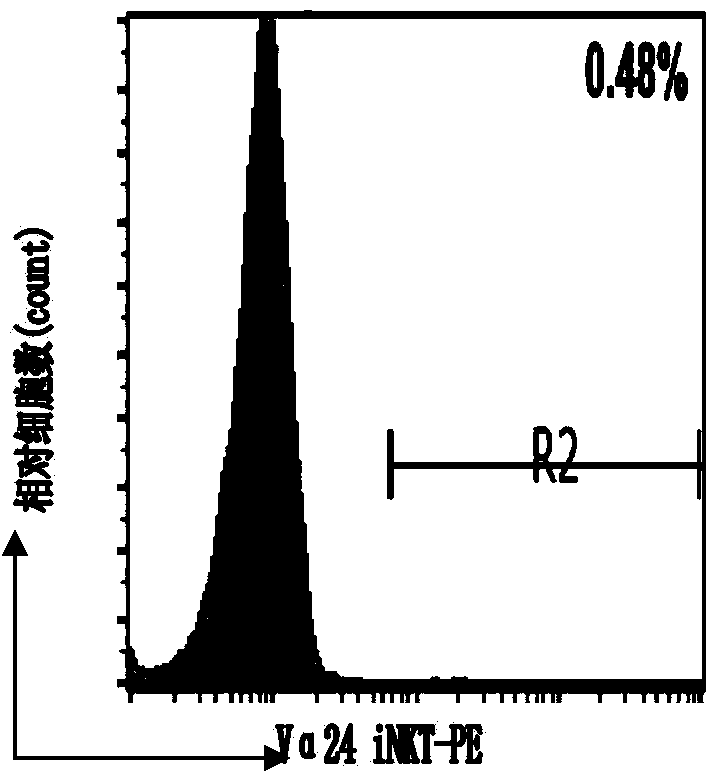
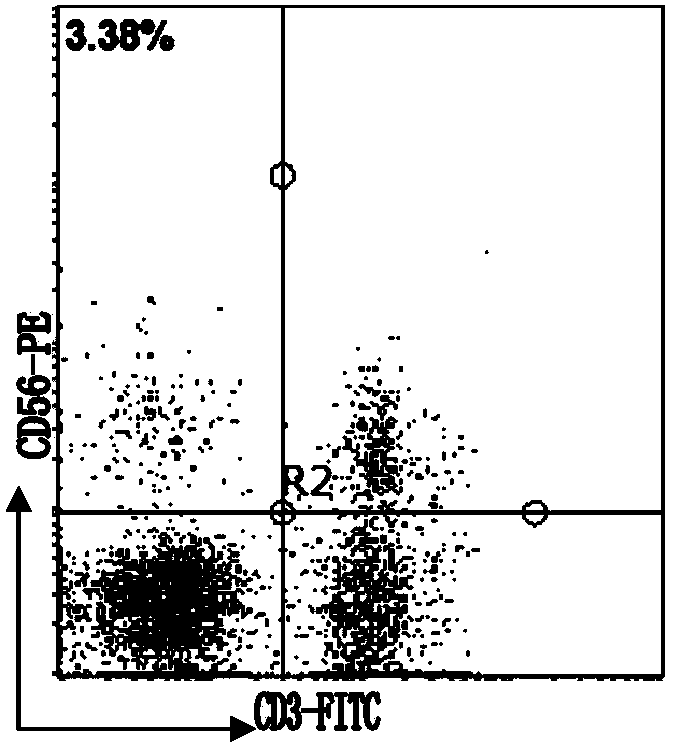
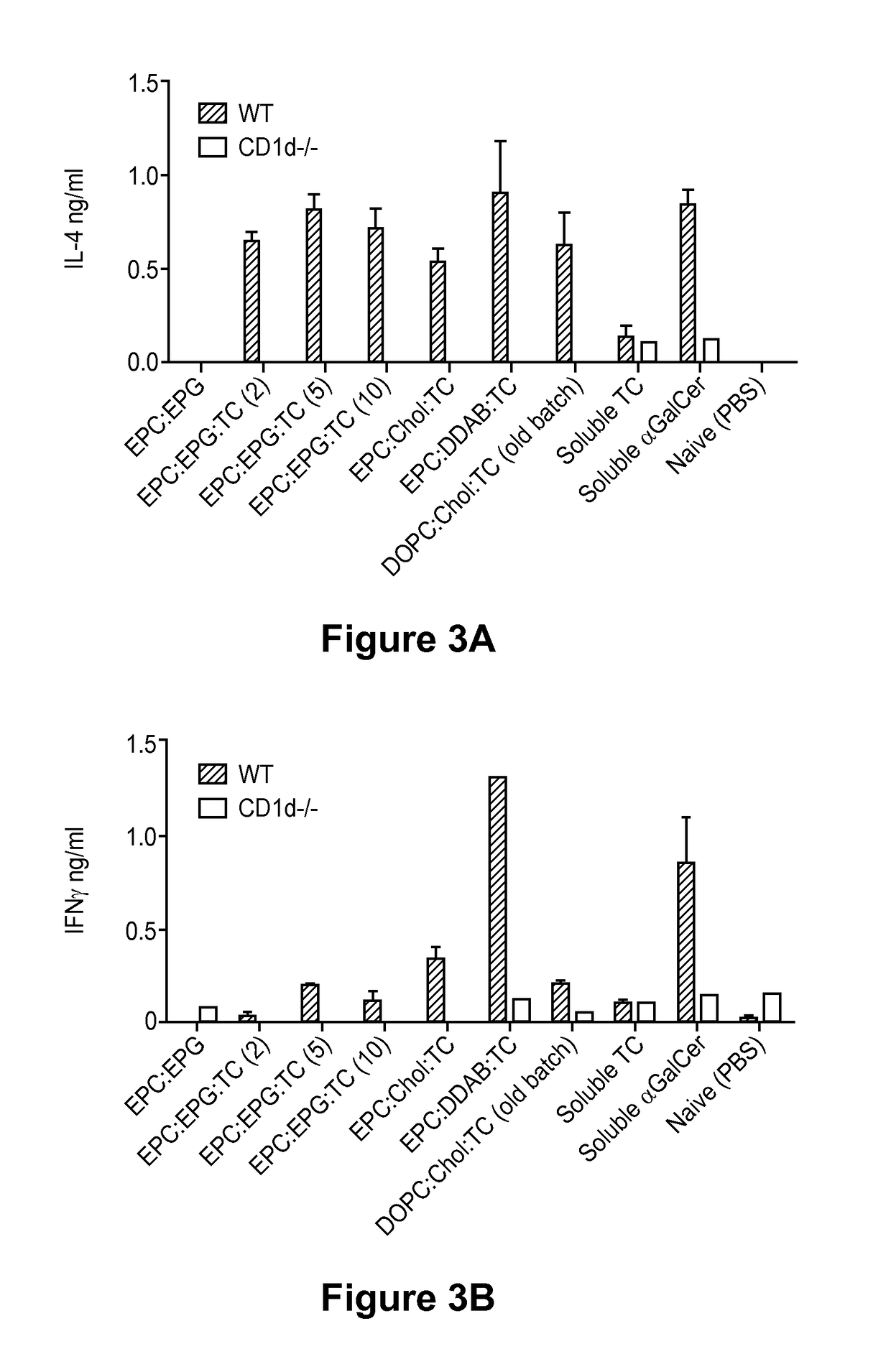
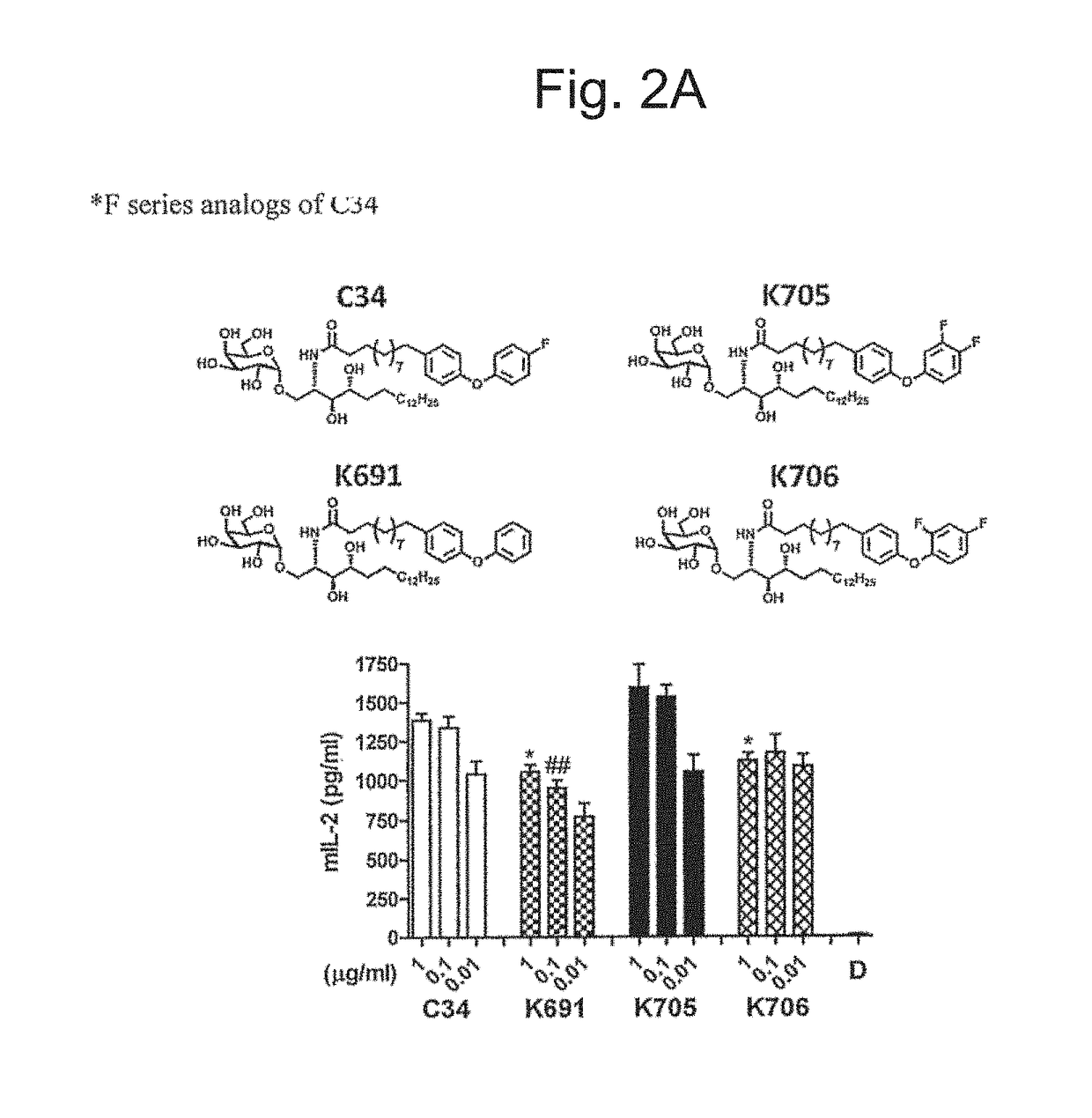
Patents
Literature
44 results about "INKT Cells" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
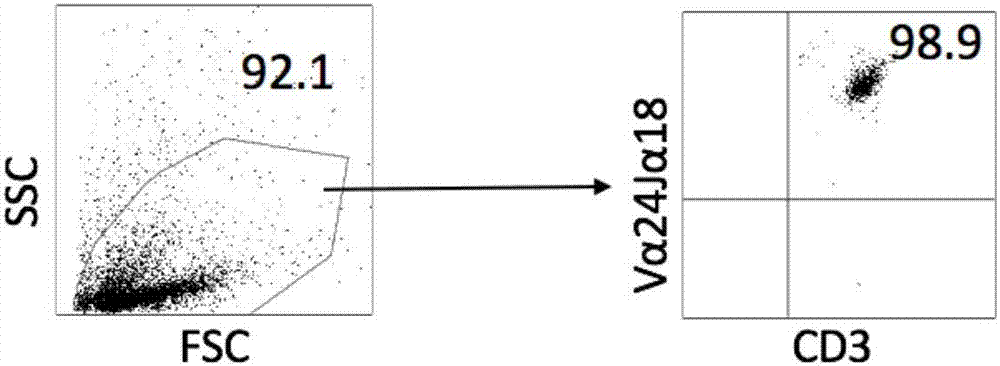
Invariant natural killer T (iNKT) cells, also known as type I or classical NKT cells, are a distinct population of T cells that express an invariant aβ T-cell receptor (TCR) and a number of cell surface molecules in common with natural killer (NK) cells.
Anticancer agent comprising Anti-pd-1 antibody or Anti-pd-l1 antibody
ActiveUS20120237522A1Recovery of iNKT cell responsivenessAntibody ingredientsImmunoglobulinsLymphatic SpreadAnticarcinogen
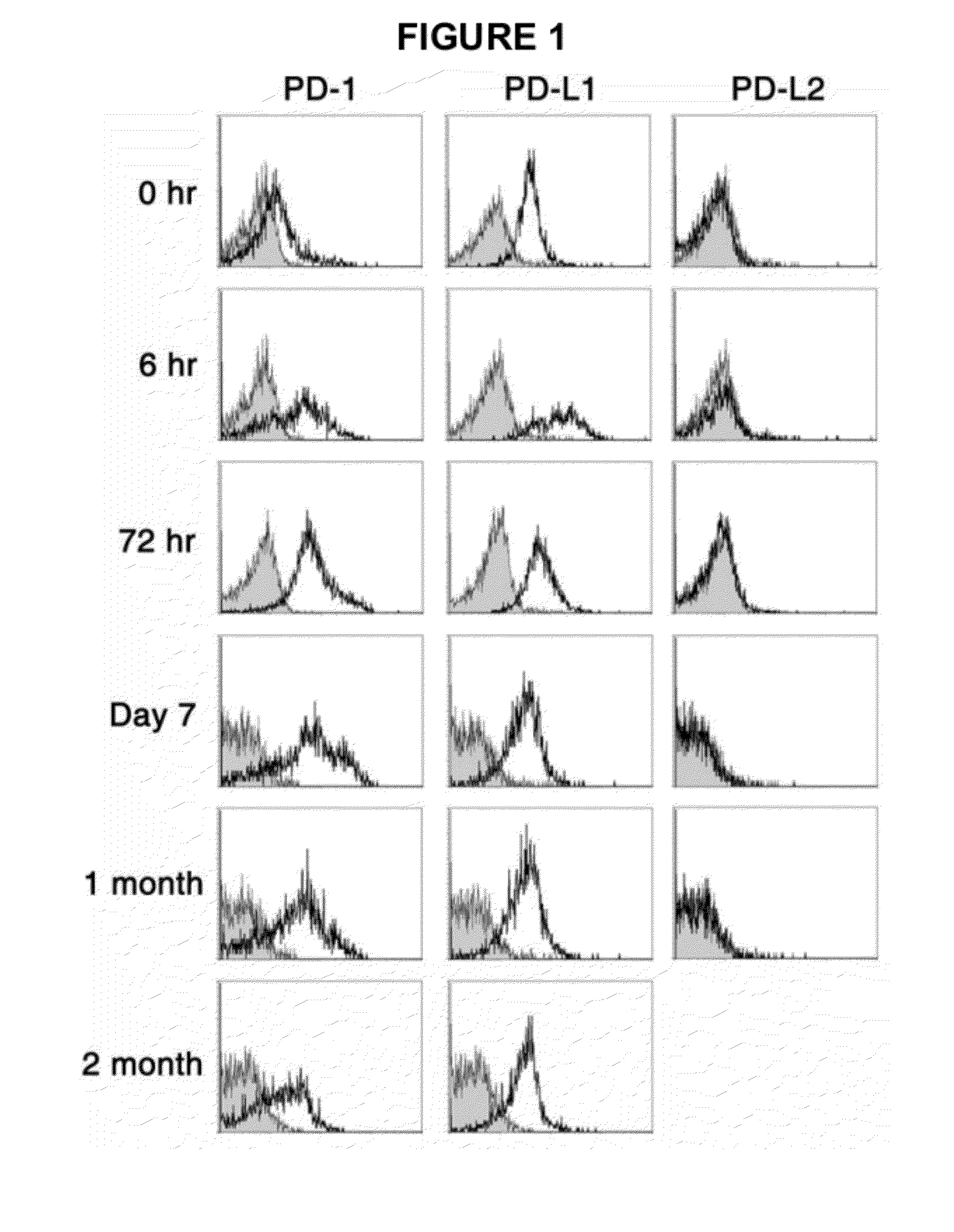
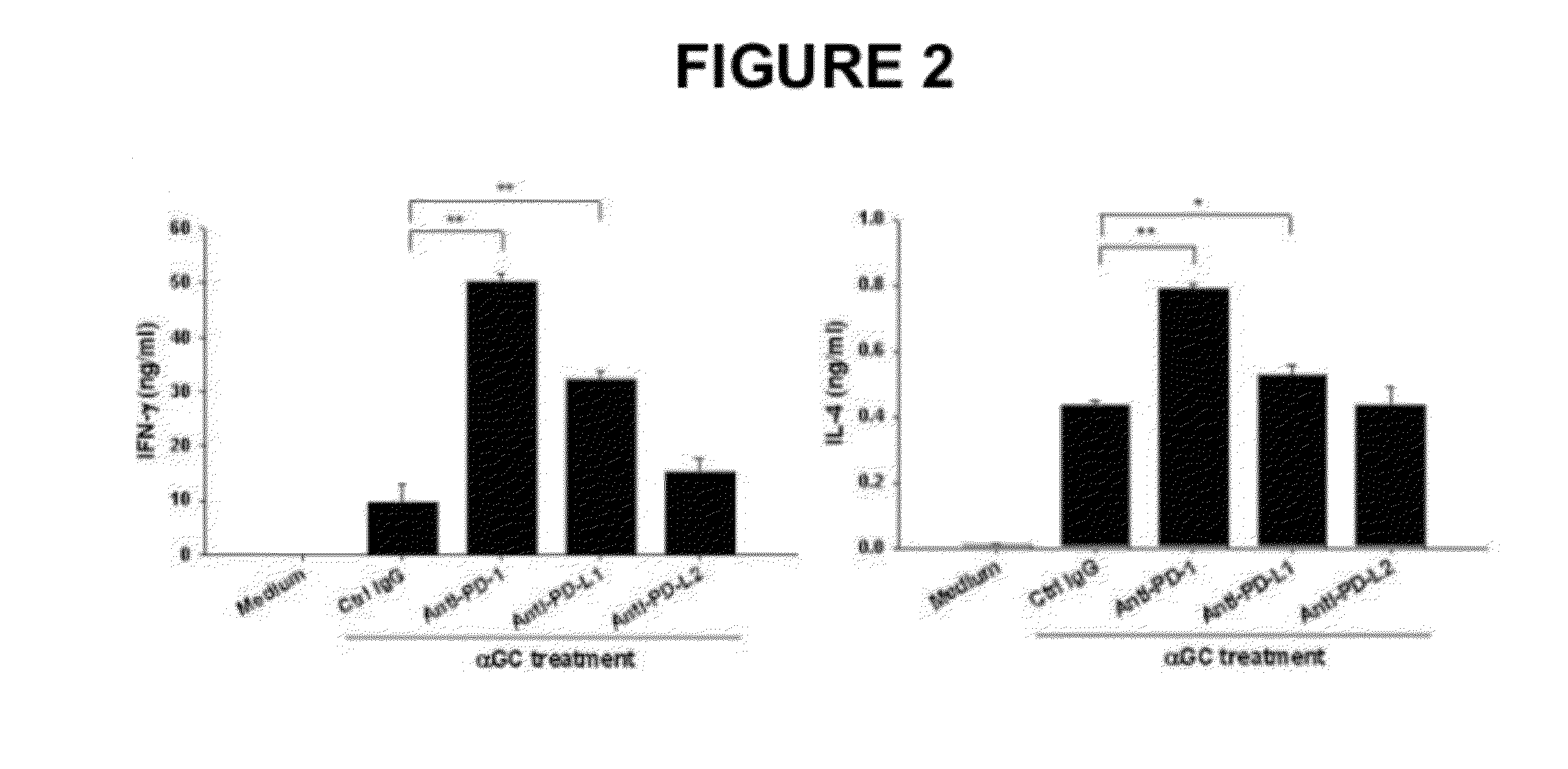
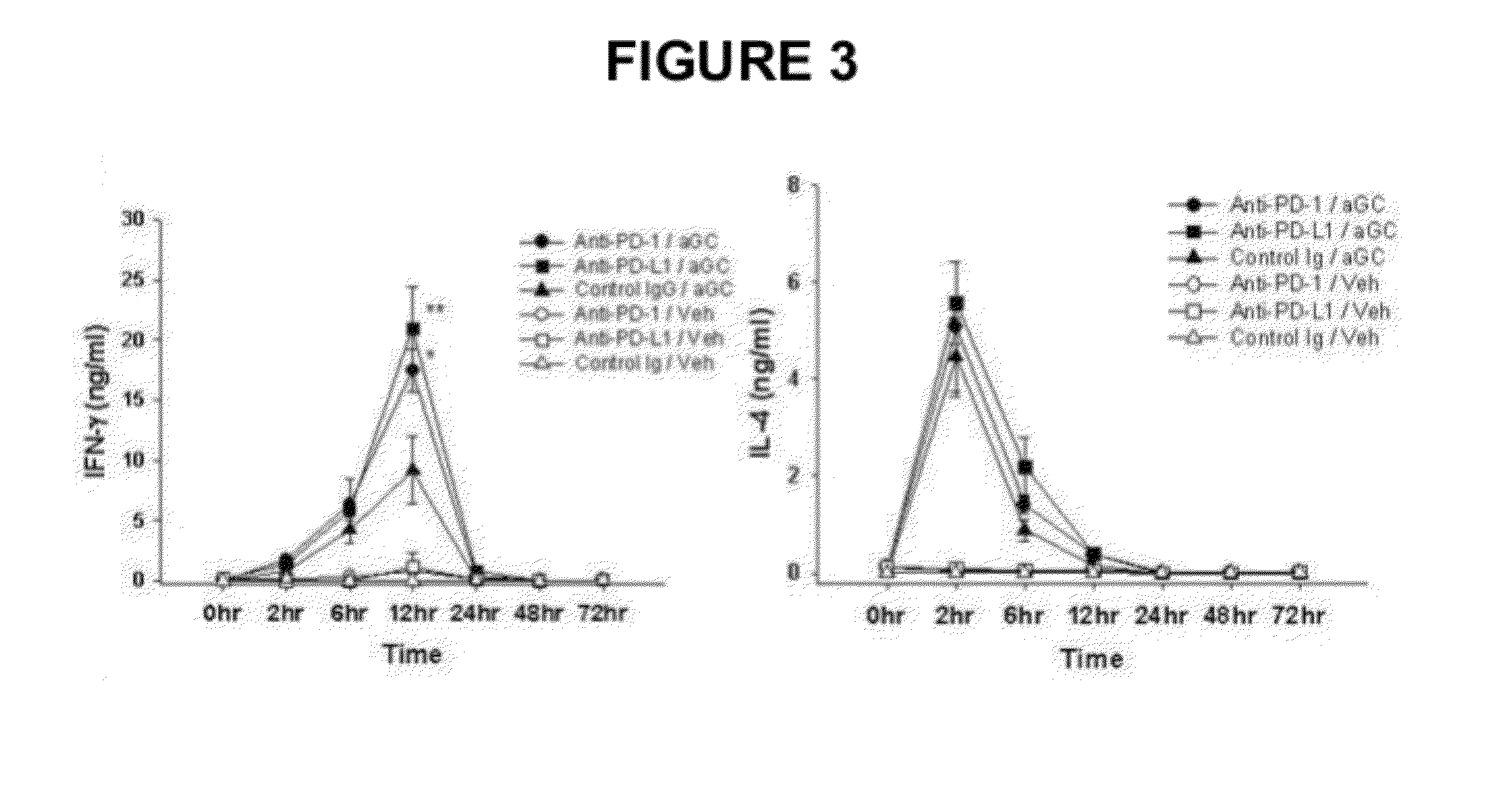
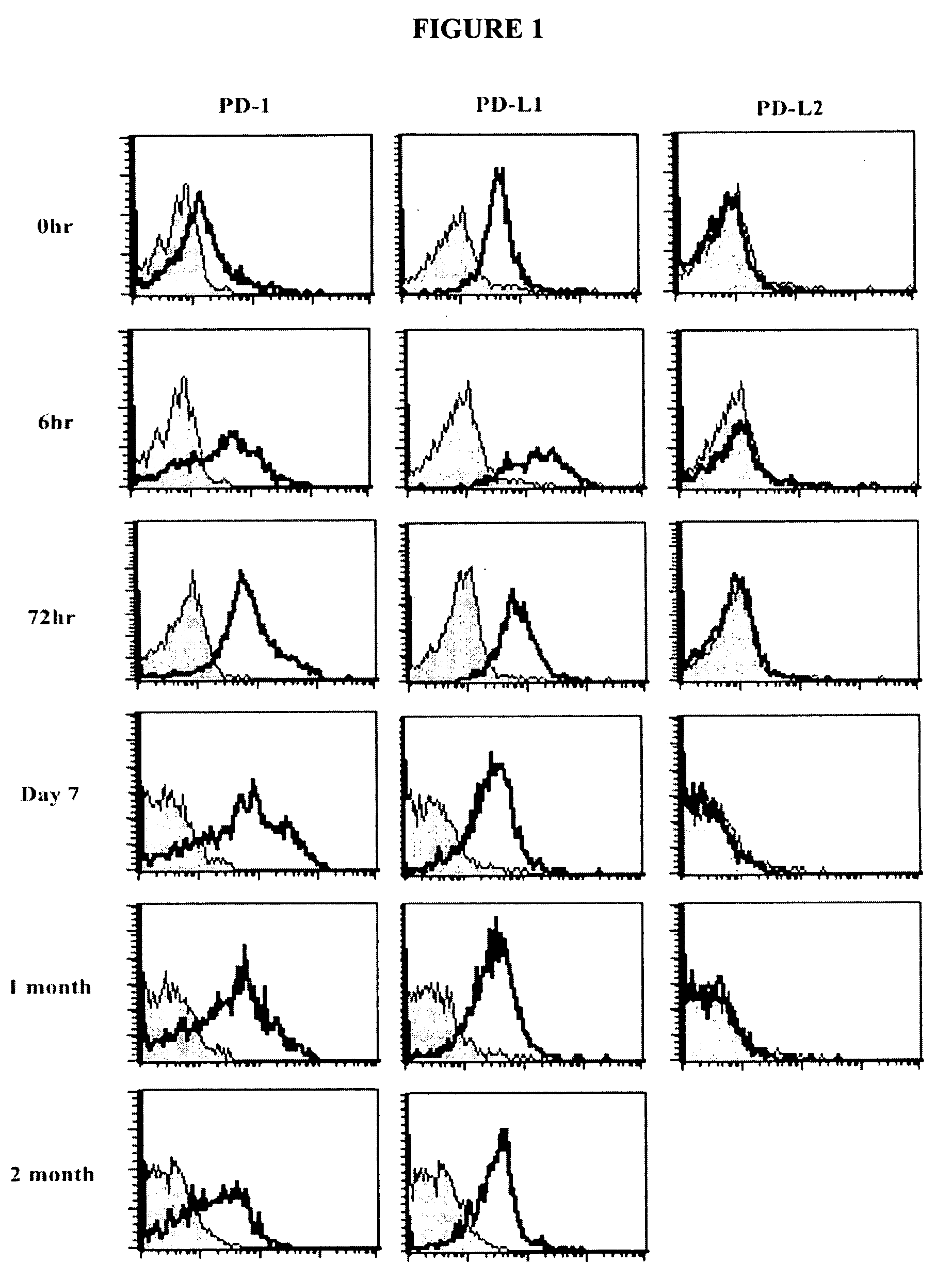
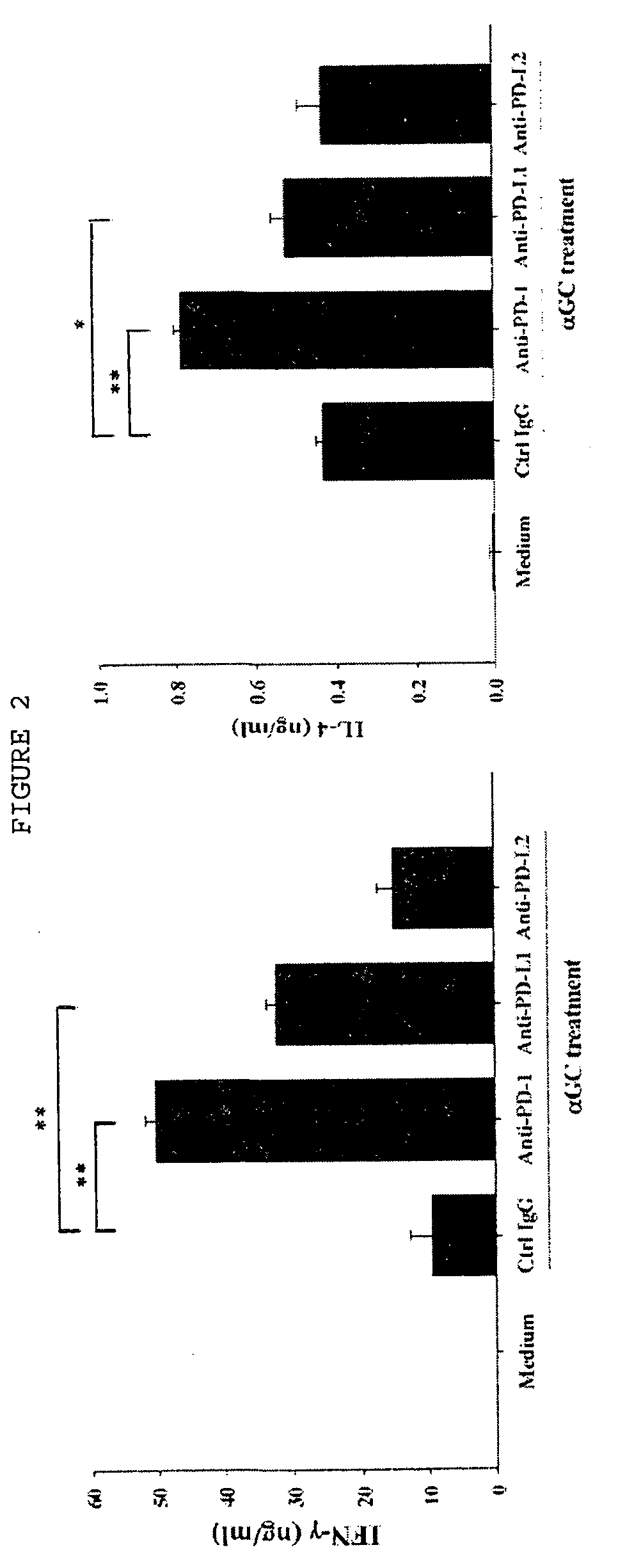
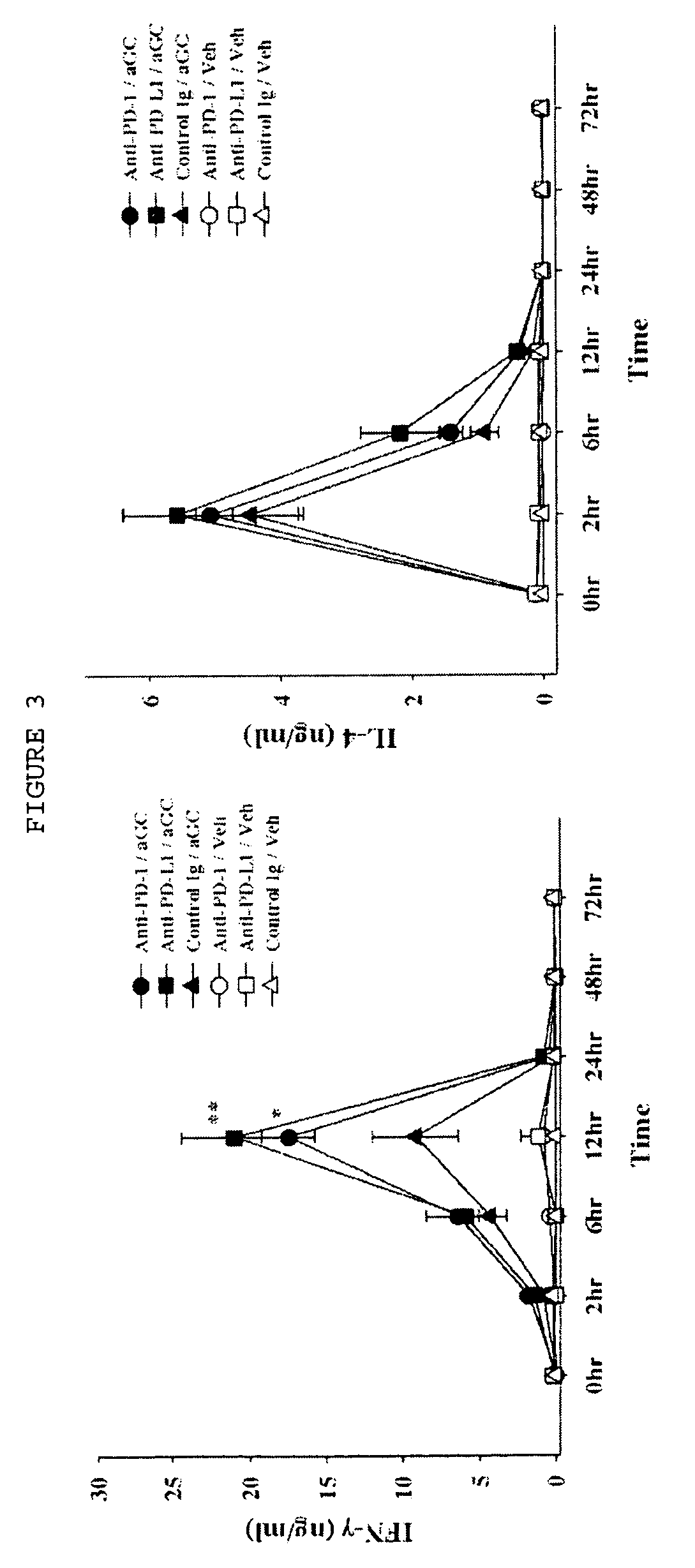
Provided is an anticancer agent which comprises an anti-PD-1 antibody or an anti-PD-L1 antibody as an active ingredient, functioning to reverse the unresponsiveness of iNKT cells in which anergy has been induced by administration with an iNKT cell ligand. The anti-PD-1 or anti-PD-L1 antibody blocks the PD-1 / PD-L1-mediated signaling pathway not only to prevent the iNKT cell ligand-induced iNKT cell anergy, but also to reverse the unresponsiveness of already anergic iNKT cells to produce cytokines. In addition, the anti-PD1 or anti-PD-L1 antibody ensures the potent anti-tumor activity of iNKT cells as demonstrated by a significant reduction in the number of metastatic nodules in B16F10 melanoma metastasis models in vivo. Collectively, the anticancer agent can be very useful in the treatment of cancer, particularly metastatic cancer.
Owner:SEOUL NAT UNIV R&DB FOUND
Anticancer agent comprising Anti-pd-1 antibody or Anti-pd-l1 antibody
InactiveUS20100086550A1Recovery of iNKT cell responsivenessAntibody ingredientsImmunoglobulinsAnticarcinogenLymphatic Spread
Provided is an anticancer agent which comprises an anti-PD-1 antibody or an anti-PD-L1 antibody as an active ingredient, functioning to reverse the unresponsiveness of iNKT cells in which anergy has been induced by administration with an iNKT cell ligand. The anti-PD-1 or anti-PD-L1 antibody blocks the PD-1 / PD-L1-mediated signaling pathway not only to prevent the iNKT cell ligand-induced iNKT cell anergy, but also to reverse the unresponsiveness of already anergic iNKT cells to produce cytokines. In addition, the anti-PD1 or anti-PD-L1 antibody ensures the potent anti-tumor activity of iNKT cells as demonstrated by a significant reduction in the number of metastatic nodules in B16F10 melanoma metastasis models in vivo. Collectively, the anticancer agent can be very useful in the treatment of cancer, particularly metastatic cancer.
Owner:ANTICANCER AGENT COMPRISING ANTI PD 1 ANTIBODY OR ANTI PD L1 ANTIBODY
HUMANIZED ANTIBODIES TO iNKT
InactiveUS20130136735A1Suppress immune responseSufficiently suppressAntibacterial agentsAntipyreticBlocking antibodyHumanized antibody
Methods of treatment to suppress an immune response are provided. The method comprises administering to a subject in need of treatment a naked blocking antibody that binds selectively iNKT cells in an amount effective to suppress the subject's iNKT cell function. Compositions comprising, an isolated, humanized antibody that binds selectively iNKT cells are also provided.
Owner:NKT THERAPEUTICS
Method for simultaneously inducing and amplifying V alpha<24+>iNKT cells and CD<3->CD<56+>NK cells
ActiveCN104357391AHigh purityHigh activityBlood/immune system cellsAdoptive cellular immunotherapySerum free media
The invention discloses a method for simultaneously inducing and amplifying V alpha<24+>iNKT cells and CD<3->CD<56+>NK cells. The method comprises the steps as follows: PBMC (peripheral blood mononuclear cells) are separated from peripheral blood, the concentration of the PBMC is adjusted to 2*10<6> / ml by a serum-free medium containing autologous plasma; an Anti-CD<16> antibody, -GalCer, IL-2, IL-18 and IL-21 are added, and then the mixture is transferred into a culture flask for culture; an Anti-CD3 antibody is added in a cell suspension in 24 hours; a serum-free medium containing IL-2, IL-18 and IL-21 is supplemented every two days according to the cell growth condition; the cell concentration is controlled to be 1.5*10<6> / ml; and after continuous culture is performed for 14-21 days, large quantities of high-purity V alpha<24+>iNKT cells and CD<3->CD<56+>NK cells can be obtained simultaneously, and the total cell quantity can reach an effective value of the cell quantity required for adoptive cellular immunotherapy clinically for tumor. The method for simultaneous and efficient amplification of the V alpha<24+>iNKT cells and the CD<3->CD<56+>NK cells is simple, convenient and effective.
Owner:HRYZ (SHENZHEN) BIOTECH CO +1
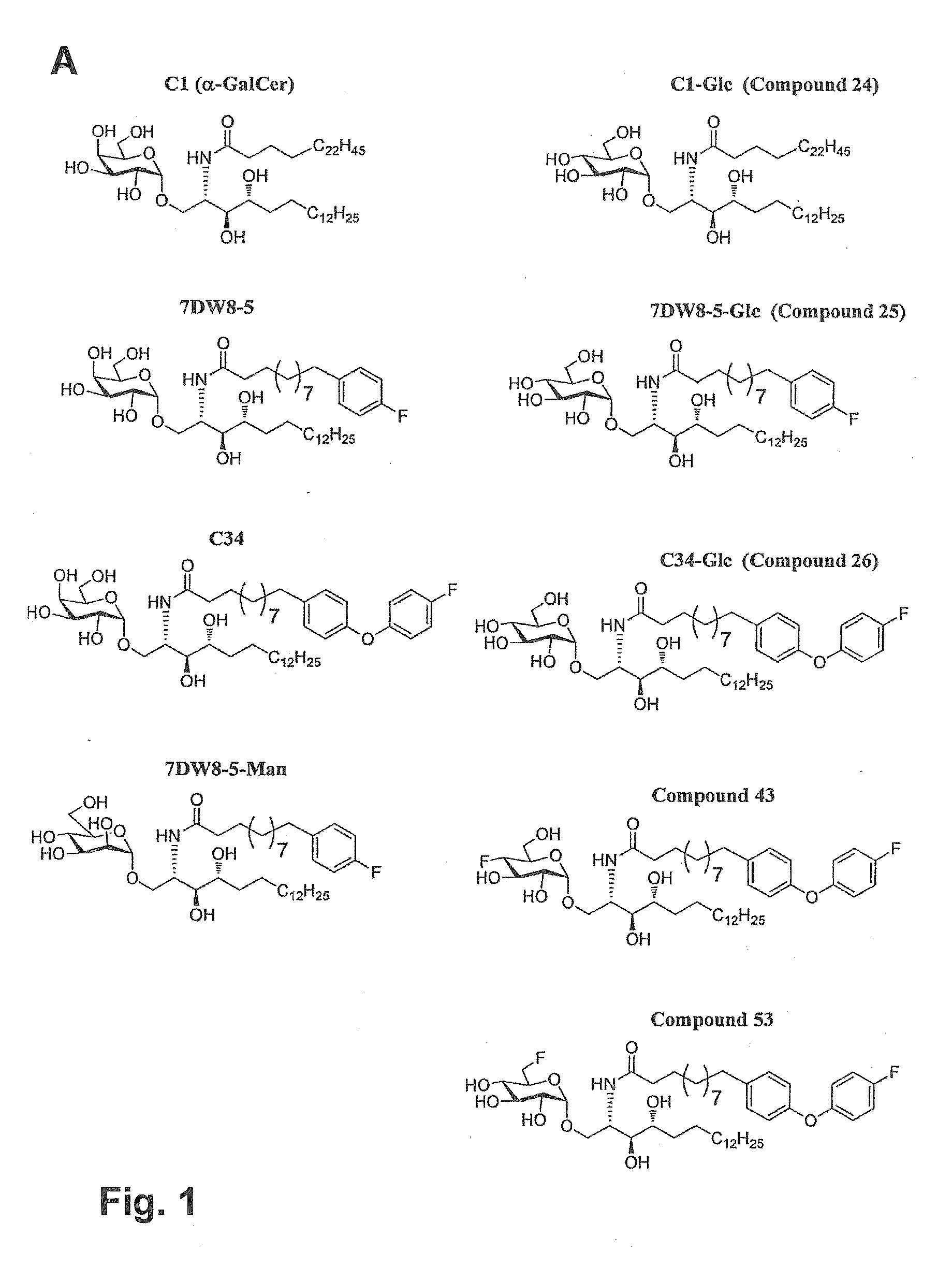
Human iNKT cell activation using glycolipids with altered glycosyl groups
ActiveUS9782476B2More potent anticancer activitiesImprove protectionAntibacterial agentsOrganic active ingredientsHuman useGlycosphingolipid
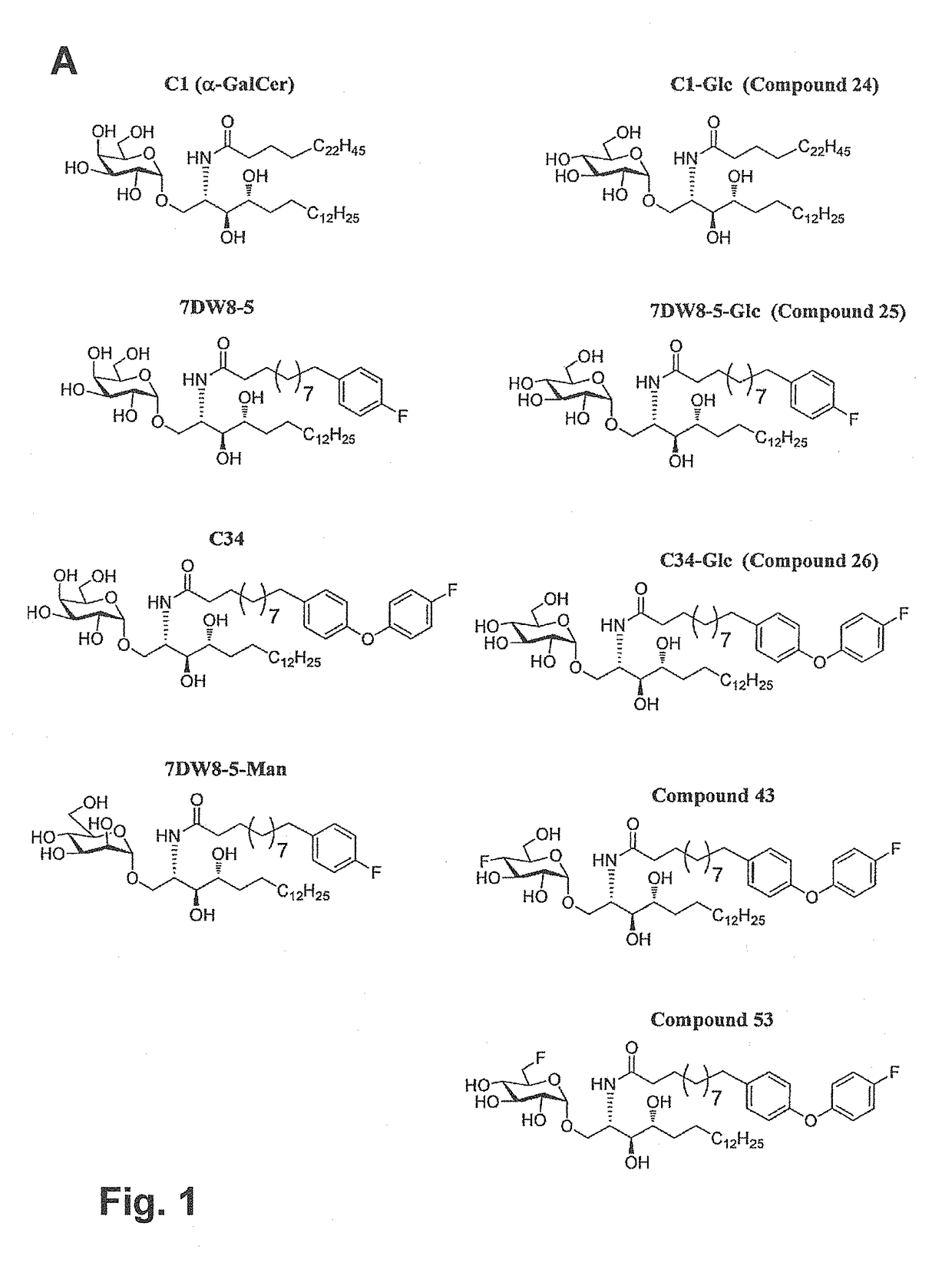
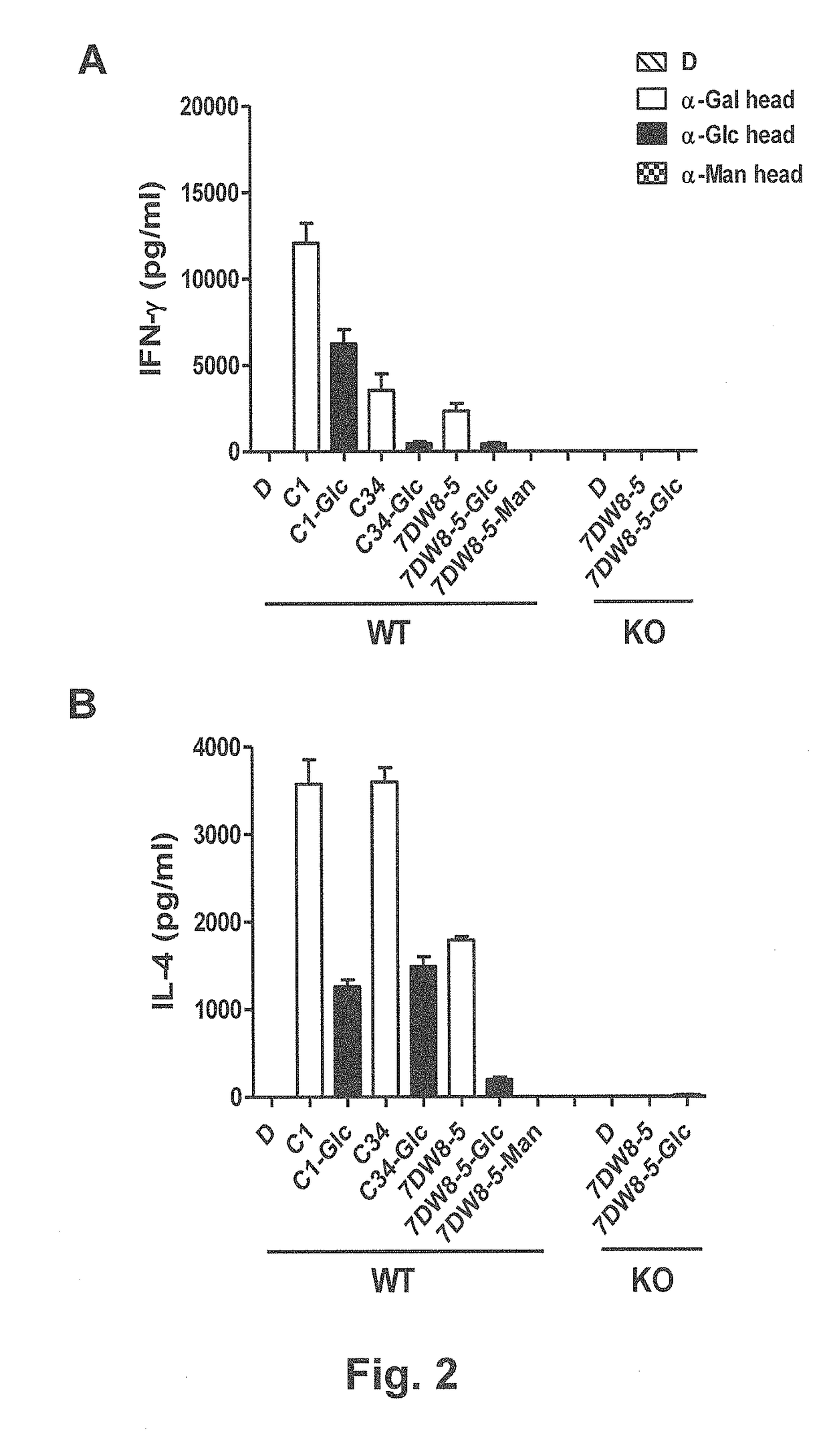
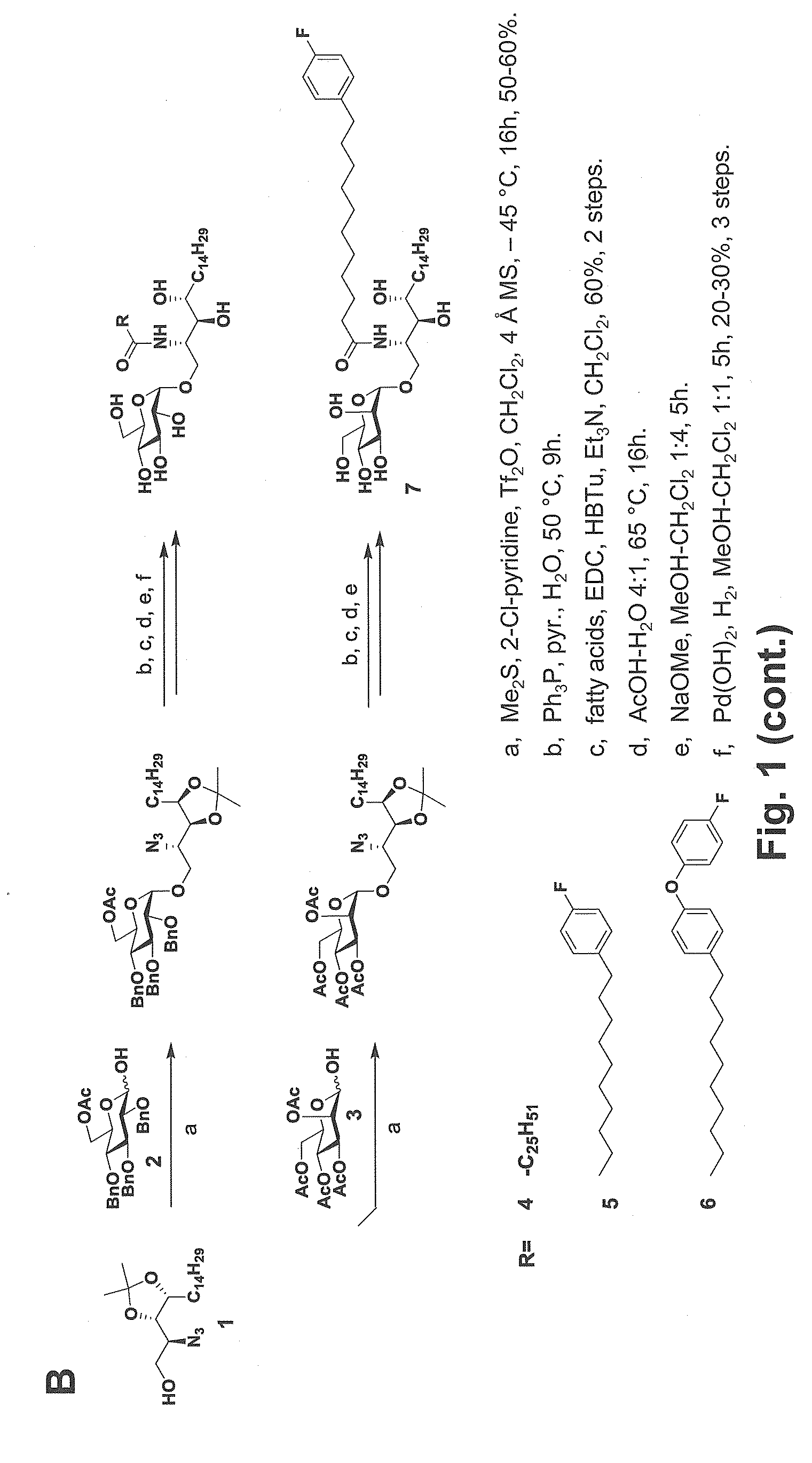
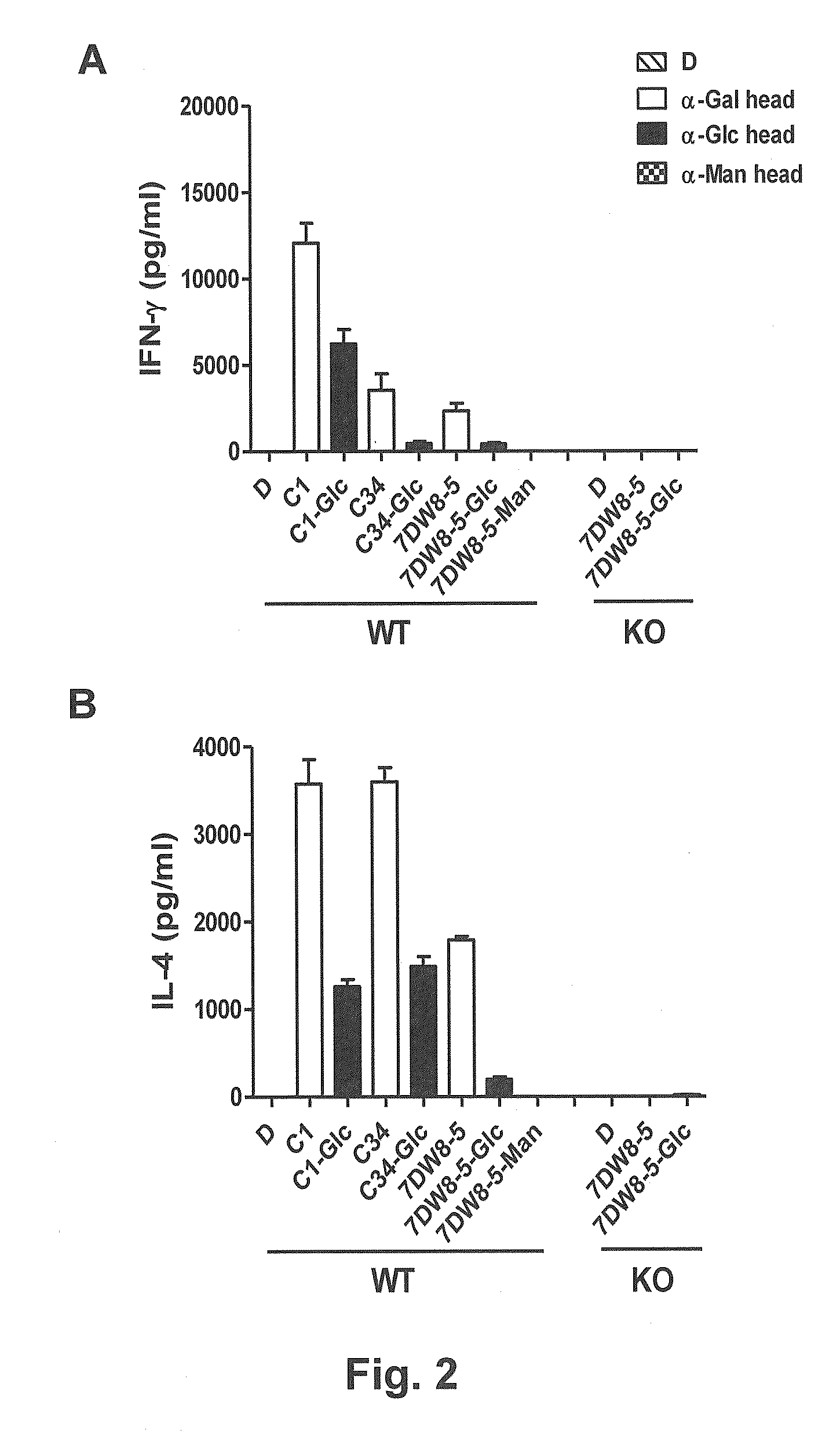
Glycosphingolipids (GSLs) bearing α-glucose (α-Glc) that preferentially stimulate human invariant NKT (iNKT) cells are provided. GSLs with α-glucose (α-Glc) that exhibit stronger induction in humans (but weaker in mice) of cytokines and chemokines and expansion and / or activation of immune cells than those with α-galactose (α-Gal) are disclosed. GSLs bearing α-glucose (α-Glc) and derivatives of α-Glc with F at the 4 and / or 6 positions are provided. Methods for iNKT-independent induction of chemokines by the GSL with α-Glc and derivatives thereof are disclosed. Methods for immune stimulation in humans using GSLs with α-Glc and derivatives thereof are provided.
Owner:ACAD SINIC
Treatment of airway hyperreactivity
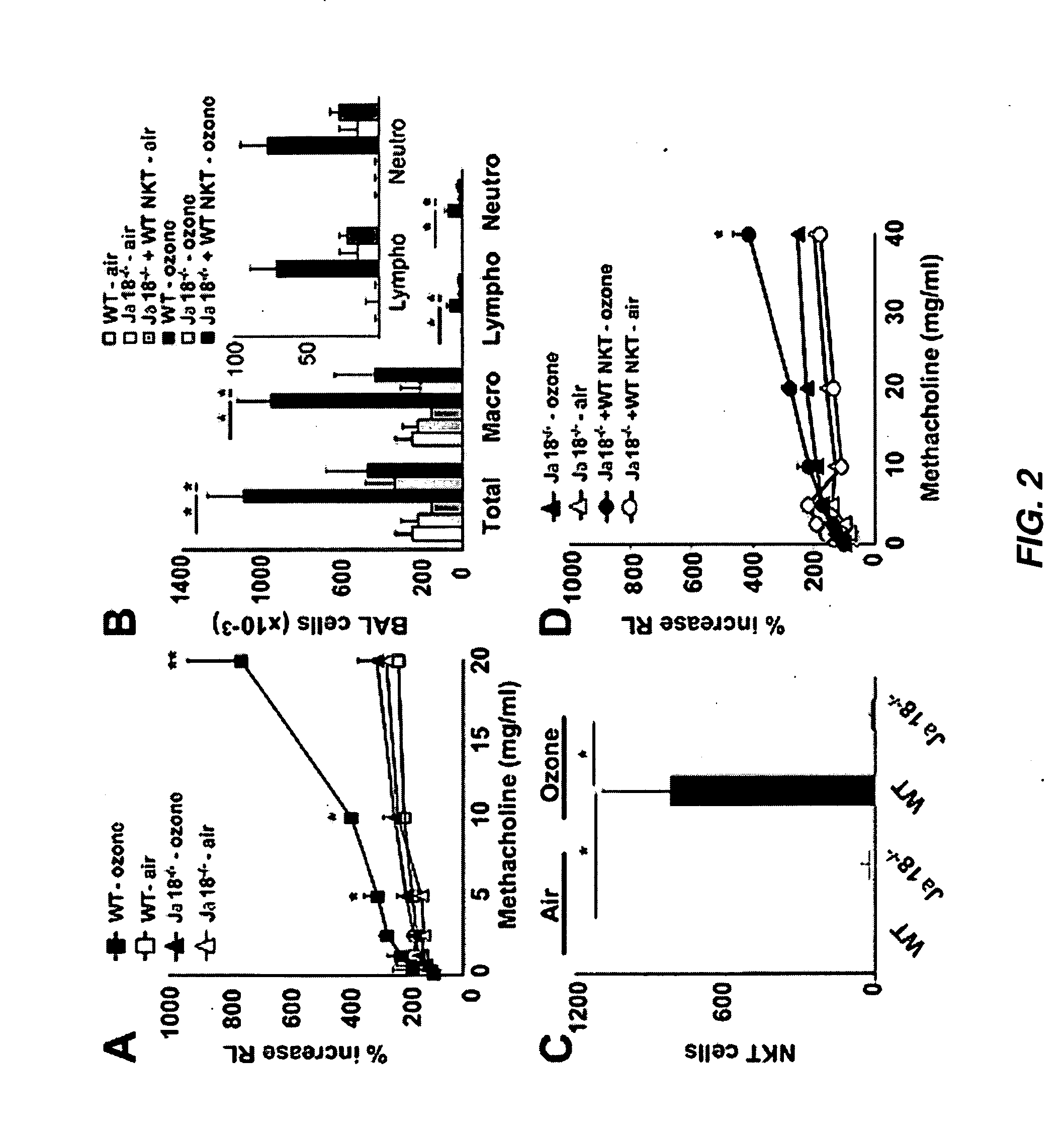
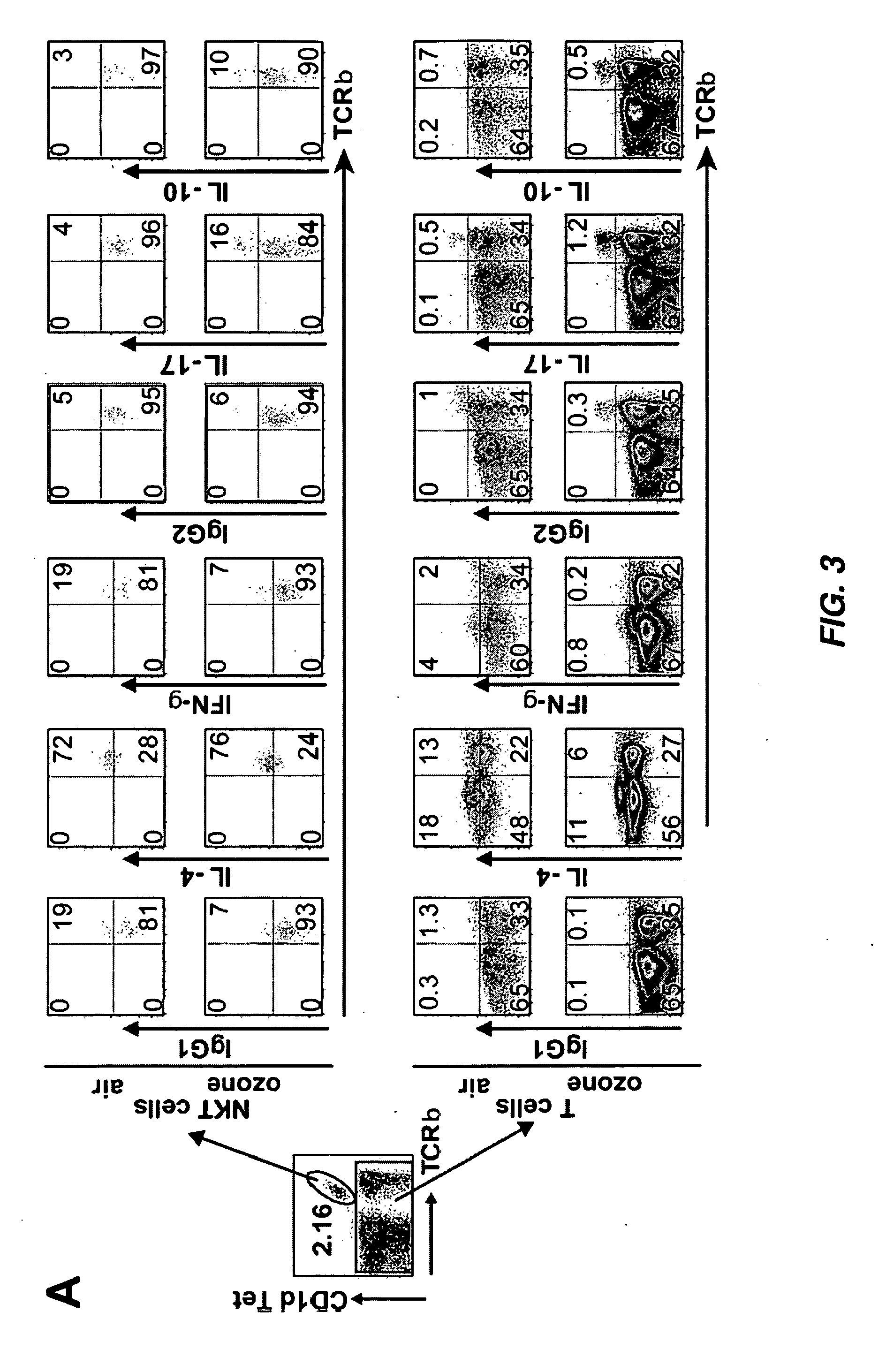
InactiveUS20110300154A1Avoid developmentOrganic active ingredientsPeptide/protein ingredientsMedicineRadiology
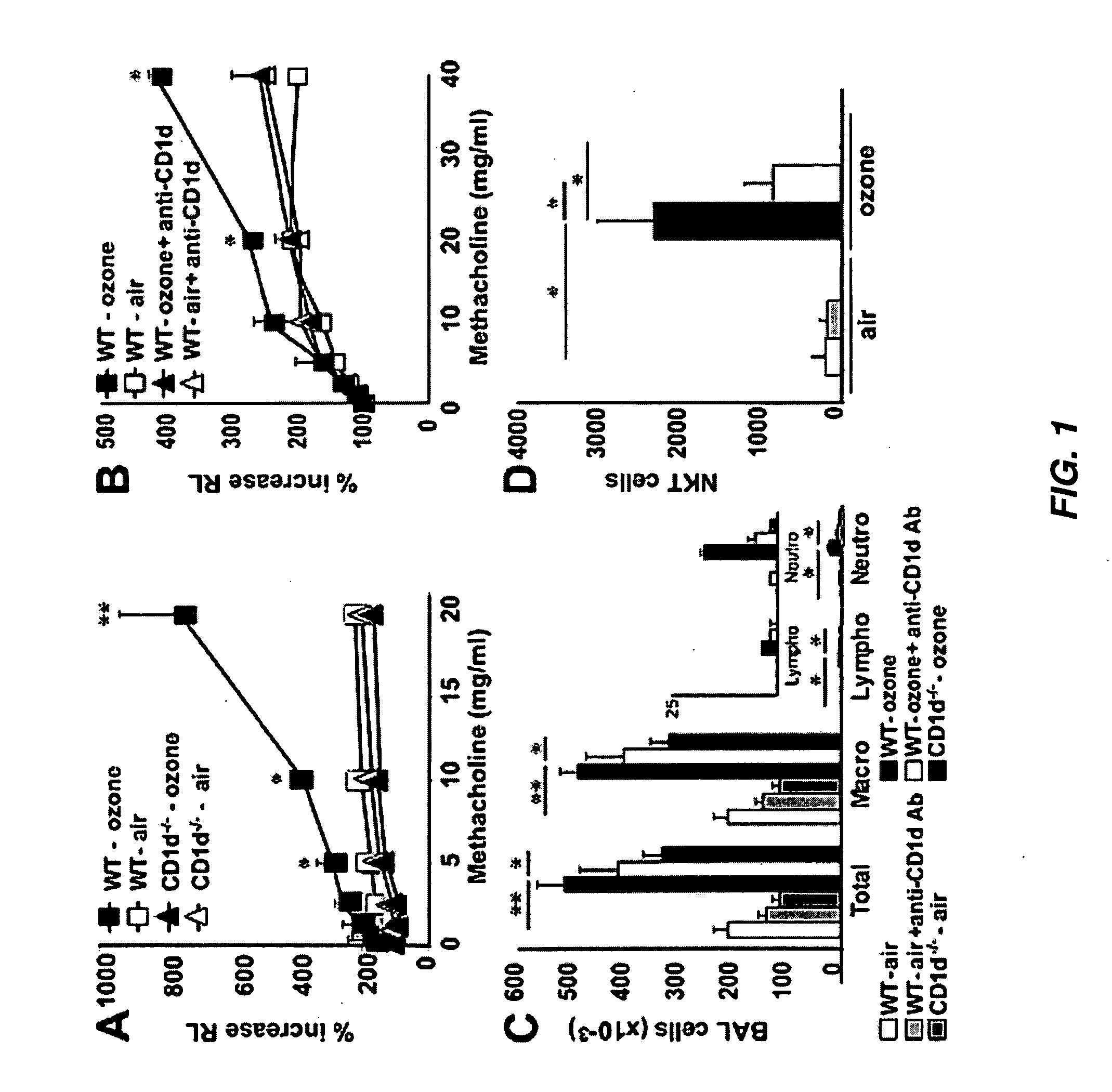
The invention provides strategies for treating and preventing airway hyperreactivity and non-allergic asthma comprising antagonizing IL-17 activity and / or production by iNKT cells. Provided herein is a method of diagnosing non-allergic asthma and airway hyperreactivity comprising neutrophils quantification in sputum.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +2
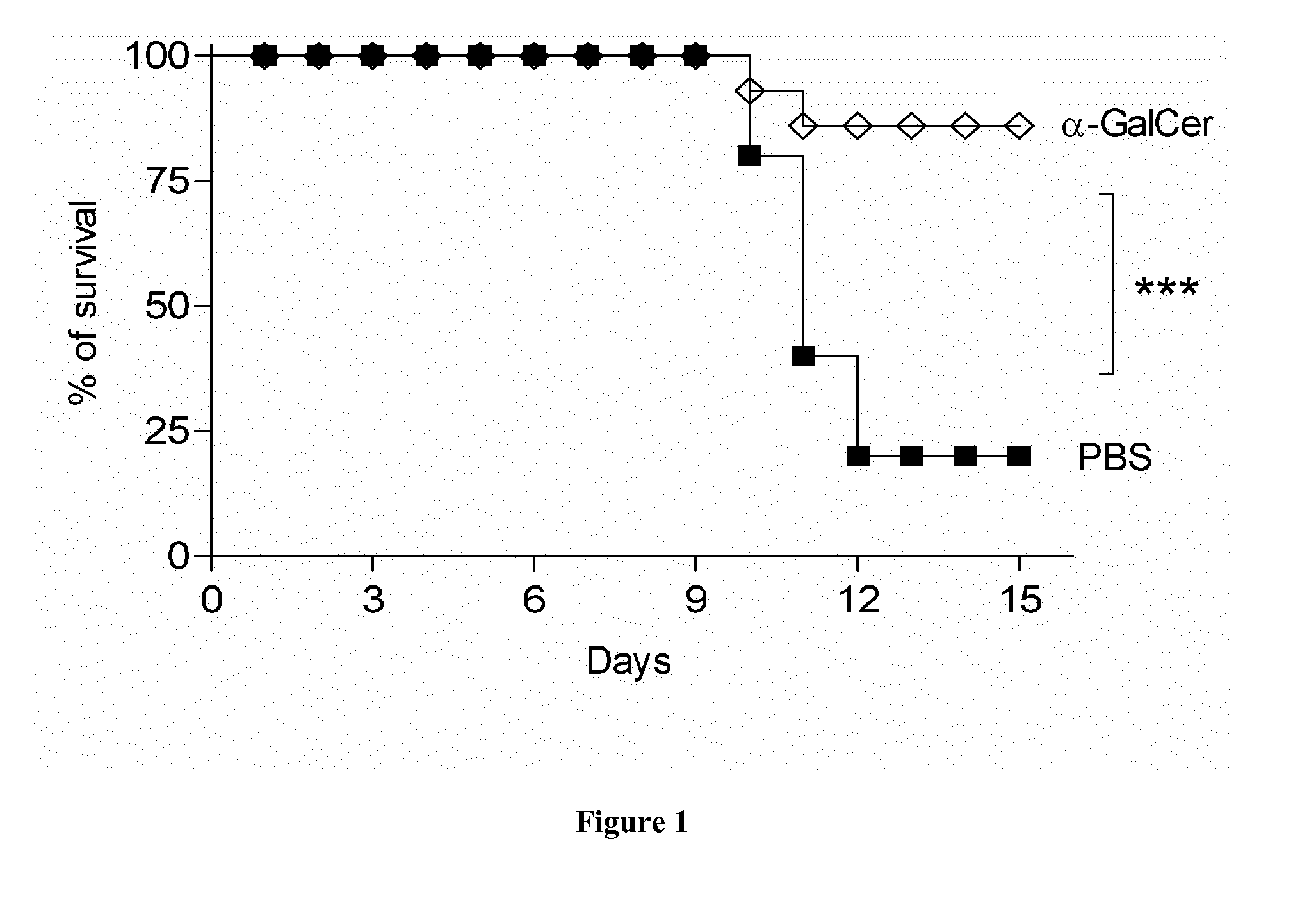
Methods and pharmaceutical compositions for the prophylactic treatment of bacterial superinfections post-influenza with invariant NKT cell agonists
The present invention relates to methods and pharmaceutical compositions for the prophylactic treatment of bacterial superinfections post-influenza with iNKT cell agonists.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +4
Invariant natural killer T (iNKT) cell expressing targeted GPC3 chimeric antigen receptor and preparation and application for invariable natural killer T (iNKT) cell
ActiveCN107384949AImmunoglobulins against cell receptors/antigens/surface-determinantsMammal material medical ingredientsSingle-Chain AntibodiesHinge region
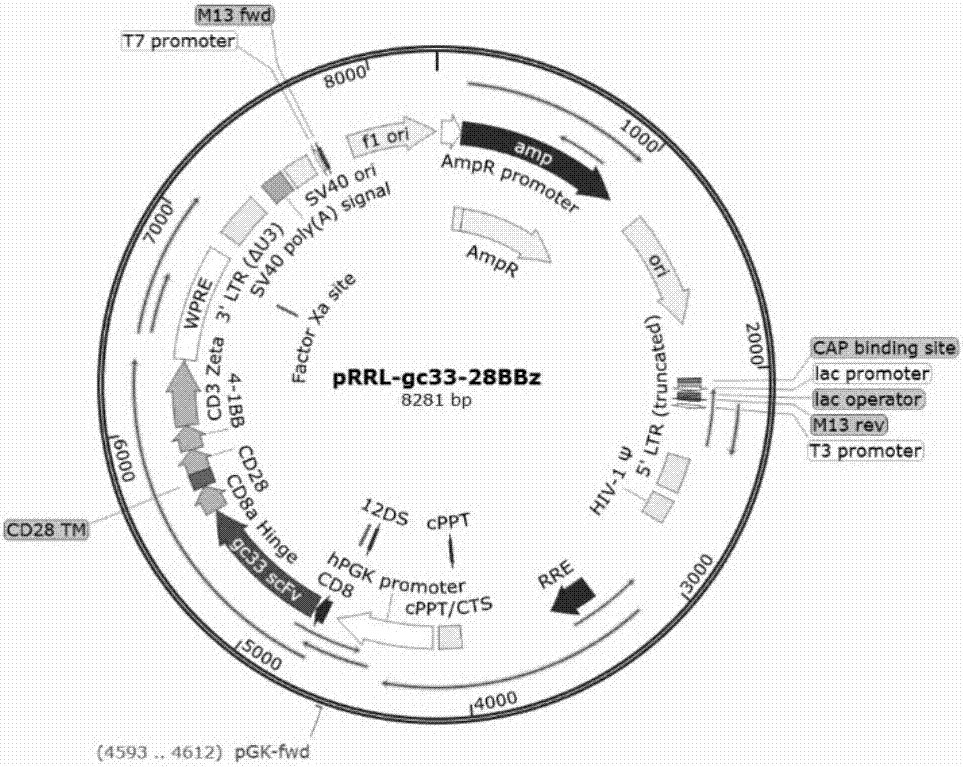
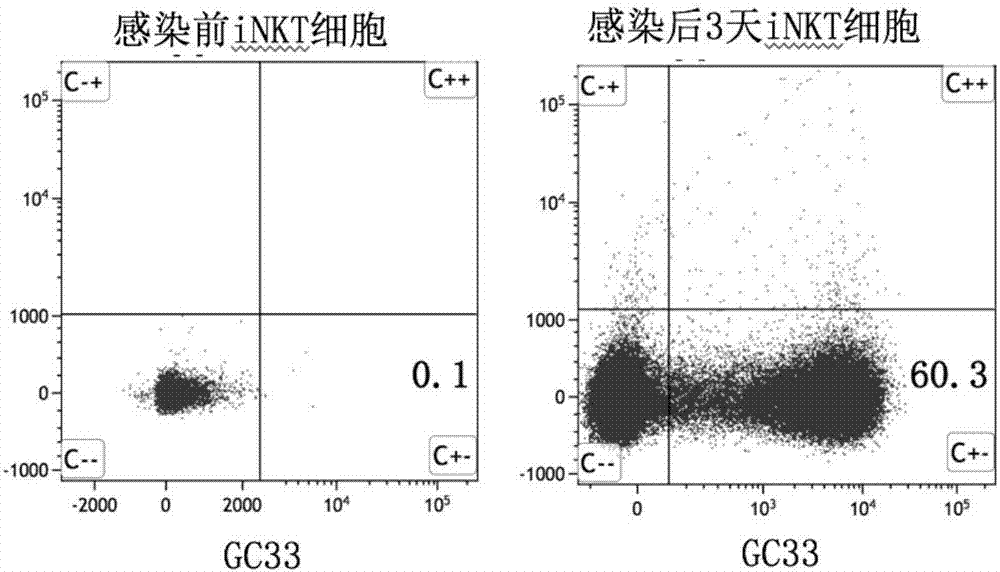
The invention discloses an invariant natural killer T (iNKT) cell expressing a targeted GPC3 chimeric antigen receptor and preparation and application for the iNKT cell. The chimeric antigen receptor contains a hinge region, a transmembrane region and an intracellular signal region of a single chain antibody GPC3-ScFv, CD8 which recognize a C tail end epitope of GPC3, wherein the hinge region, the transmembrane region and the intracellular signal region are connected according to series structure domains. Preparation of the iNKT cell modified by the chimeric antigen receptor comprises the following steps that a chimeric antigen receptor pRRL-gc33-28BBz is built, and the iNKT cell is infected; and after special amplification in vitro, the GPC3-targetted iNKT cell is obtained. According to the iNKT cell expressing the targeted GPC3 chimeric antigen receptor and preparation and application for the iNKT cell, the nucleic acids encoding the chimeric antigen receptor protein, a plasmid containing the nucleic acids, a virus containing the plasmid and the transgenic iNKT lymphocyte transduced by the virus can be effectively applied to tumor immunotherapy.
Owner:BEIJING GENE KEY LIFE TECH CO LTD
Liposomal formulation of nonglycosidic ceramides and uses thereof
ActiveUS20170105936A1Promote growthReduce spreadCancer antigen ingredientsLiposomal deliveryDiseaseDendritic cell
The invention provides liposomes containing nonglycosidic ceramides within their bilayers, and compositions thereof. These liposomes activate murine iNKT cells and induce dendritic cell (DC) maturation, both in vitro and in vivo at an efficacy that is comparable to their corresponding soluble nonglycosidic ceramides. Also provided are methods for treating diseases using the liposomes and compositions of the invention.
Owner:LUDWIG INST FOR CANCER RES
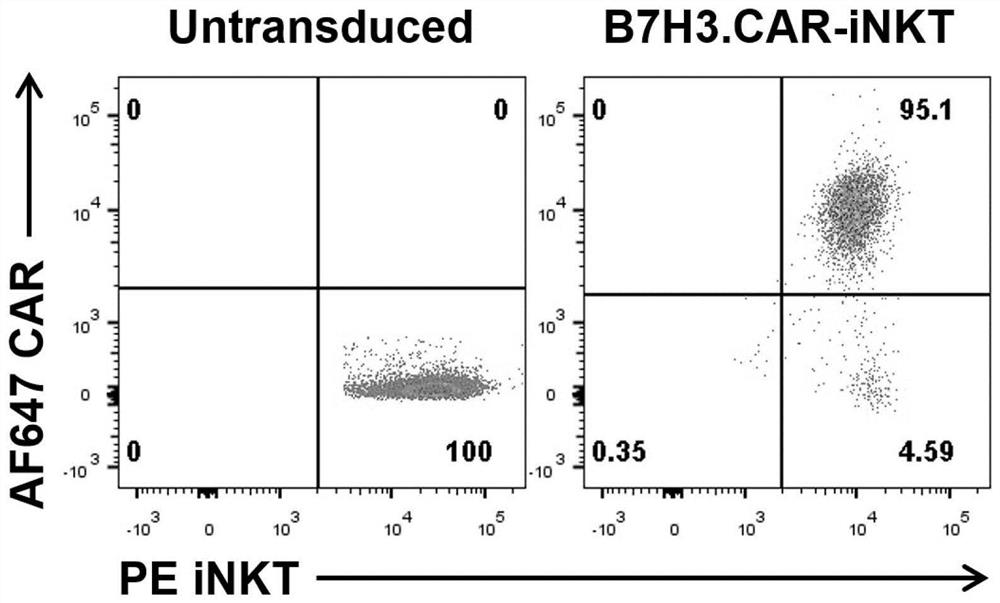
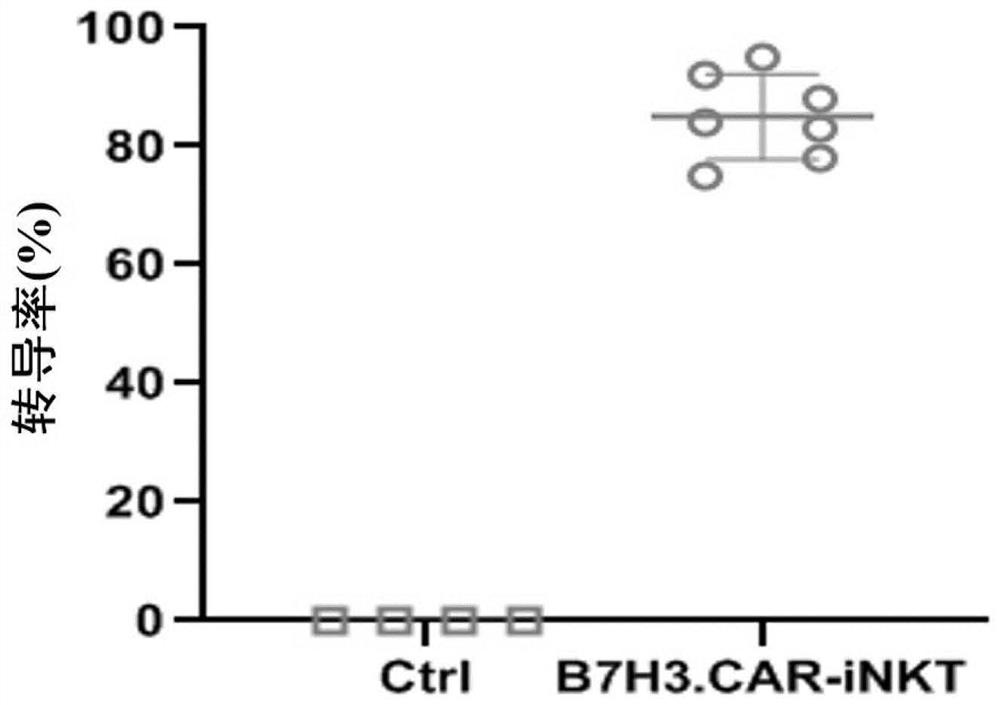
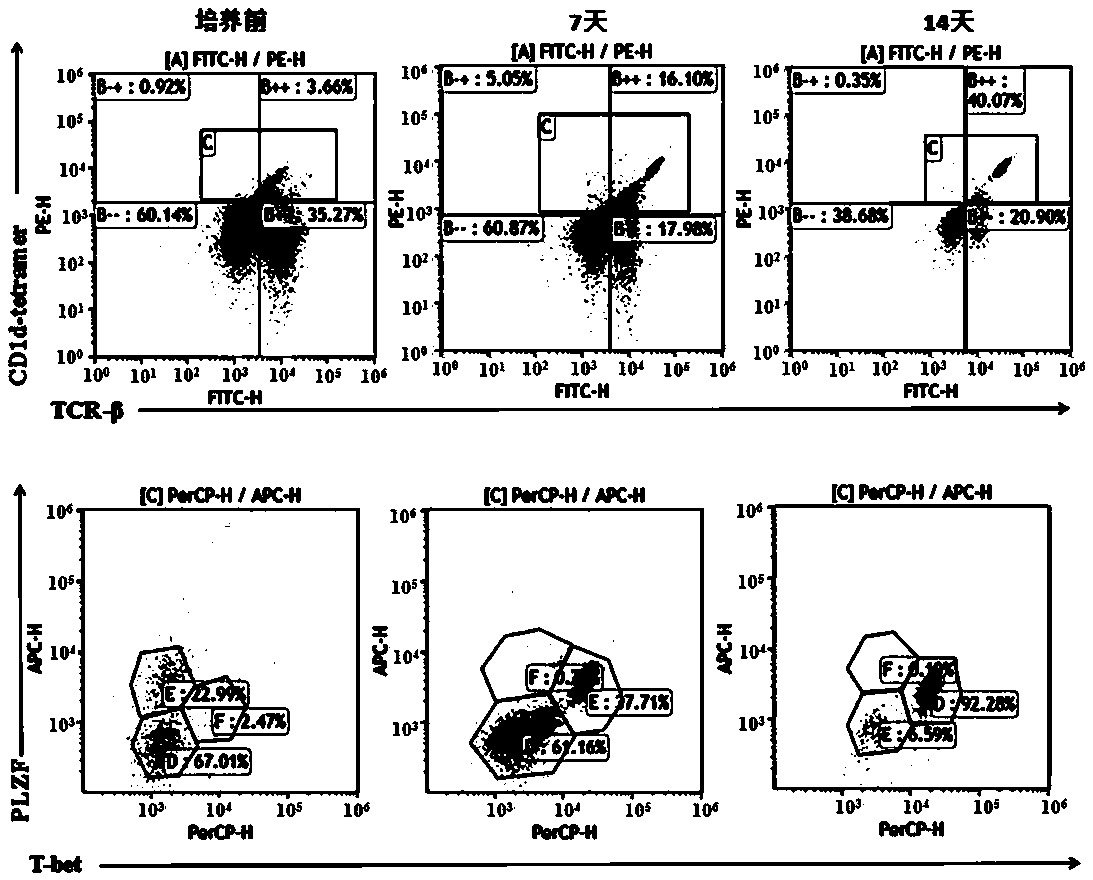
B7H3-targeted fully-humanized chimeric antigen receptor, iNKT cell and application thereof
ActiveCN113501884AGood killing effectEfficient removalNucleic acid vectorImmunoglobulinsAntigen receptorAntigen binding
The invention discloses a B7H3-targeting fully human chimeric antigen receptor, an iNKT cell and application thereof, and the chimeric antigen receptor comprises a B7H3 antigen binding structural domain, a transmembrane structural domain, an intracellular signal structural domain and IL-15, experiments prove that the CAR-iNKT cell targeting B7H3 prepared by the invention has strong proliferation ability, cytokine release ability and tumor cell killing ability, can effectively remove tumor cells, and has important application prospects in the field of tumor cell immunotherapy.
Owner:江苏集萃崛创生物科技研究所有限公司
Methods for determining the risk of acute graft versus host disease
ActiveUS20150301022A1Increased riskBiocidePhosphorous compound active ingredientsCandidate donorSub populations
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +4
HUMAN iNKT CELL ACTIVATION USING GLYCOLIPIDS WITH ALTERED GLYCOSYL GROUPS
ActiveUS20150071960A1Superior tumour protectionGood choiceAntibacterial agentsBiocideHuman useGlycophorin
Glycosphingolipids (GSLs) bearing α-glucose (α-Glc) that preferentially stimulate human invariant NKT (iNKT) cells are provided. GSLs with α-glucose (α-Glc) that exhibit stronger induction in humans (but weaker in mice) of cytokines and chemokines and expansion and / or activation of immune cells than those with α-galactose (α-Gal) are disclosed. GSLs bearing α-glucose (α-Glc) and derivatives of α-Glc with F at the 4 and / or 6 positions are provided. Methods for iNKT-independent induction of chemokines by the GSL with α-Glc and derivatives thereof are disclosed. Methods for immune stimulation in humans using GSLs with α-Glc and derivatives thereof are provided.
Owner:ACAD SINIC
Humanized antibodies to iNKT
Methods of treatment to suppress an immune response are provided. The method comprises administering to a subject in need of treatment a naked blocking antibody that binds selectively iNKT cells in an amount effective to suppress the subject's iNKT cell function. Compositions comprising, an isolated, humanized antibody that binds selectively iNKT cells are also provided.
Owner:NKT THERAPEUTICS
CAR-iNKT with high amplification, survival capacity and tumour killing effect, and application
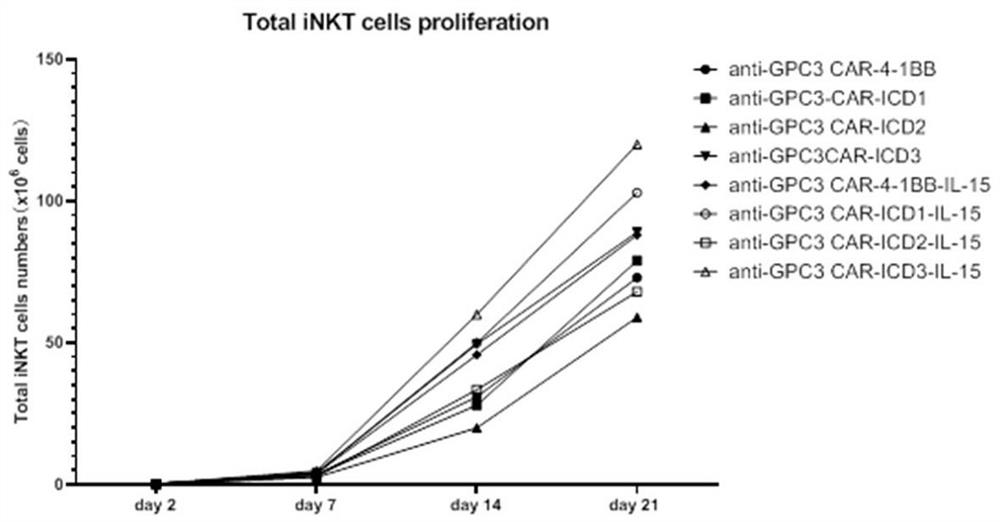
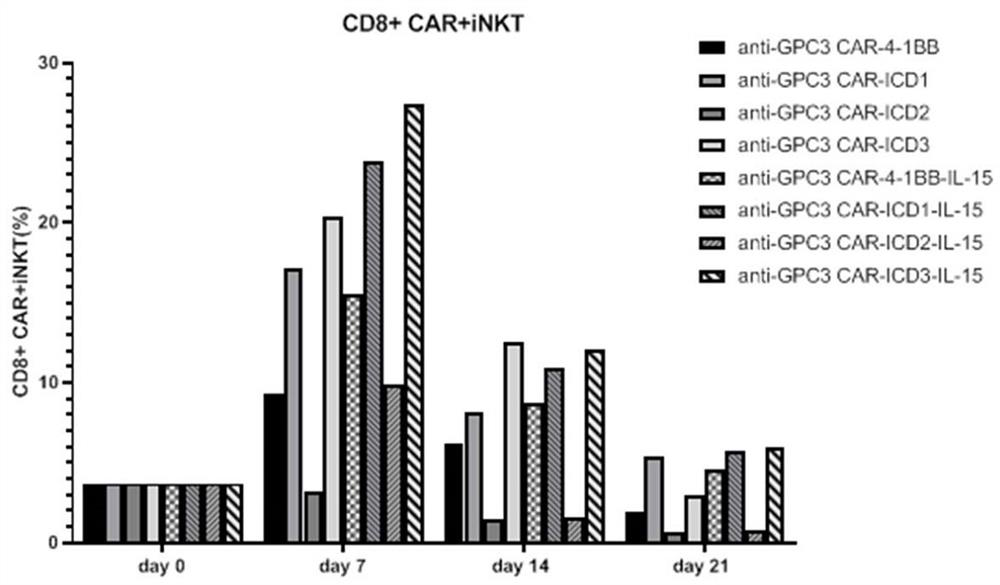
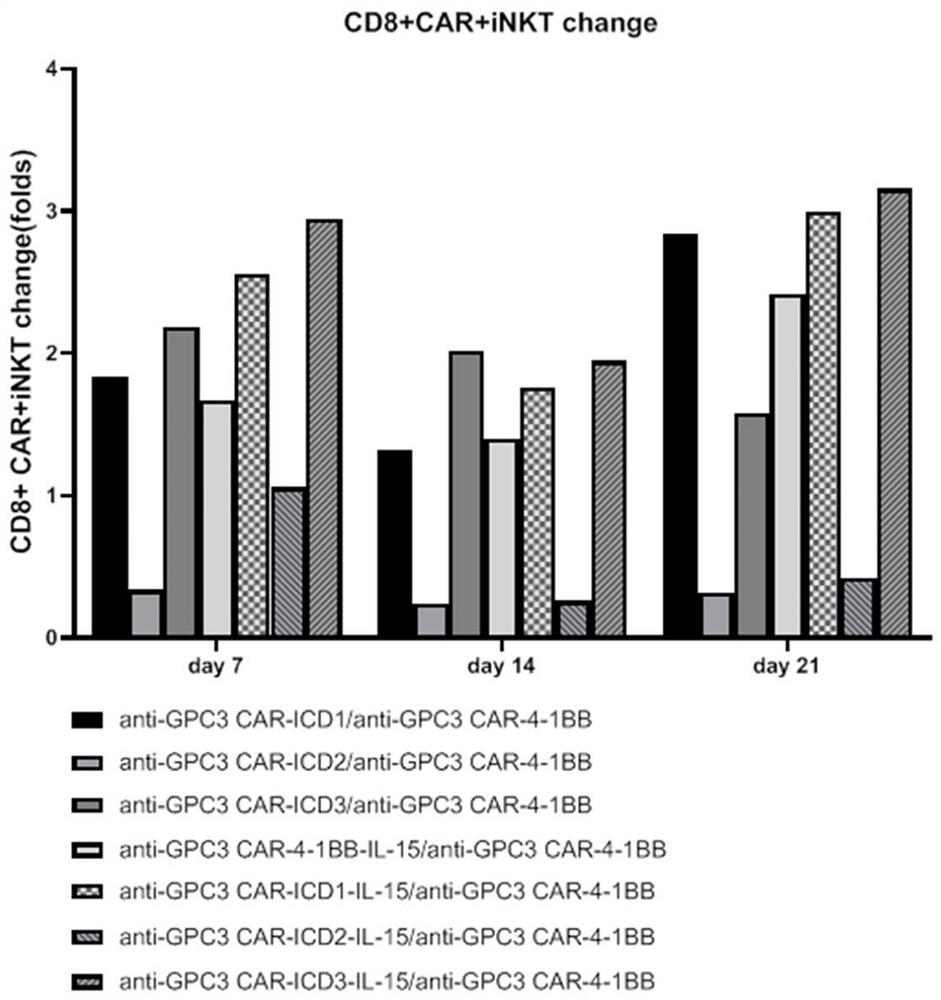
ActiveCN112225822AReduce exhaustExtended durationVirusesAntibody mimetics/scaffoldsAntigen receptorIntracellular
The invention provides a chimeric antigen receptor. The chimeric antigen receptor comprises a GPC3 antigen binding structural domain, an ICD1, ICD2 or ICD3 intracellular signal stimulation domain withamino acid sequences of SEQ ID NO.29, 31 and 33 and IL-15-IL-15alpha fusion protein with an amino acid sequence of SEQ ID NO.7. After the chimeric antigen receptor is transferred into immune cells, especially iNKT cells, the cell proliferation speed, the survival time and the tumour killing effect can be effectively improved. The invention further provides a corresponding expression vector, a transduction system, pharmaceutical application, independent ICD1, ICD2 and ICD3 intracellular signal stimulation domains and IL-15-IL-15alpha fusion protein.
Owner:BEIJING GENE KEY LIFE TECH CO LTD
In-vitro amplification method of iNKT cells
InactiveCN110872575AIncrease the amplification factorIncrease lethalityCulture processBlood/immune system cellsDendritic cellDendrite
Owner:中冠赛尔生物科技(北京)有限公司
Method for generation of regulatory T-cells using factors secreted by iNKT cells
Owner:UNIV OF FLORIDA RES FOUNDATION INC
METHOD FOR GENERATION OF REGULATORY T-CELLS USING FACTORS SECRETED BY iNKT CELLS
ActiveUS20150361397A1Mammal material medical ingredientsBlood/immune system cellsRegulatory T cellCellular secretion
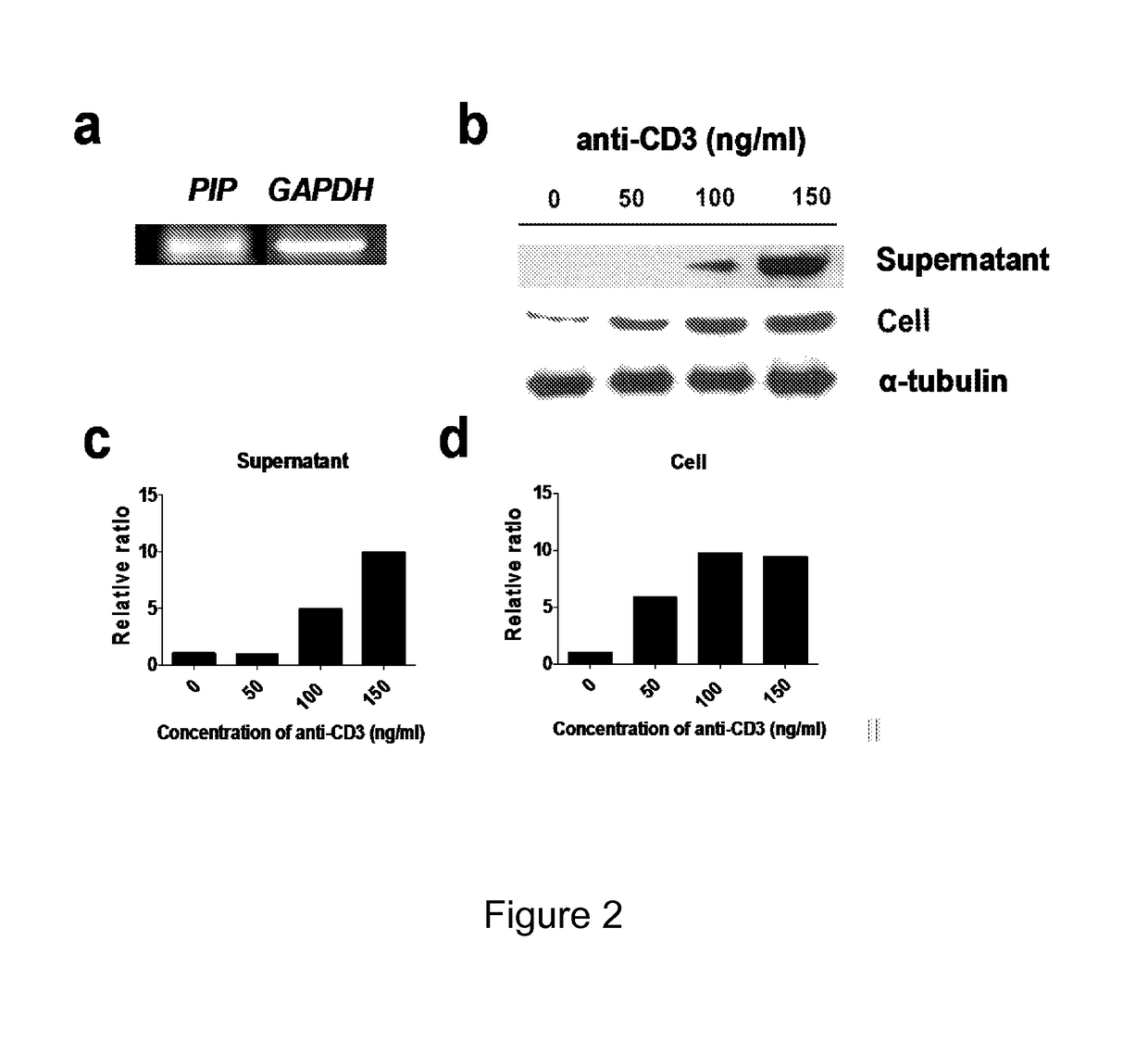
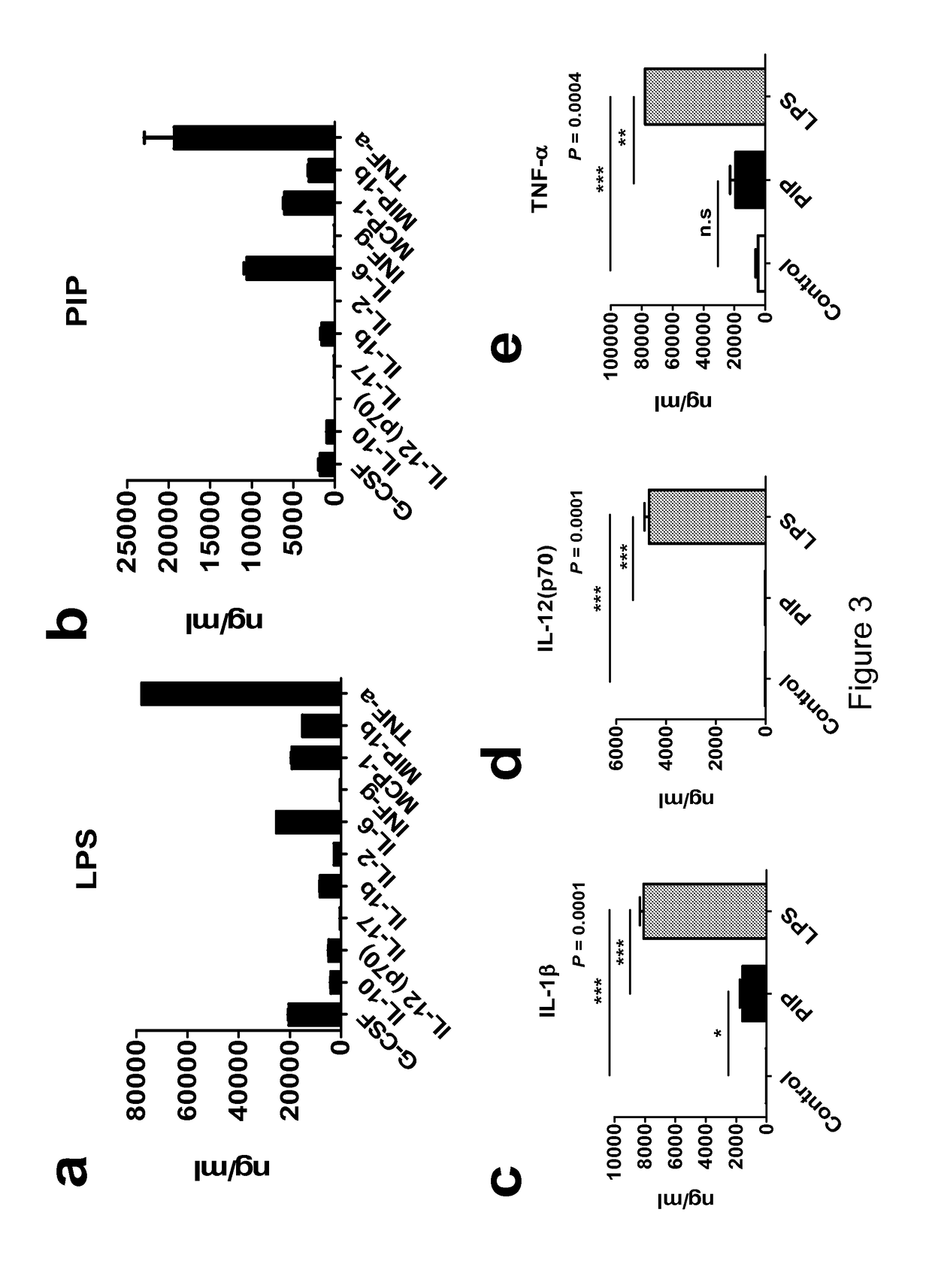
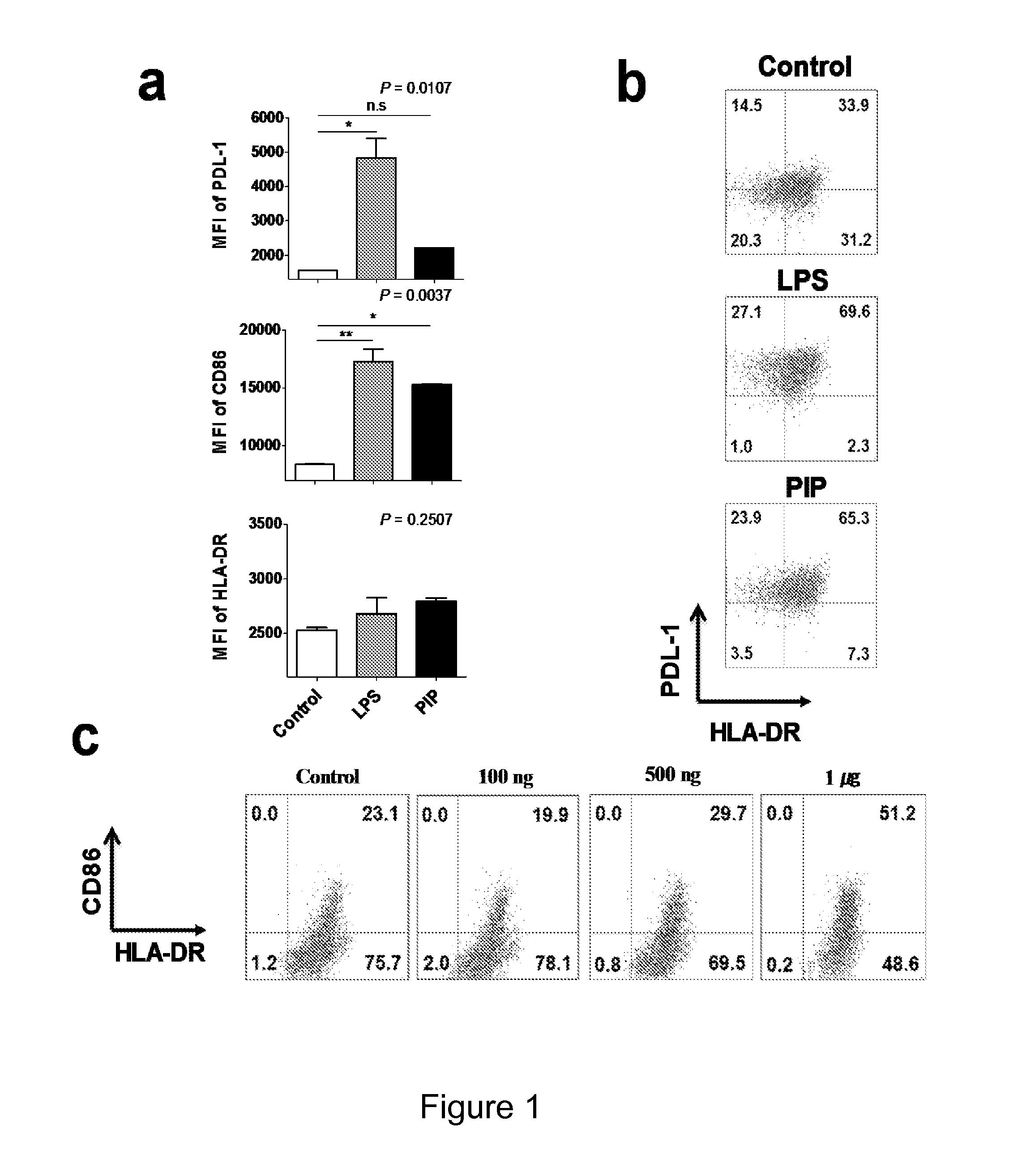
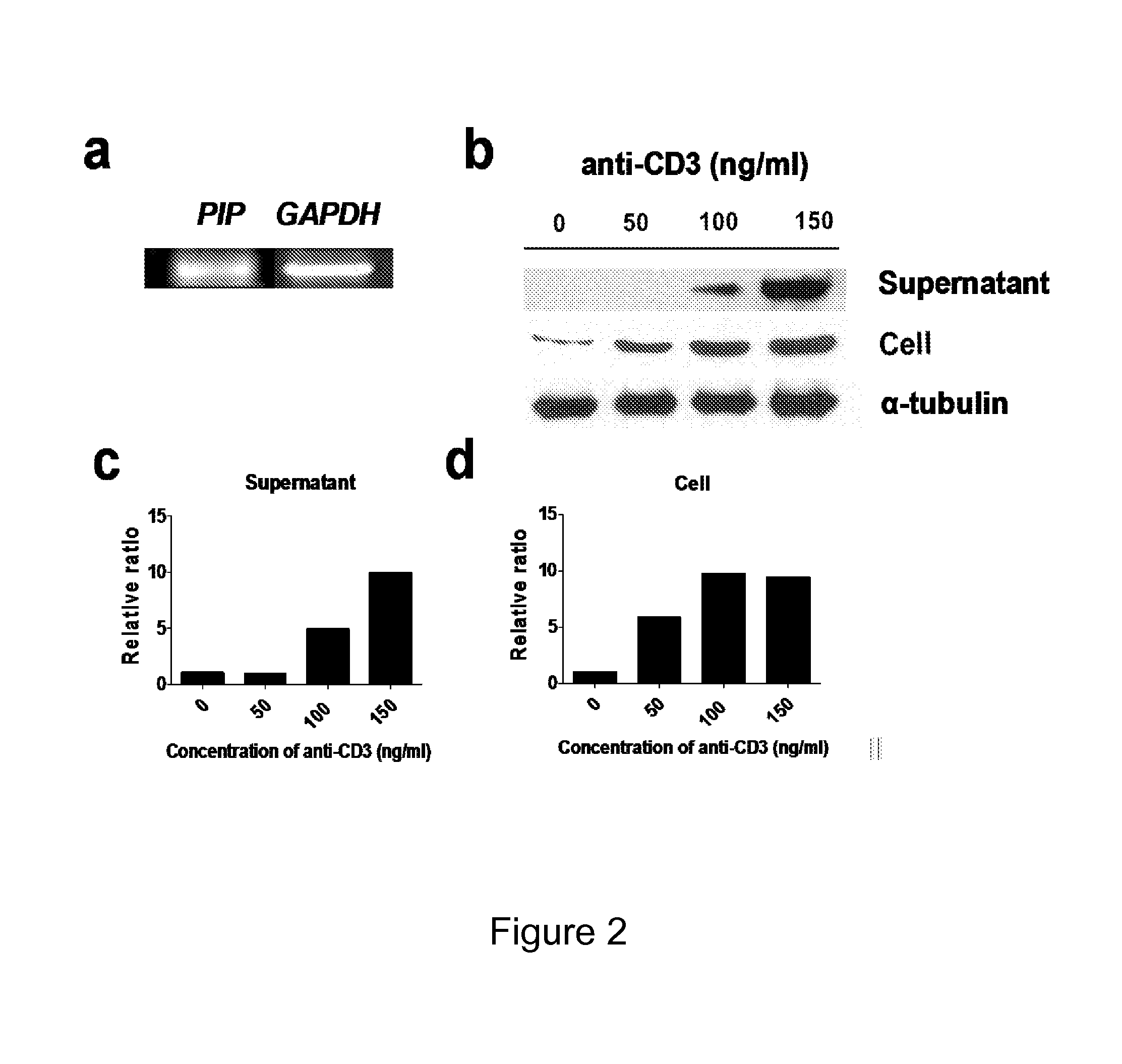
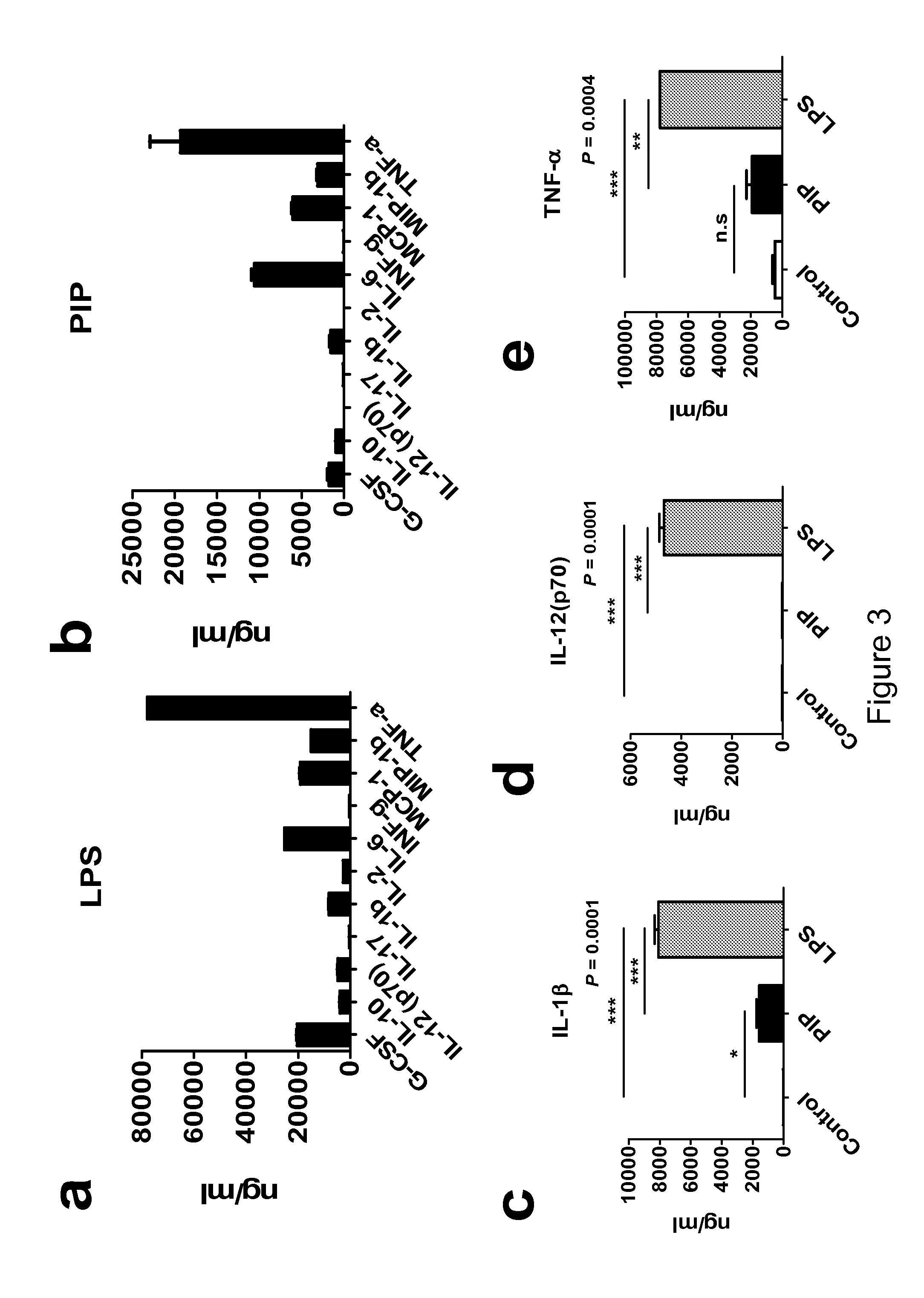
The current invention concerns the use of PIP or S100A8 for the generation of regulatory T cells. The current invention provides a method of generating regulatory T cells by contacting immature DC with PIP or S100A8 to produce tolerogenic DC and further contacting naïve T cells to the tolerogenic DC to produce regulatory T cells. The invention also concerns PIP protein where immature DC contacted with PIP produce tolerogenic DC that induce conversion of naïve T-cells into regulatory T cells. The invention also concerns S100A8 protein where immature DC contacted with S100A8 produce tolerogenic DC that induce conversion of naïve T-cells into regulatory T cells. The invention also provides methods for identification of factors secreted by iNKT cells that induce conversion of immature DC into tolerogenic DC. The subject invention also concerns using regulatory T cells for treatment and / or management of cancer or the diseases of the immune system.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
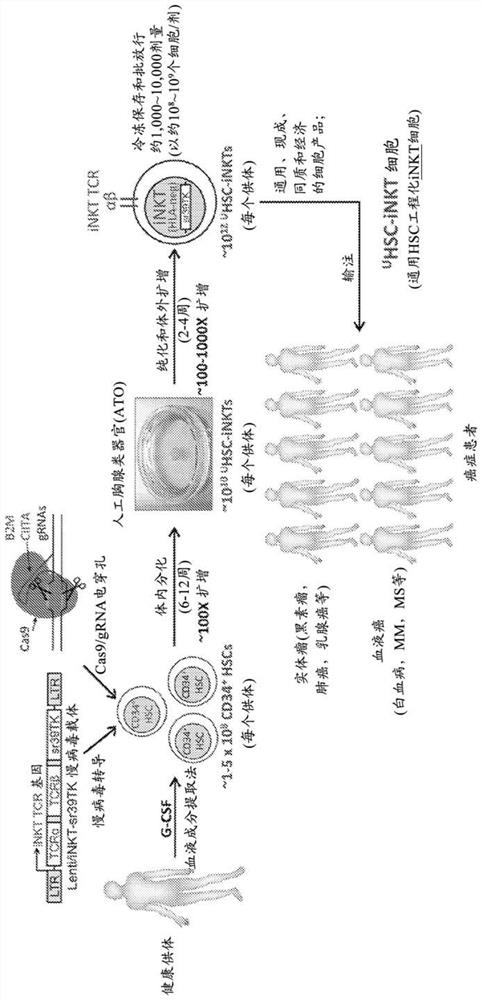
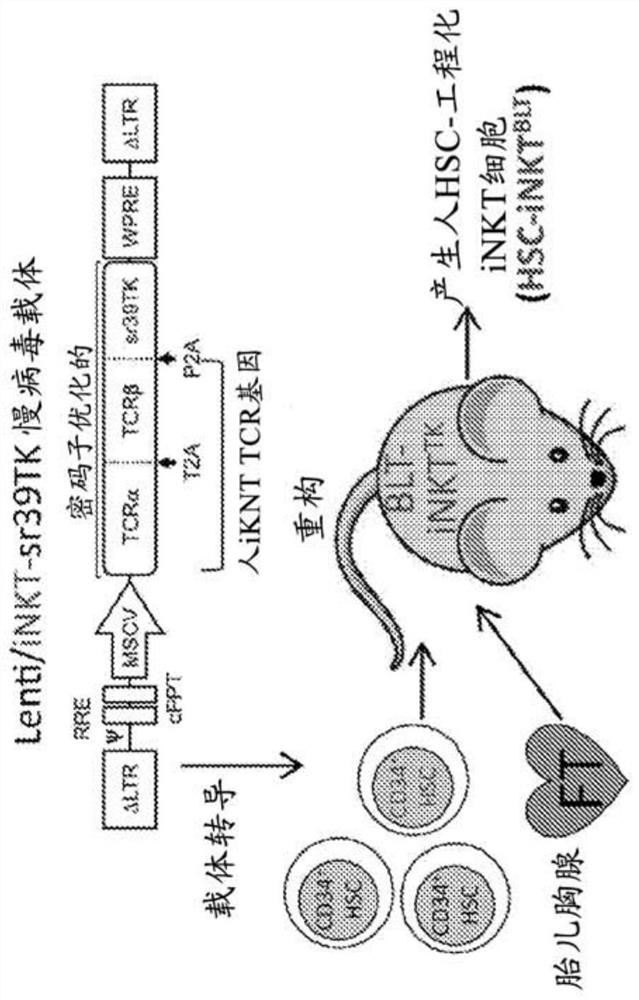
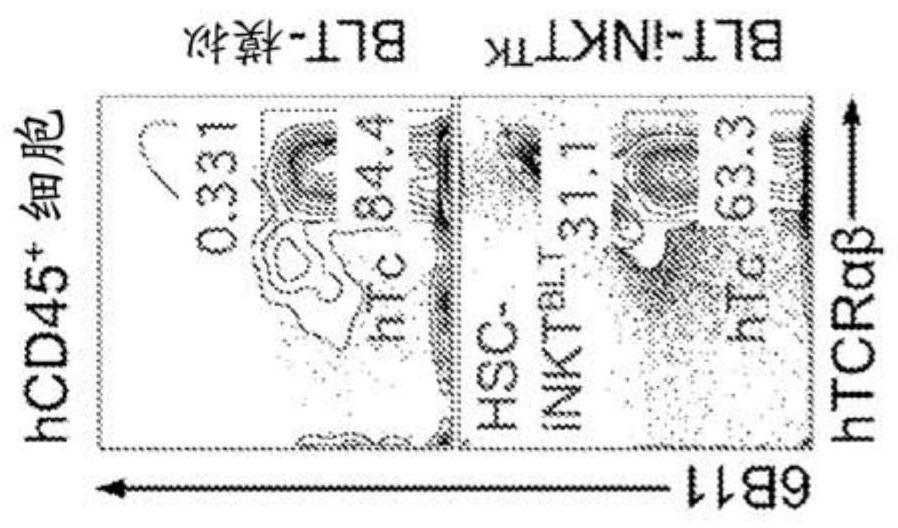
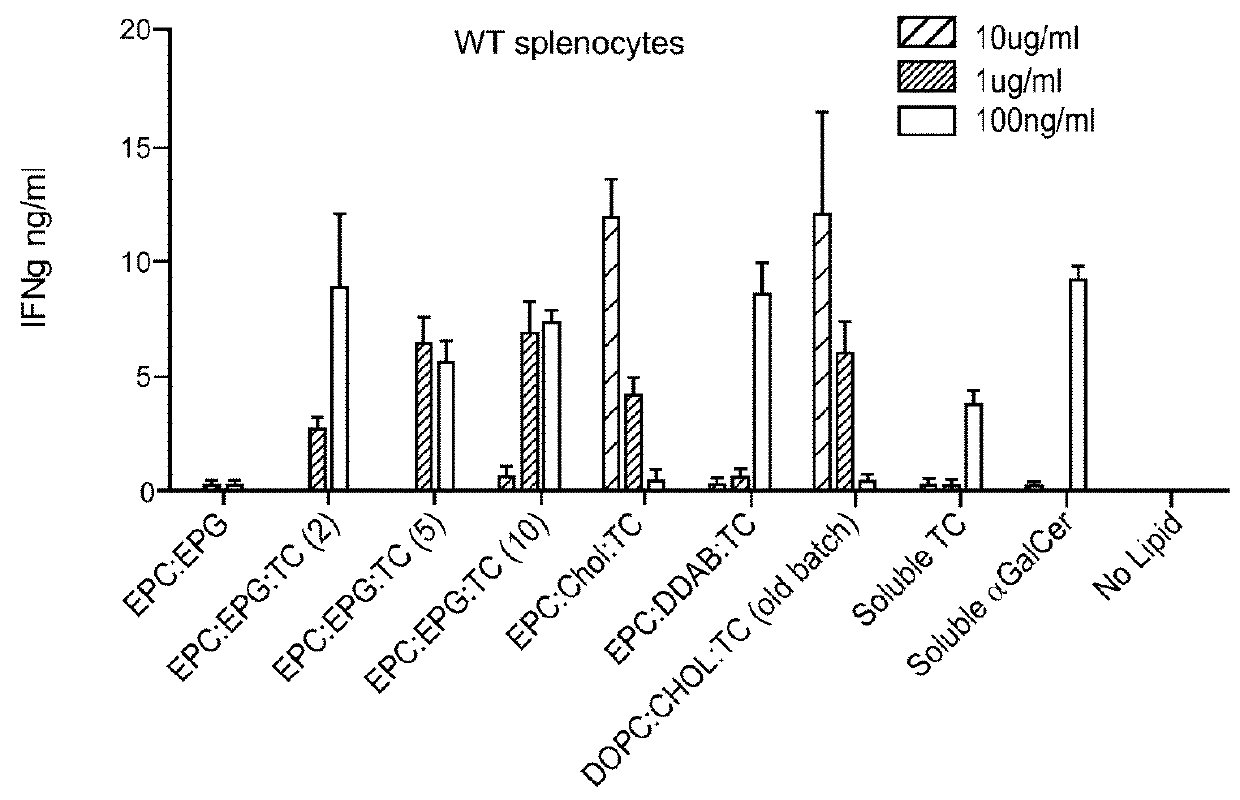
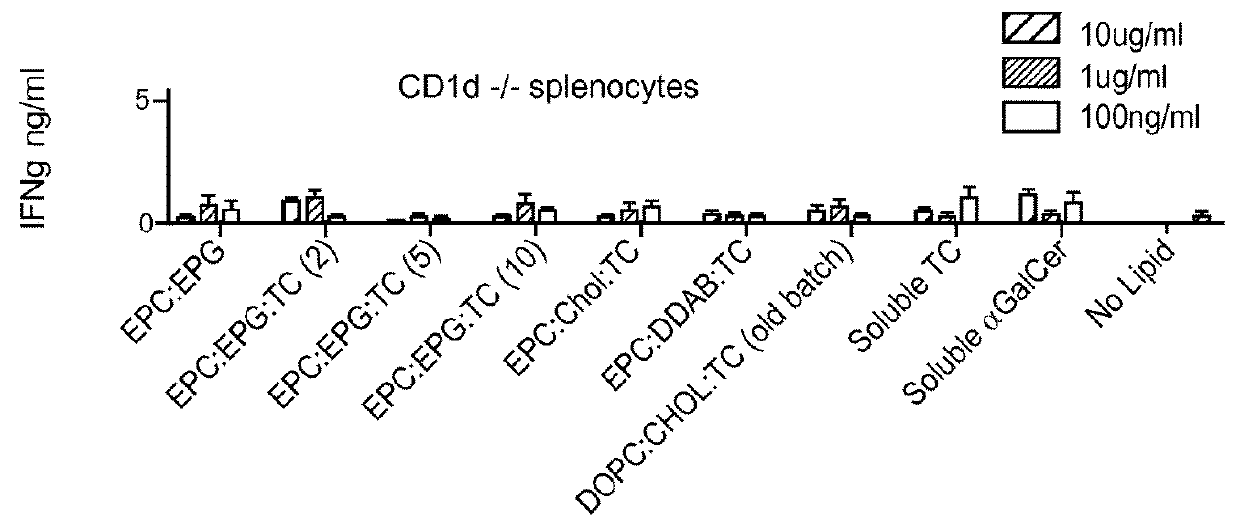
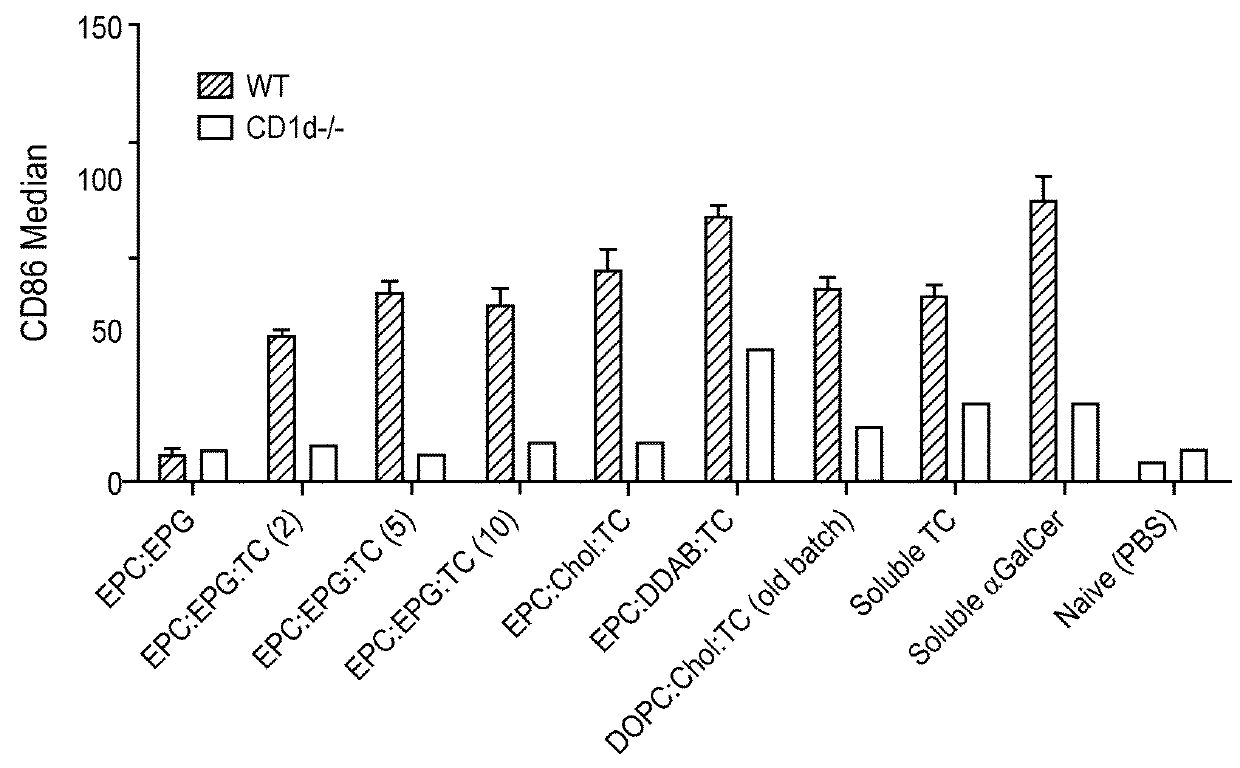
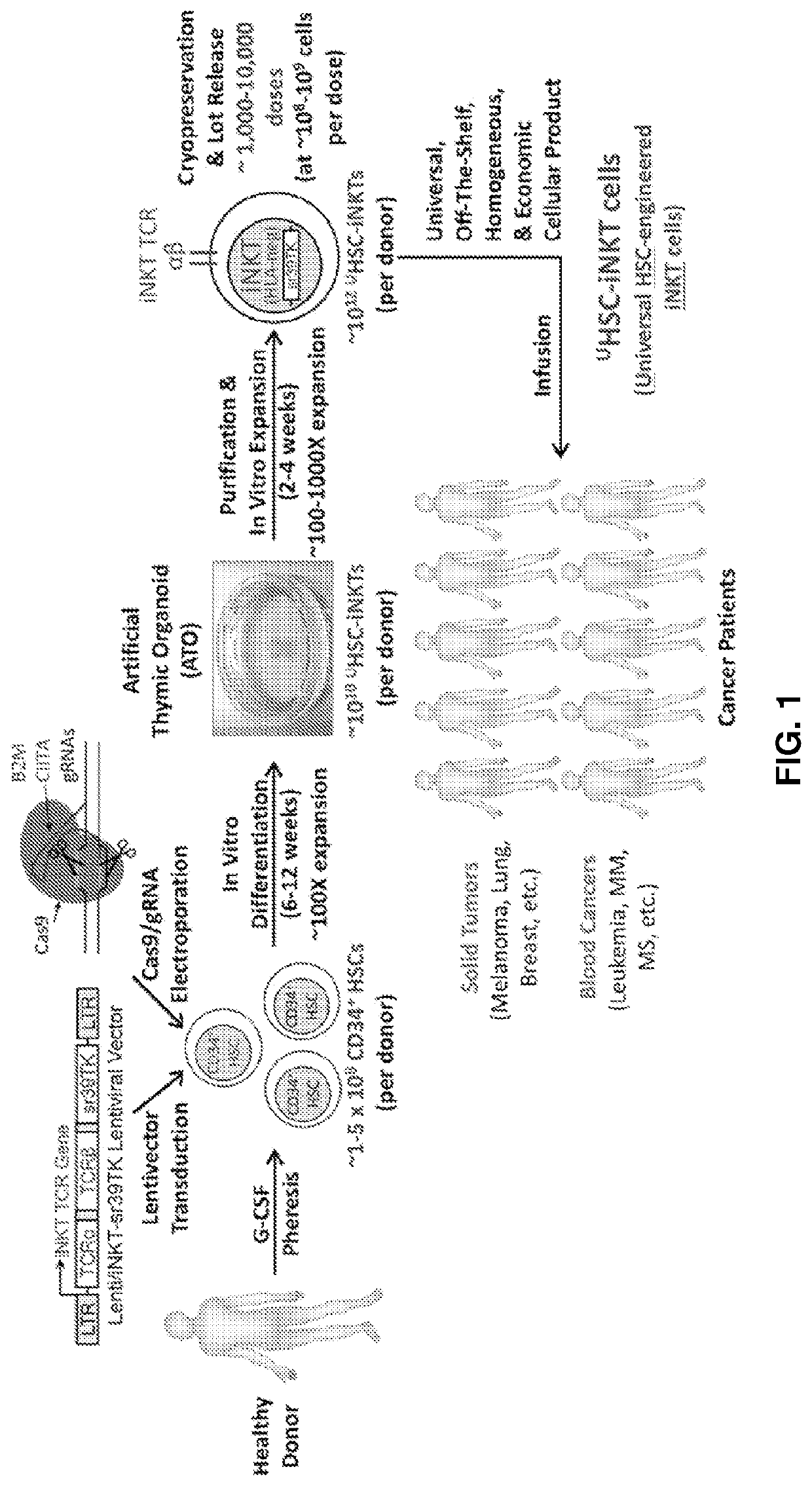
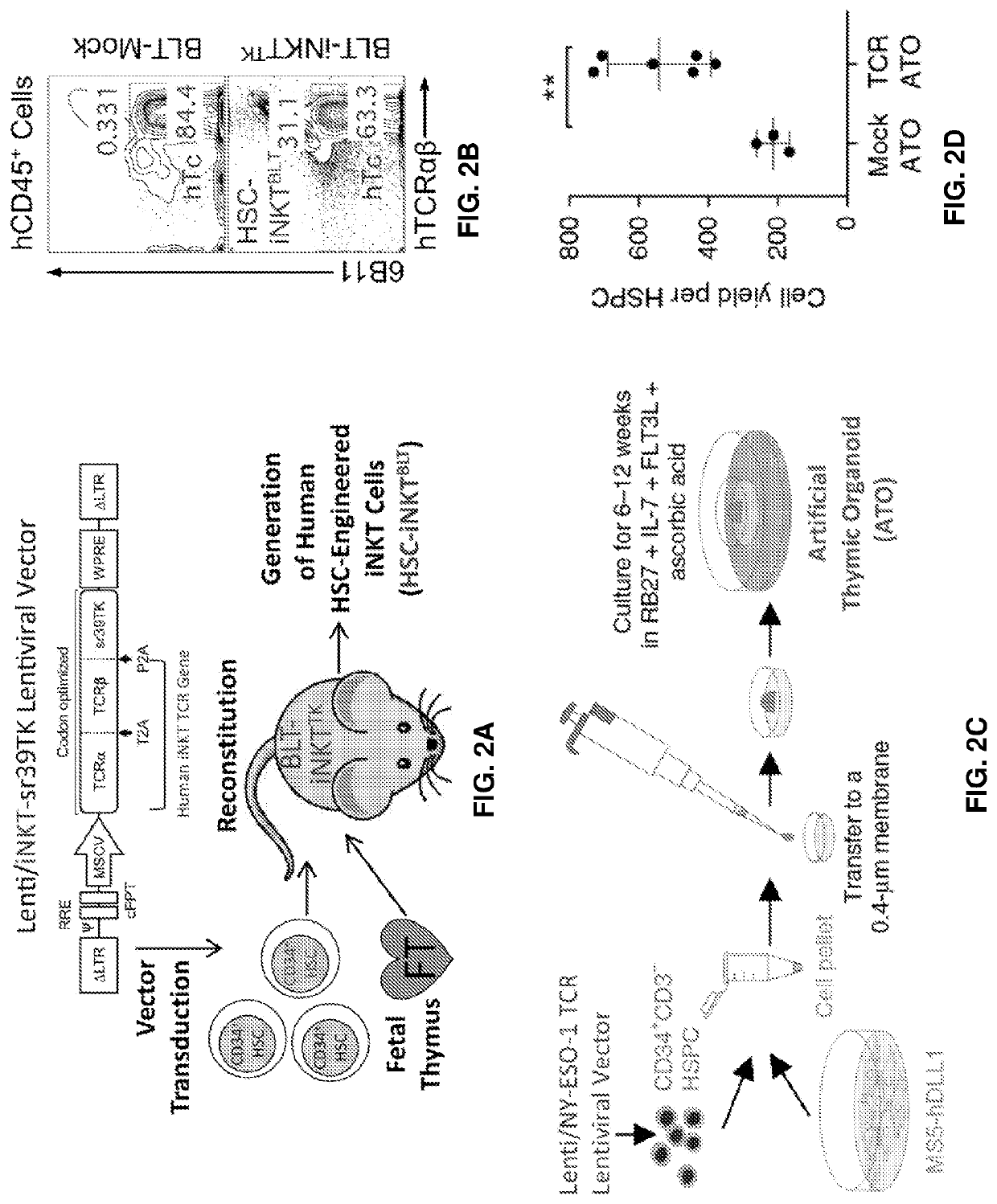
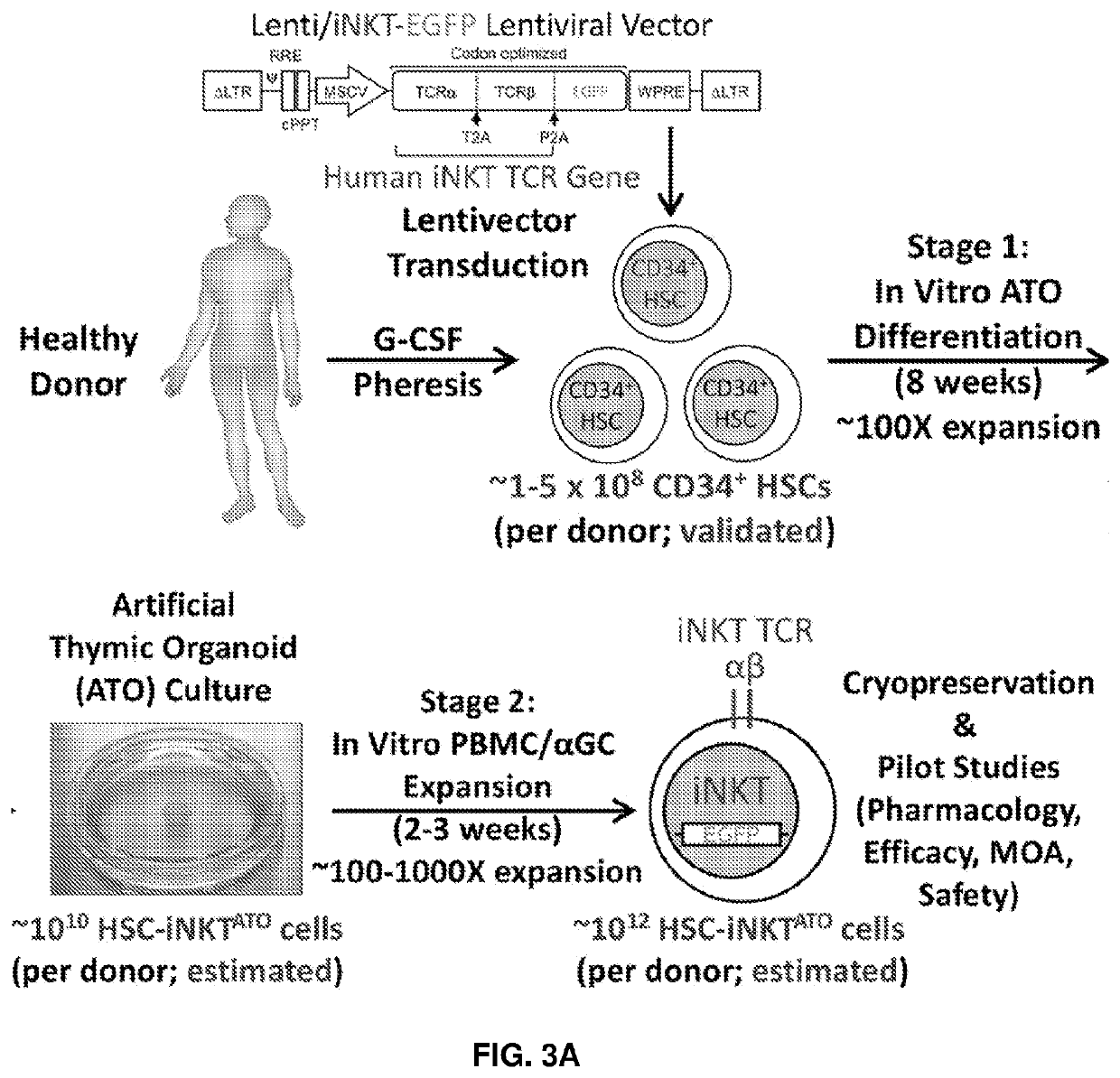
Stem cell-engineered inkt cell-based off-the-shelf cellular therapy
Embodiments of the disclosure include compositions and methods related to engineered invariant natural killer T (iNKT) cells for off-the-shelf use for clinical therapy. In particular embodiments, theiNKT cells are produced from hematopoietic stem progenitorcells and also are suitable for allogeneic cellular therapy because they are HLA negative. In specific embodiments, the cells are cultured ina particular in vitro three-dimensional artificial thymic organoid system and the cells have imaging and suicide targeting capabilities.
Owner:RGT UNIV OF CALIFORNIA
Method for orient induction amplification of thymus gland-derived iNKT (Invariant Natural Killer) cell
ActiveCN107904203AAchieve directed differentiationIncrease the number of cellsCulture processBlood/immune system cellsSpecific functionStimulant
The invention provides a method for orient induction amplification of thymus gland-derived iNKT (Invariant Natural Killer) cells. The method comprises two stages, namely a first stage, including stepsof performing in-vivo stimulation of iNKT cell proliferation, namely stimulating in-vivo iNKT cell proliferation of mice by using a specific stimulant alpha-Galcer; a second stage, including steps ofperforming in-vitro orient induction of iNKT cell proliferation, namely stimulating thymus glands of the mice by using alpha-Galcer, preparing a single-cell suspension, adding alpha-Galcer and an induction agent, assisting iNKT cell amplification, and inducing cell differentiation. With the combination of in-vivo proliferation stimulation and in-vitro induction amplification, orient iNKT cell induction is achieved, and the purpose that a great amount of orient differentiation iNKT cells with specific functions are obtained within a short time is achieved. Complex and tedious steps of in-vitrocell amplification culture are effectively reduced, the cell culture time is shortened, good repeatability is achieved, and the cost is relatively low.
Owner:HEBEI UNIVERSITY
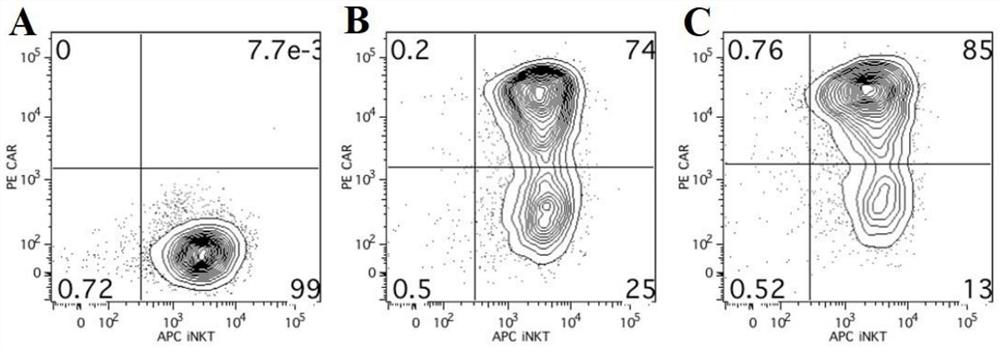
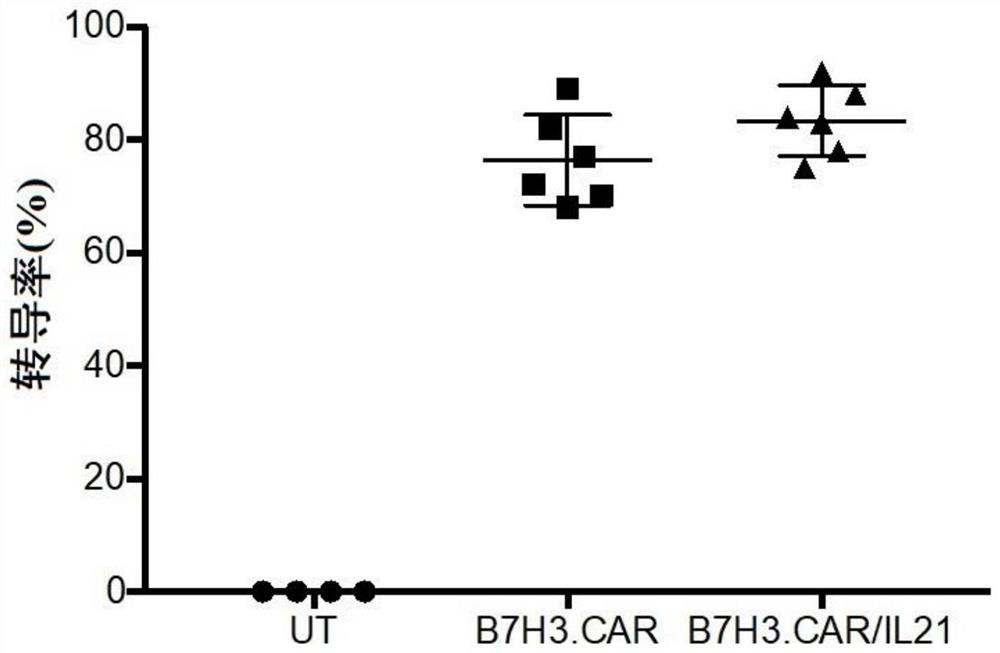
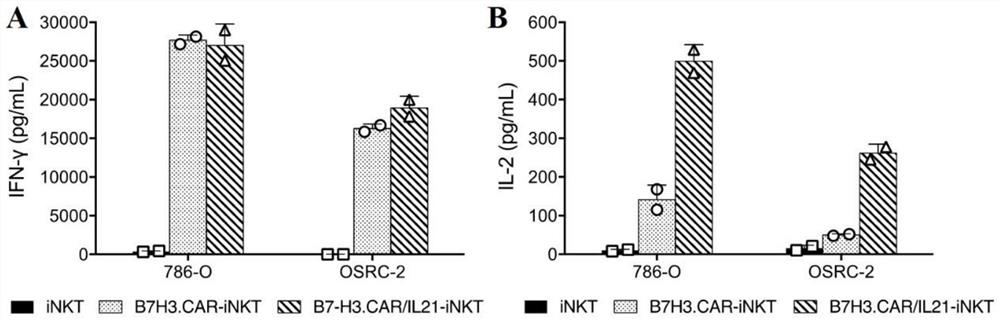
A fully human chimeric antigen receptor targeting b7h3 co-expressing IL-21, inkt cells and its use
ActiveCN113402619BGood killing effectEfficient removalNucleic acid vectorImmunoglobulinsAntigen receptorAntigen binding
The invention discloses a fully human chimeric antigen receptor targeting B7H3 and co-expressing IL-21, iNKT cells and applications thereof. The chimeric antigen receptor comprises a B7H3 antigen binding domain, a transmembrane domain, a cell Inner signaling domain and IL-21, it has been verified by experiments that the B7H3.CAR / IL-21-iNKT cells targeting B7H3 prepared by the present invention have strong cytokine release ability and tumor cell killing ability, and can effectively eliminate tumor cells , has important application prospects in the field of tumor cell immunotherapy.
Owner:XUZHOU MEDICAL UNIV
Liposomal formulation of nonglycosidic ceramides and uses thereof
The invention provides liposomes containing nonglycosidic ceramides within their bilayers, and compositions thereof. These liposomes activate murine iNKT cells and induce dendritic cell (DC) maturation, both in vitro and in vivo at an efficacy that is comparable to their corresponding soluble nonglycosidic ceramides. Also provided are methods for treating diseases using the liposomes and compositions of the invention.
Owner:LUDWIG INST FOR CANCER RES LTD
Stem cell-engineered inkt cell-based off-the-shelf cellular therapy
PendingUS20210123022A1Improve availabilityImprove the usefulnessImmunoglobulin superfamilyPeptide/protein ingredientsAllogeneic cellNatural killer cell
Embodiments of the disclosure include compositions and methods related to engineered invariant natural killer T (iNKT) cells for off-the-shelf use for clinical therapy. In particular embodiments, the iNKT cells are produced from hematopoietic stem progenitor cells and also are suitable for allogeneic cellular therapy because they are HLA negative. In specific embodiments, the cells are cultured in a particular in vitro three-dimensional artificial thymic organoid system and the cells have imaging and suicide targeting capabilities.
Owner:RGT UNIV OF CALIFORNIA
HUMAN iNKT CELL ACTIVATION USING GLYCOLIPIDS
ActiveUS20180170957A1More potent anticancer activitiesImprove protectionOrganic active ingredientsSugar derivativesGlycosphingolipidHuman cell
Owner:ACAD SINIC
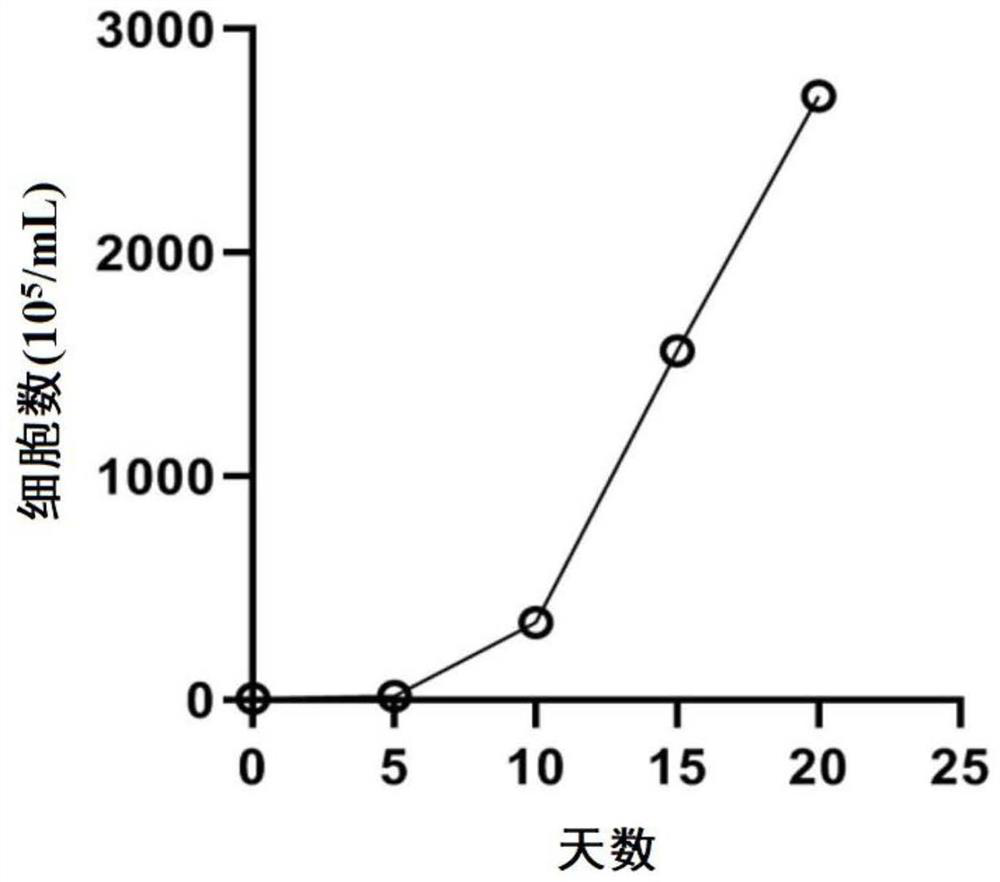
Simple and effective method for induced amplification of iNKT cells and application
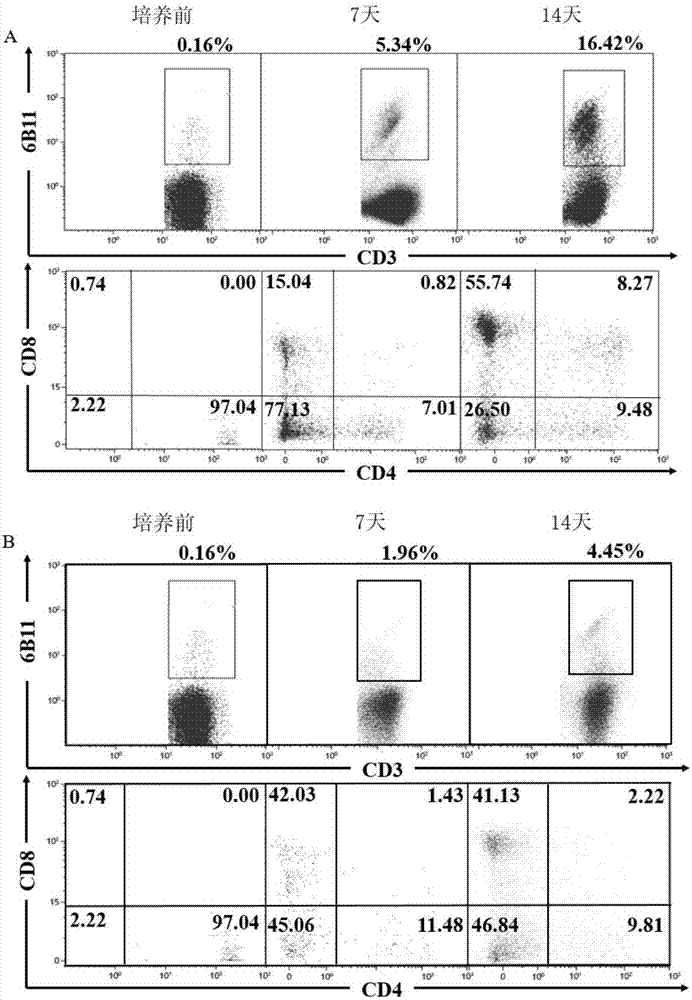
The invention provides a method for induced amplification of iNKT cells. According to the method, the initial demand quantity is small (-10<6> orders of magnitude), the proportion of the obtained iNKT cells is high, the proportion of the iNKT cells is increased to 20-80% from 0.01-1% after 7-9 days, the efficiency can be remarkably improved, and the final amplification of the iNKT cells exceeds 2000 times; and the prepared cells can secrete Th1 type cytokines, highly express CD62L, have long in-vivo survival time and can meet the requirements of clinical treatment.
Owner:XUZHOU MEDICAL UNIV
Methods for determining the risk of acute graft versus host disease
ActiveUS10151745B2Enhance and regulate activityRapid responseDisease diagnosisBiological testingCandidate donorSub populations
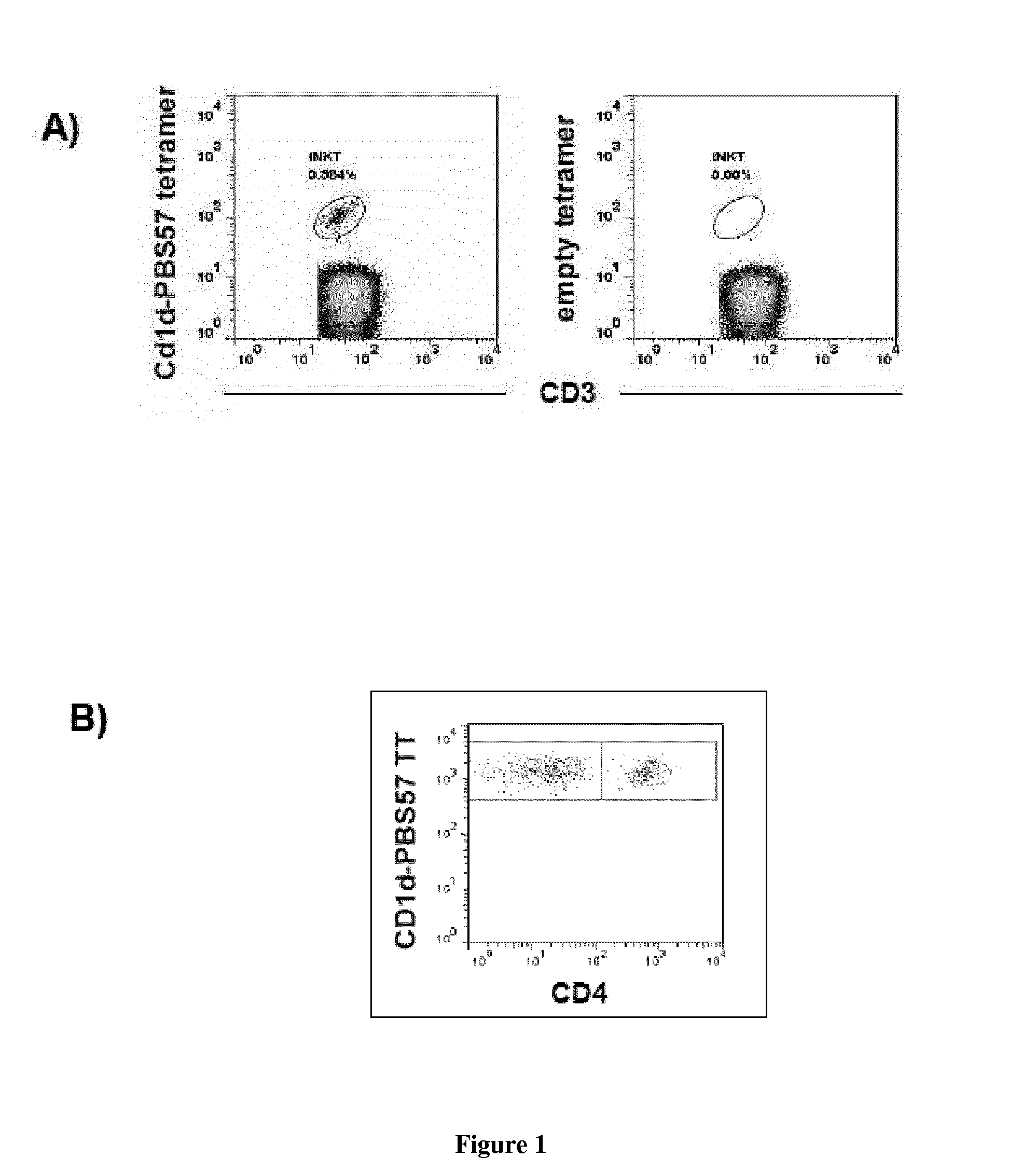
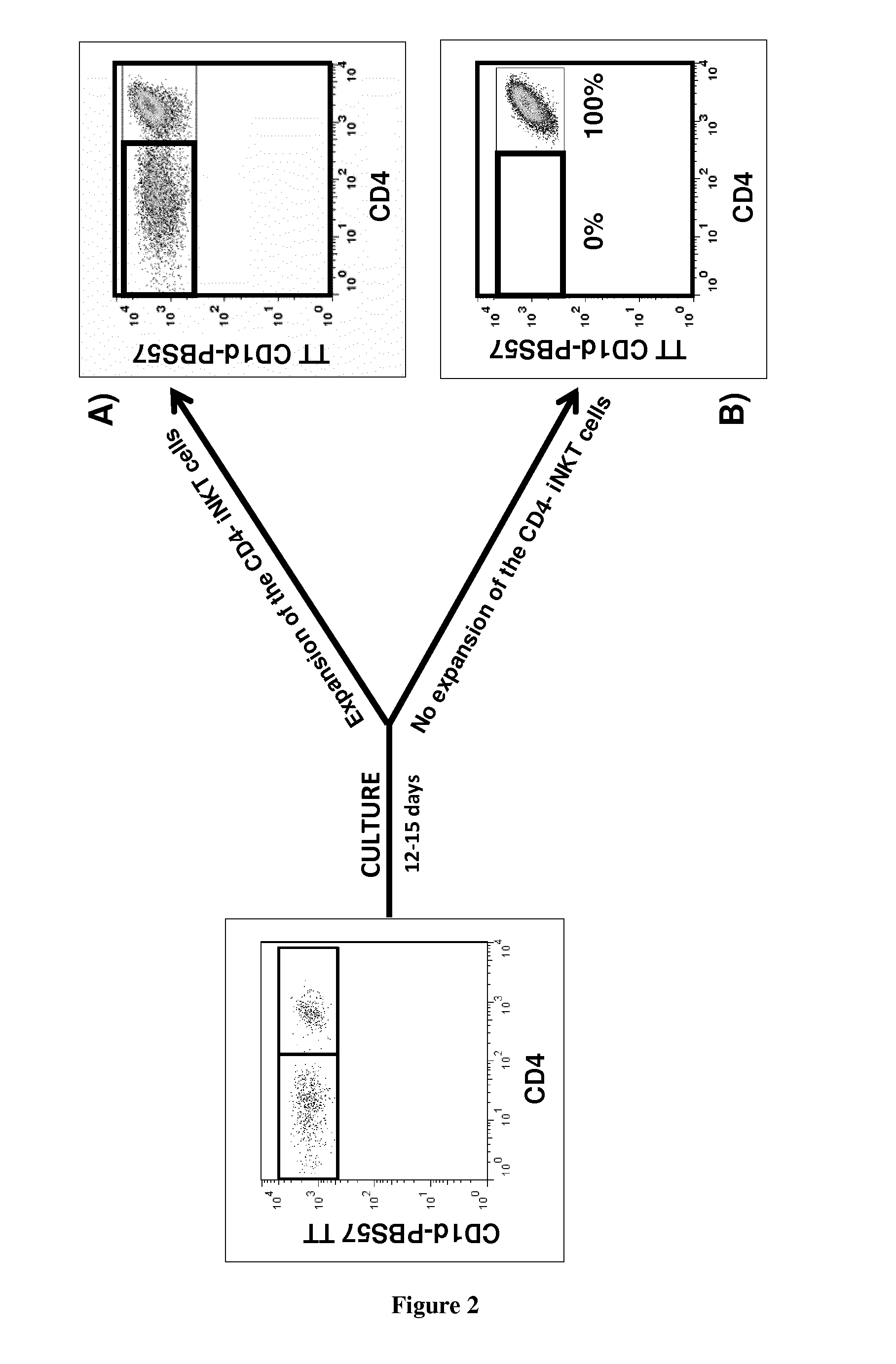
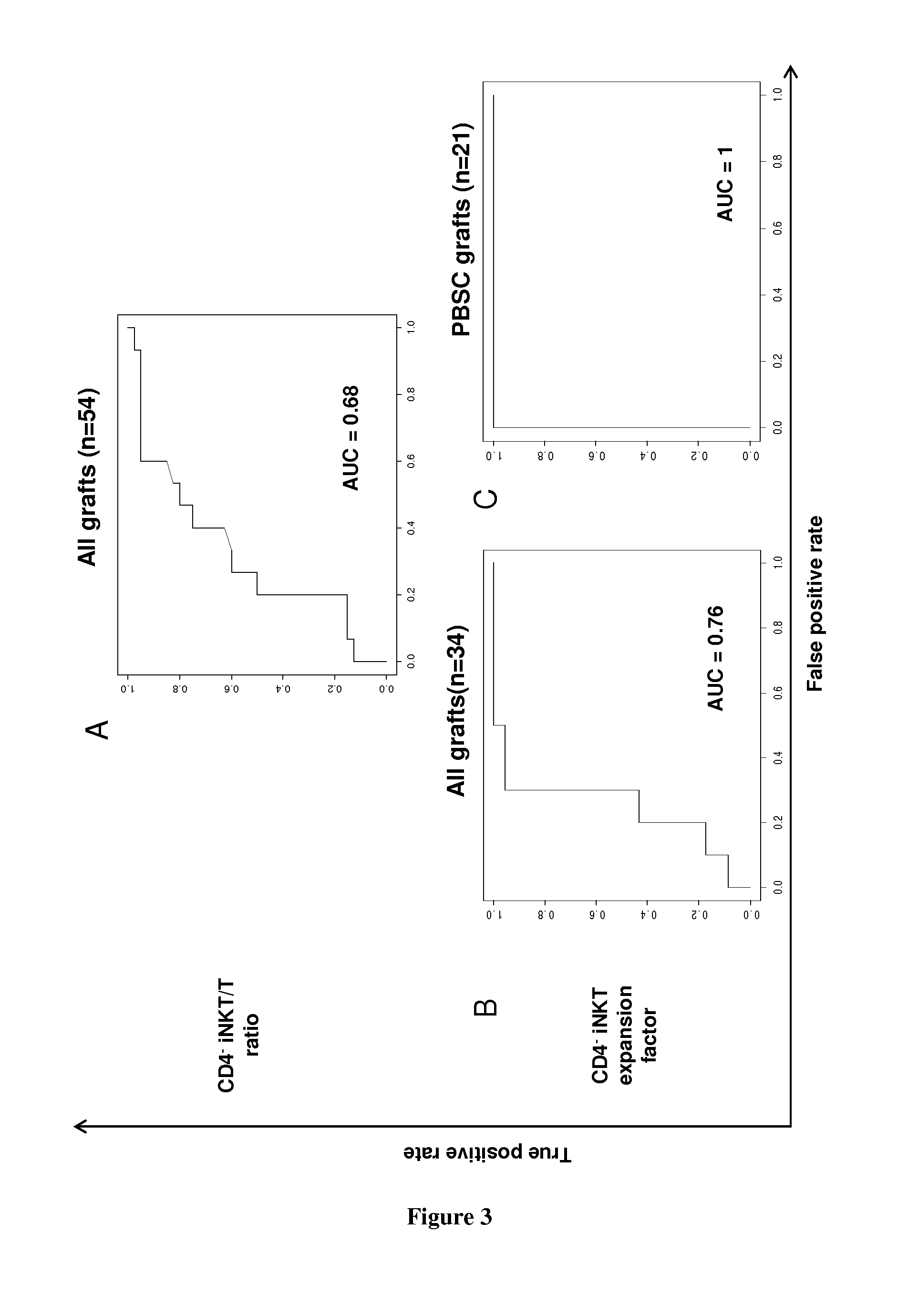
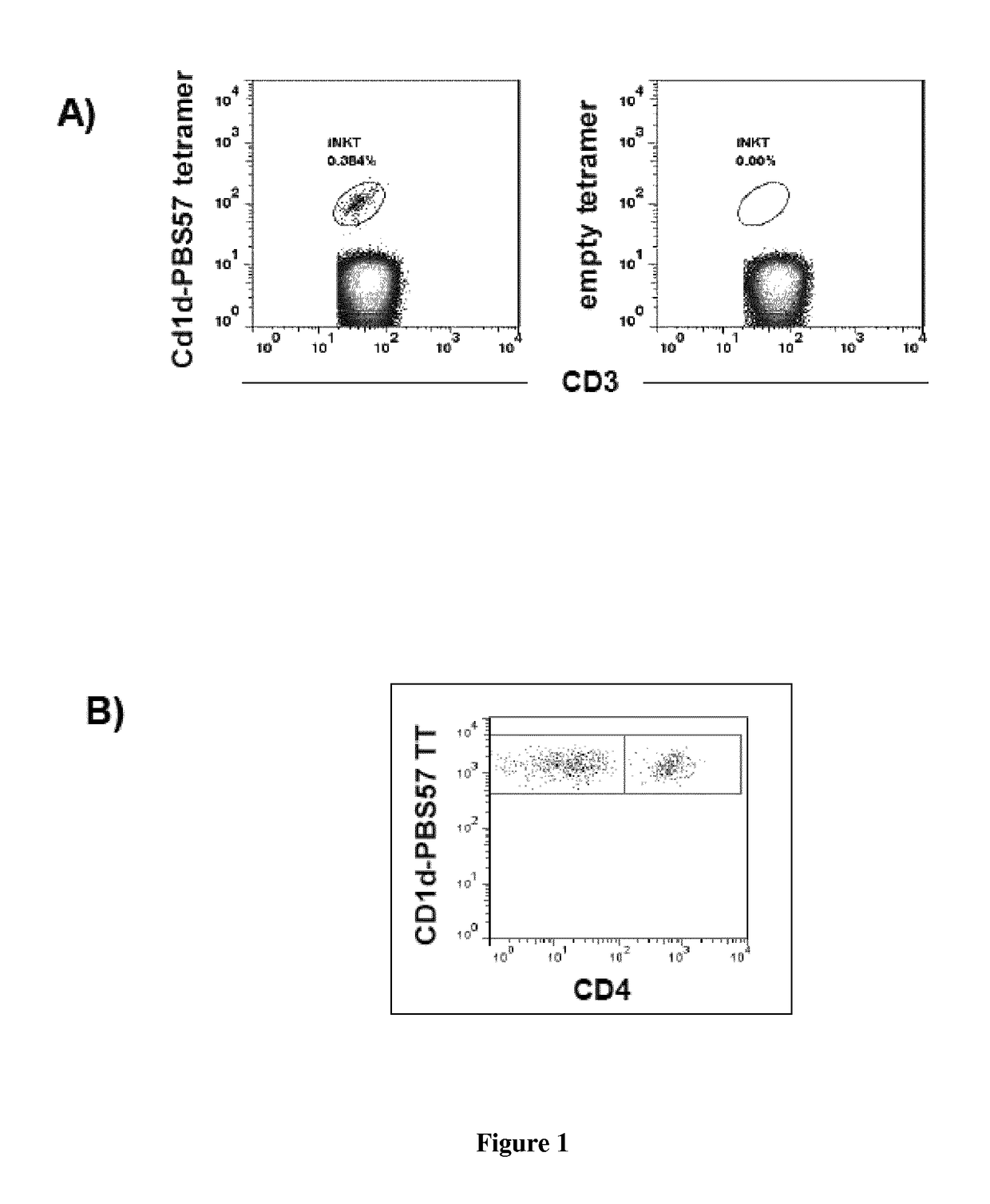
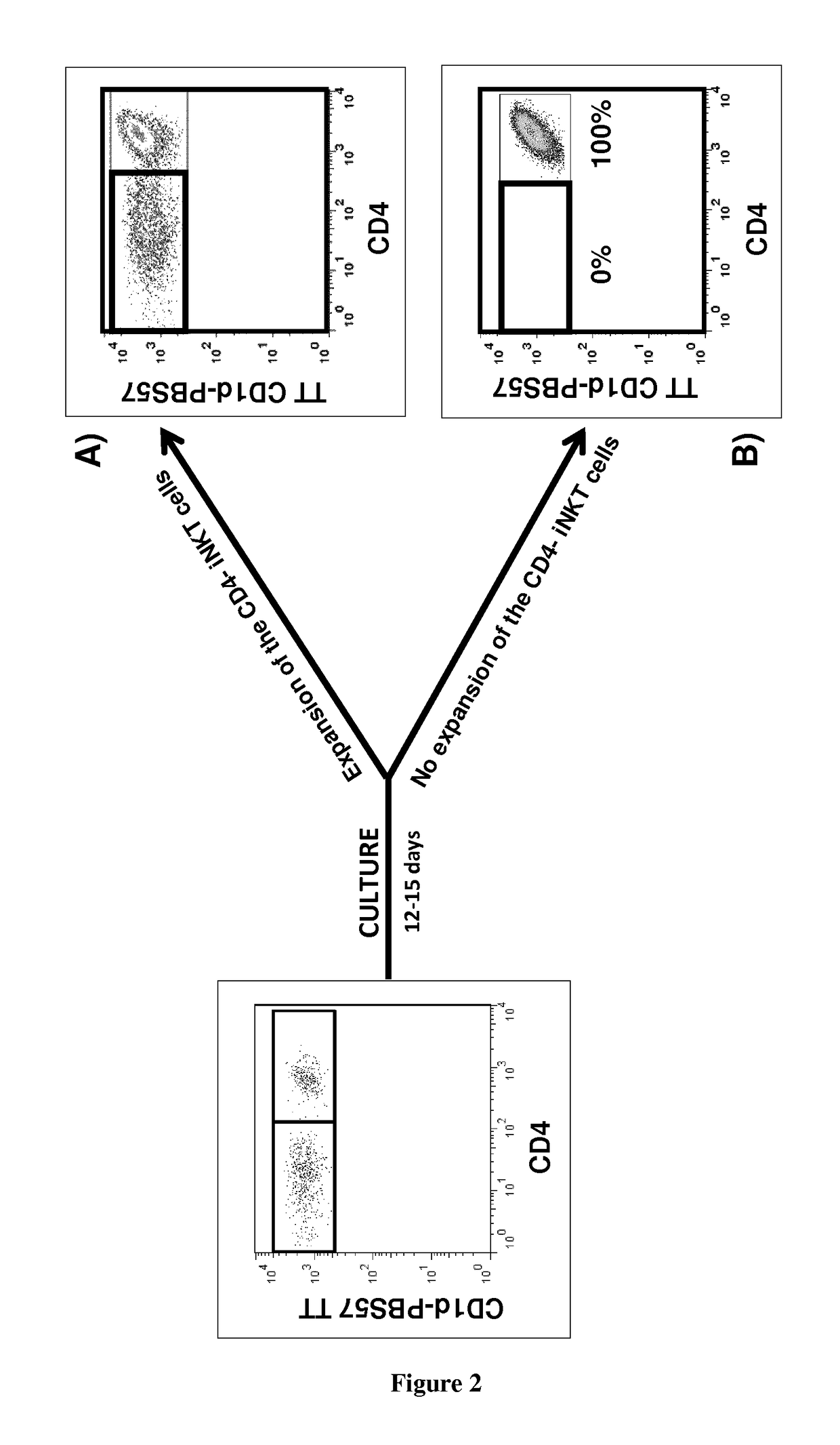
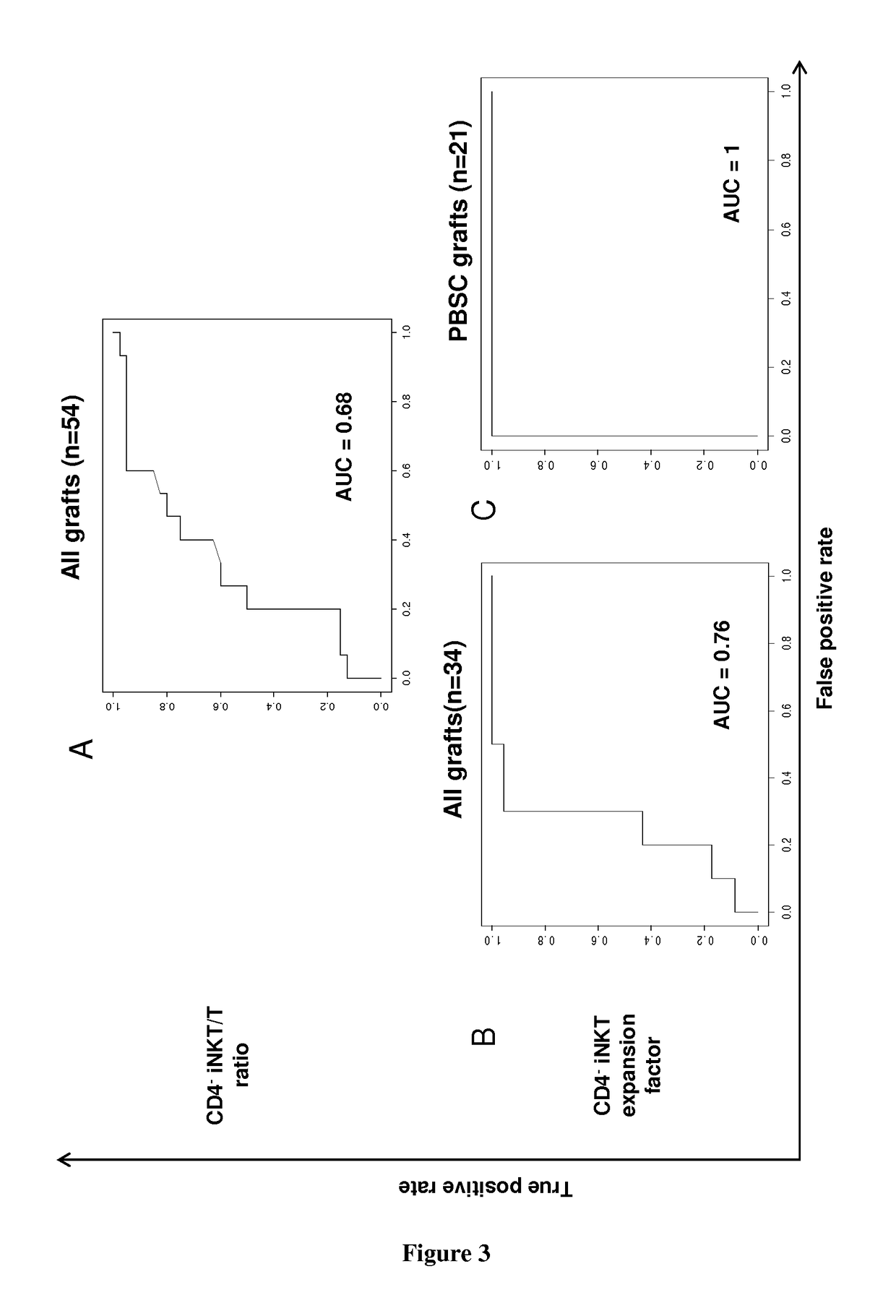
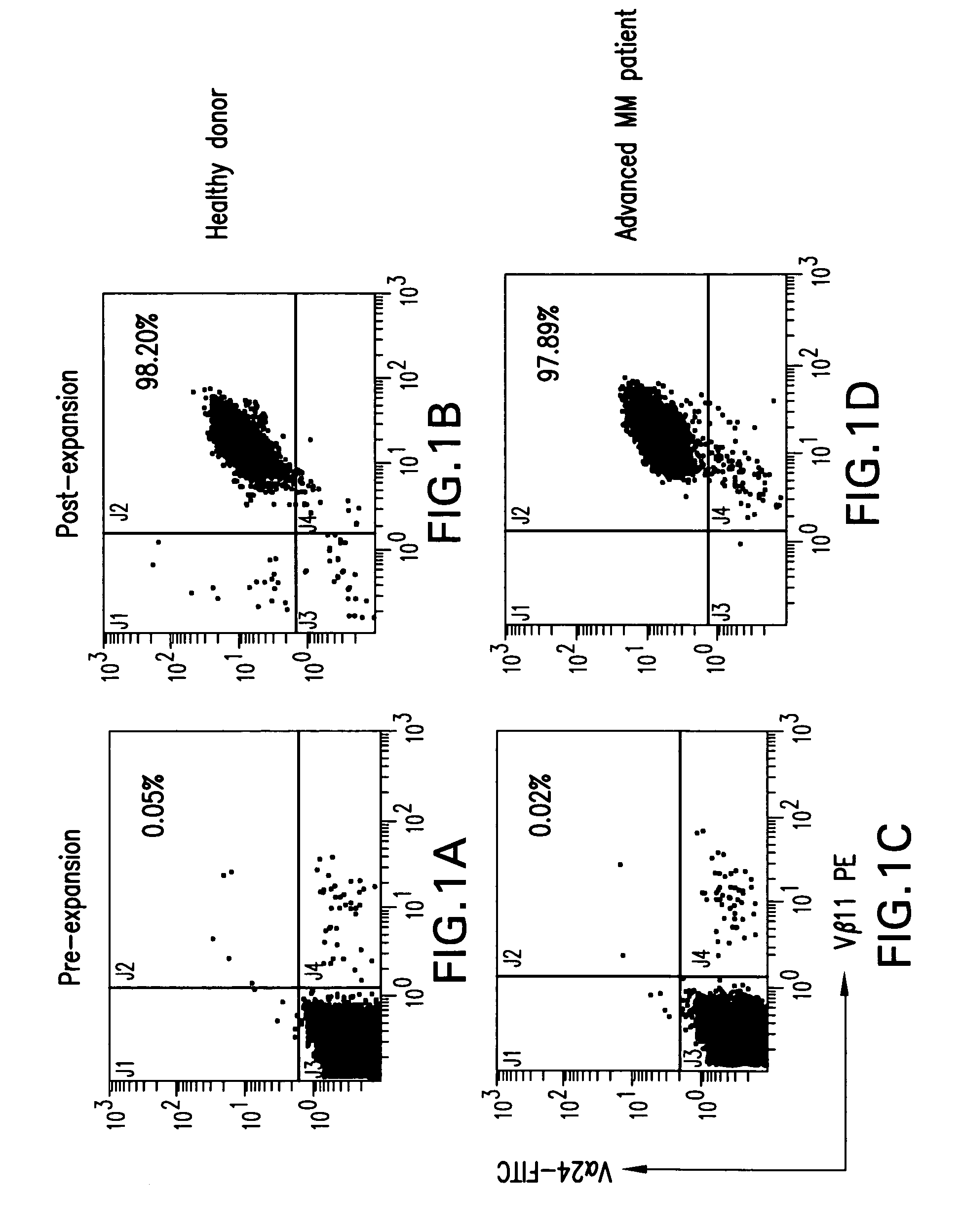
The present invention relates to a method for determining whether a candidate human transplant donor is at risk of inducing acute graft versus host disease (aGVHD) in a human transplant recipient, which may in turn allow the selection of a donor exhibiting no risk for the recipient. The present invention also relates to a method for adjusting the immunosuppressive treatment administered to a human transplanted recipient following its graft transplantation after having performing the method for determining risk of the invention. The methods comprise expanding the candidate donor's iNKT cells (invariant NKT cells) and determining the presence or absence of expansion of the CD4(−) iNKT cell sub-population. In particular, CD3+CD4− TCRV[alpha]24V[beta]11 cells are determined. Kits are disclosed.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +4
Multicell conjugates for activating antigen-specific t cell responses
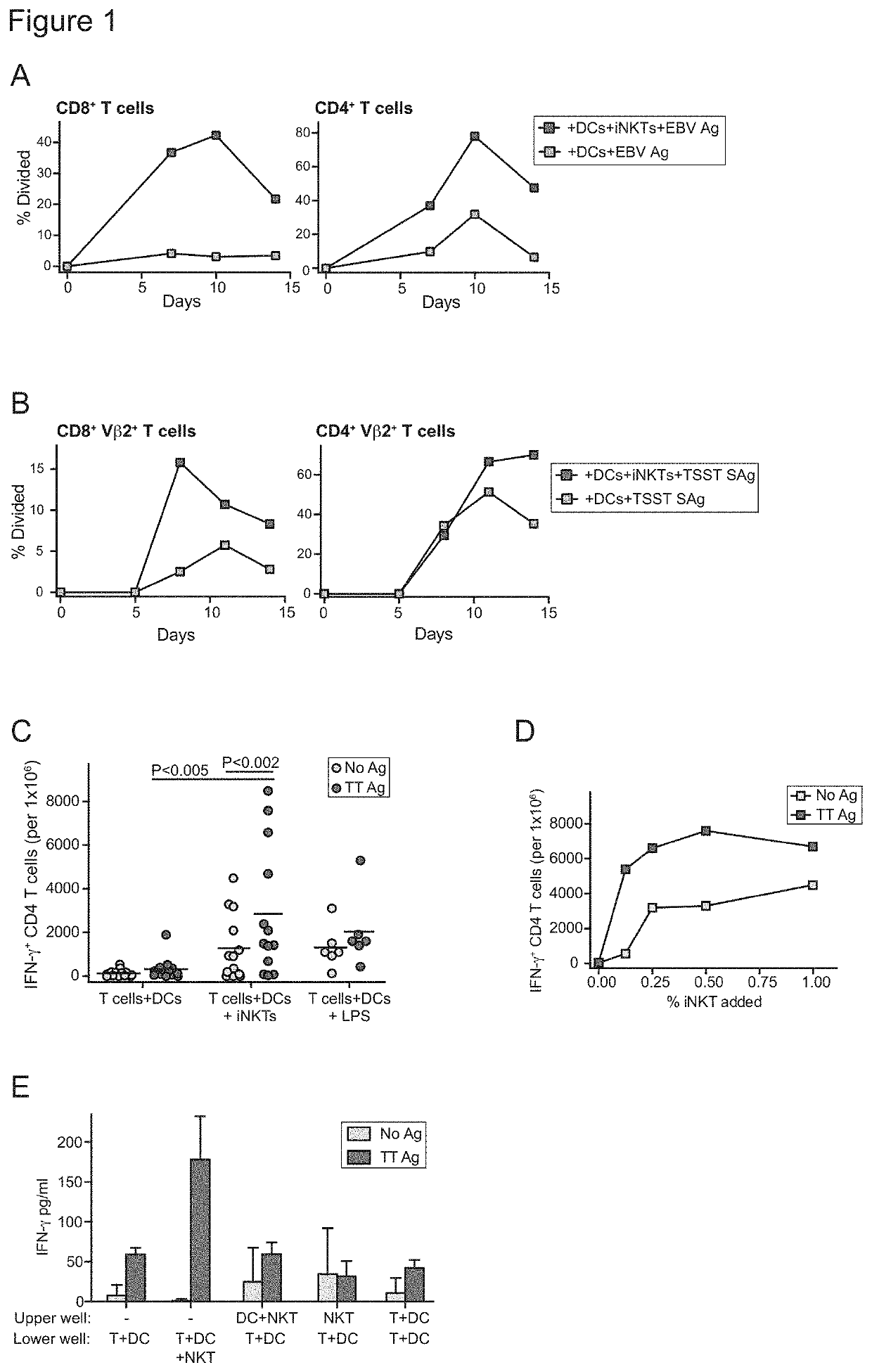
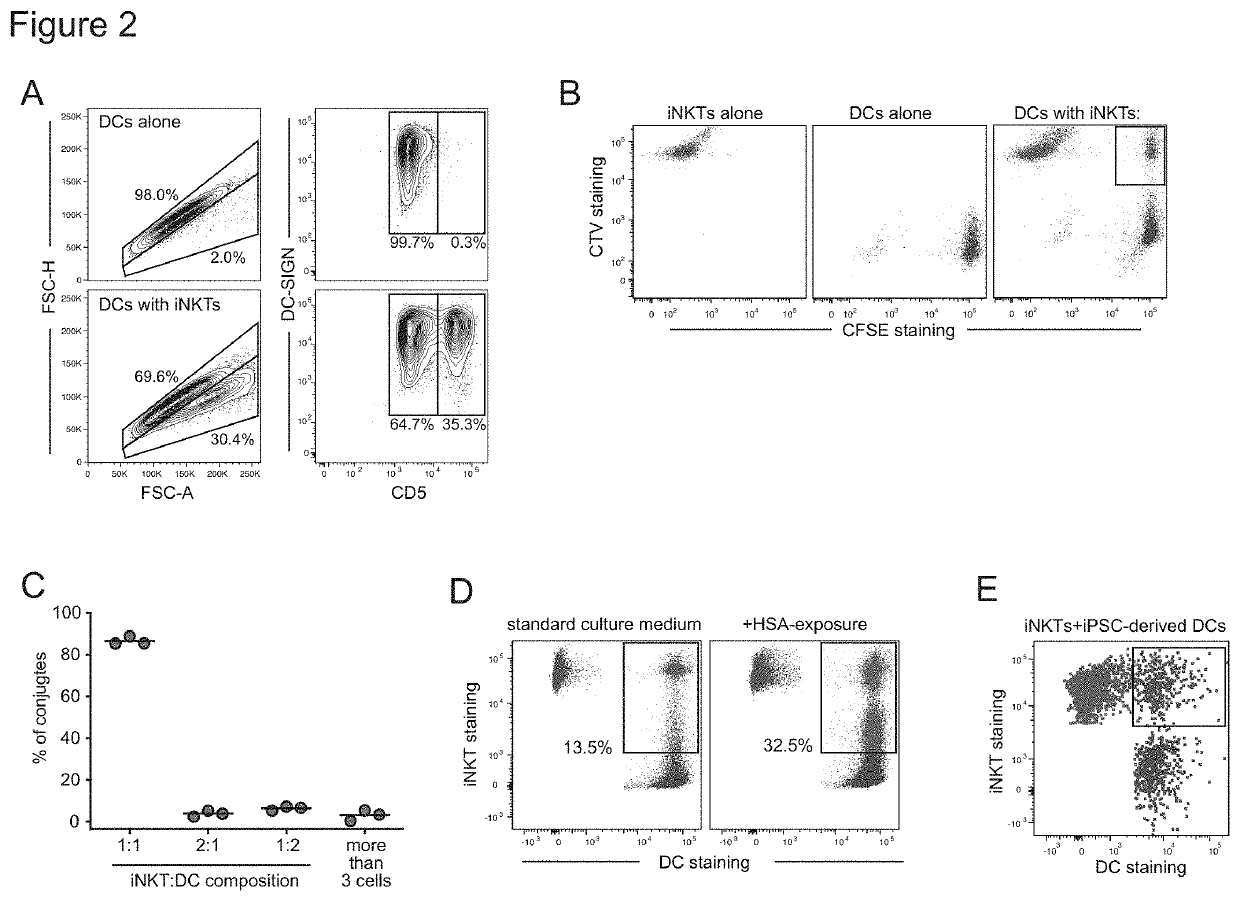
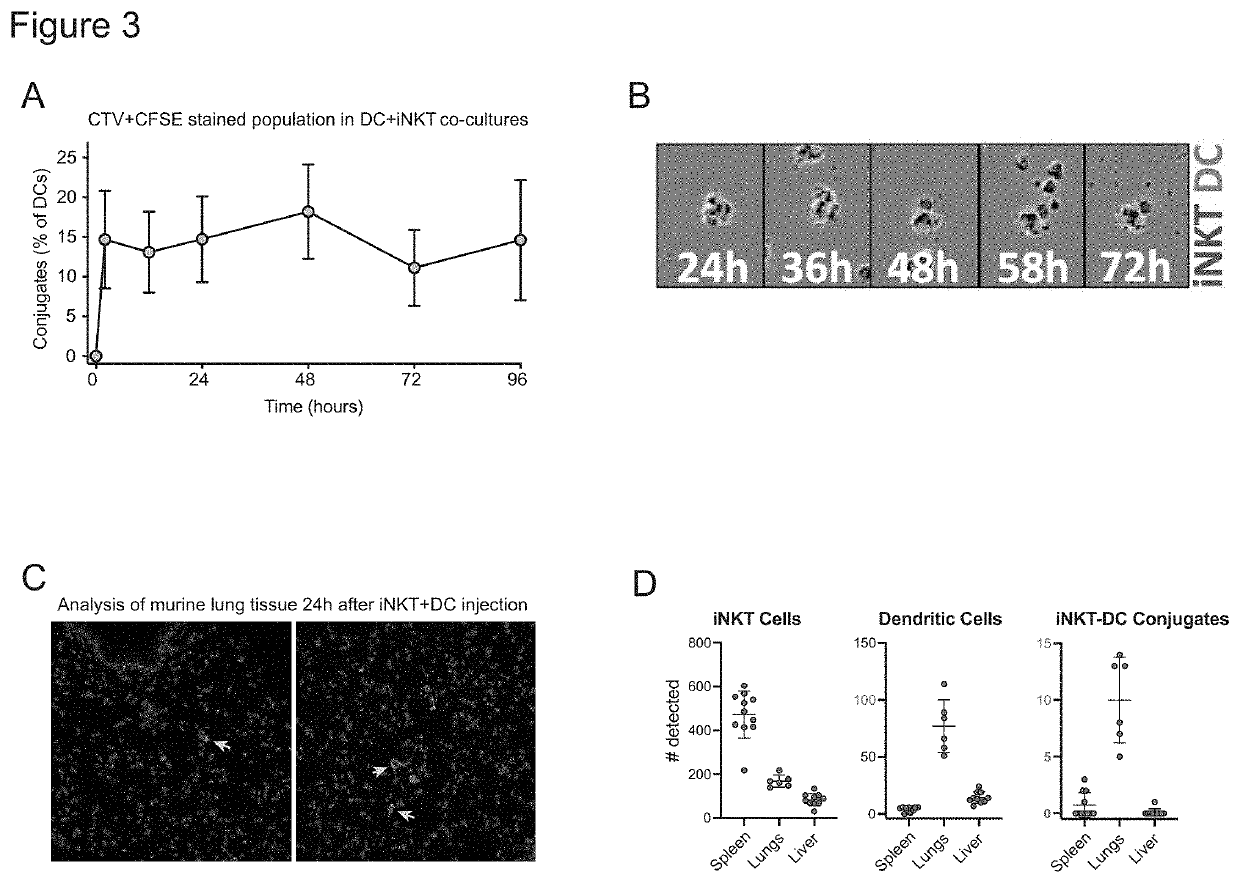
The present invention provides in vitro derived a multicell conjugate comprising an iNKT cell and a dendritic cell (DC). The invention also provides methods of making the multicell conjugate and methods of using the multicell conjugate and compositions comprising the same to treat one or more conditions associated with an antigen or methods of activating an immune response.
Owner:WISCONSIN ALUMNI RES FOUND
Preparation method and application of combination of tumor antigen-targeting antibody and iNKT cell
PendingCN111166878AStrong specificityEnhance immune functionPharmaceutical delivery mechanismMammal material medical ingredientsTumor targetAntiendomysial antibodies
The invention provides application of a combination of a tumor antigen-targeting antibody and an immune cell iNKT in preparation of anti-tumor drugs. The invention also provides a preparation method of the combination. The iNKT cell is adopted as an effector cell of the combination, and the tumor antigen-targeting antibody and the iNKT cell are combined, so that higher killing activity on tumor target cells is obtained, and the anti-tumor ability is further improved.
Owner:SHANGHAI SINOBAY BIOTECH CO LTD
Culturing method and application of CD19CAR-iNKT cell
ActiveCN108165568AMammal material medical ingredientsNGF-receptor/TNF-receptor superfamilySingle-Chain AntibodiesHinge region
The invention relates to a targeting CD19CAR-iNKT cell and application thereof. Specifically, the invention provides a polynucleotide sequence, and activation culture and application thereof. The polynucleotide sequence is selected from (1) a coding sequence containing, successively connected, a coding sequence of an anti-CD19 single-chain antibody, a coding sequence of the hinge region of human CD8alpha, a coding sequence of the transmembrane region of human CD8, a coding sequence of the intracellular region of human 41BB and a coding sequence of the intracellular region of human CD3zeta; and(2) complementary sequences of the polynucleotide sequence described in (1). The invention also provides related fusion proteins, vectors containing the coding sequences, and application of the fusion proteins, the coding sequences and the vectors. The invention also provides activation and culture methods and the application scope of iNKT cells.
Owner:HRAIN BIOTECHNOLOGY CO LTD
Concentration gradient rhIL-2 dependent iNKT (invariant natural killer) cell amplification method and application thereof
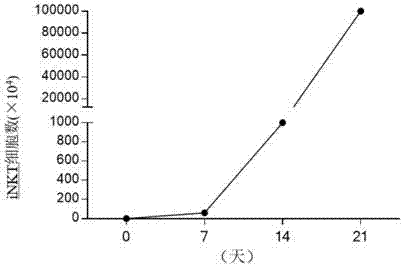
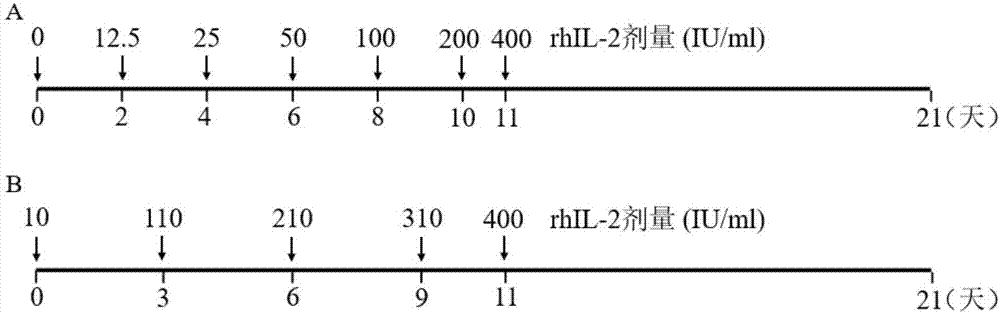
ActiveCN107502591AAddressing Purity IssuesMeet the needs of clinical treatmentMammal material medical ingredientsCell culture supports/coatingAbnormal tissue growthPeripheral blood mononuclear cell
The invention discloses a concentration gradient rhIL-2 dependent iNKT (invariant natural killer) cell amplification method and an application thereof. The method includes: 1), extracting PBMCs (peripheral blood mononuclear cell); 2), adding alpha-GalCer 100ng / ml in final concentration to the extracted PBMCs in the step 1) for culture for 48 hours; gradually increasing concentration of rhIL-2 in a culture medium in a gradient manner in the cultured cells in the step 2). The iNKT cells are obtained on the 21 day of culture, the number of the iNKT cells is increased by 100,000 times, more than 90% of the cells are CD4-iNKT cells, and functions of immune reaction up-regulation and direct tumor killing are achieved; the method is higher in amplification efficiency in stimulating and amplifying the iNKT cells, Th1 sampled iNKT cells are selectively amplified, and immune enhancement of the in vitro amplified iNKT cells and tumor immune surveillance and killing functions are strengthened.
Owner:BEIJING GENE KEY LIFE TECH CO LTD
Immunotherapy for hematological malignancies
Methods and compositions for treating, preventing and / or managing various hematological malignancies are disclosed. Specific methods and compositions relate to use of tumor cells engineered to express antigen presenting molecules which present antigens recognized by iNKT cells and eliciting antitumor immune response from iNKT cells using such tumor cells, optionally in combination with an immunomodulatory compound.
Owner:MUNSHI NIKHIL C +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com