Stem cell-engineered inkt cell-based off-the-shelf cellular therapy
An engineering and cellular technology, applied in genetic engineering, non-embryonic pluripotent stem cells, receptors/cell surface antigens/cell surface determinants, etc., can solve cancer patient toxicity and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0208] The preparation methods of the present disclosure can produce compounds comprising at least about 10 2 to 10 6 A cloned iNKT cell population of cloned iNKT cells. The method can produce at least about 10 6 to 10 12 cell population of total cells. The resulting cell population can be frozen and then thawed. In certain instances of the method of preparation, the method further comprises introducing into the frozen and thawed population of cells one or more other nucleic acids, eg, such as one or more other nucleic acids encoding one or more therapeutic gene products.
[0209] In certain embodiments, methods of developing 3D culture compositions (eg, ATO production) involving use of human DLL1 (MS5-hDLL1, below) and mobilized peripheral cells isolated from human umbilical cord blood, bone marrow, or G-CSF can be provided blood CD34 + HSPC-transduced aggregates of the MS-5 murine stromal cell line. up to 1x10 6 Individual HSPCs were mixed with MS5-hDLL1 cells in an ...
Embodiment 1
[0324] Example 1: Hematopoietic stem cell (HSC) method for engineering off-the-shelf INKT cells
[0325] This example relates to the generation of ready-made iNKT cells comprising deficient or down-regulated surface expression of one or more HLA-I and / or HLA-II molecules. In specific embodiments, iNKT cells are expanded from healthy donor peripheral blood mononuclear cells (PBMCs) and then CRISPR-Cas9 engineered to knock out B2M and CIITA genes. Due to the high variability and low frequency of iNKT cells in the human population (approximately 0.001-0.1% in blood), it would be beneficial to create methods that allow alternative means of obtaining iNKT cells.
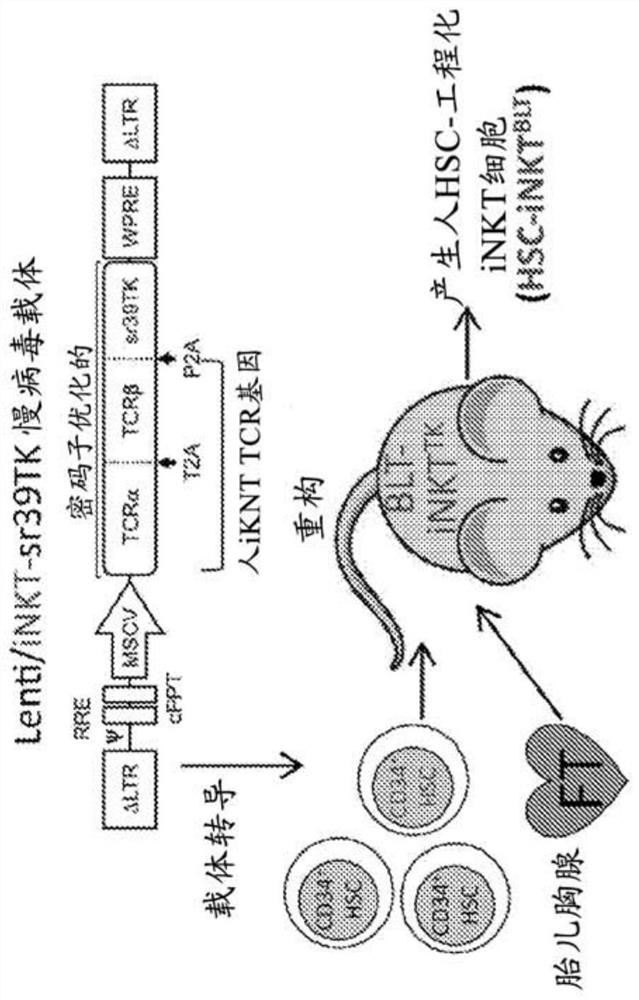
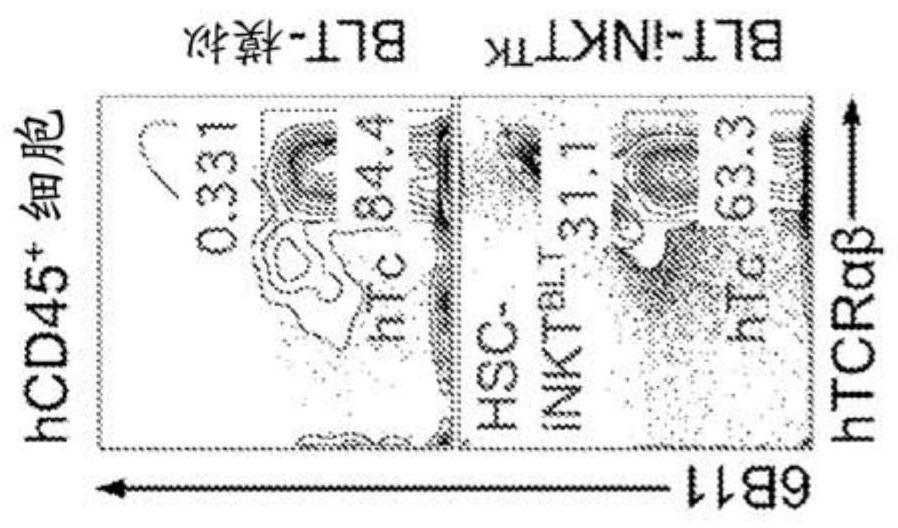
[0326] The present disclosure provides a powerful method to generate iNKT cells from hematopoietic stem cells (HSCs) by genetically engineering HSCs with iNKT TCRs and programming these HSCs to develop into iNKT cells (Smith et al., 2015). This approach exploits two molecular mechanisms that control iNKT cell development...
Embodiment approach
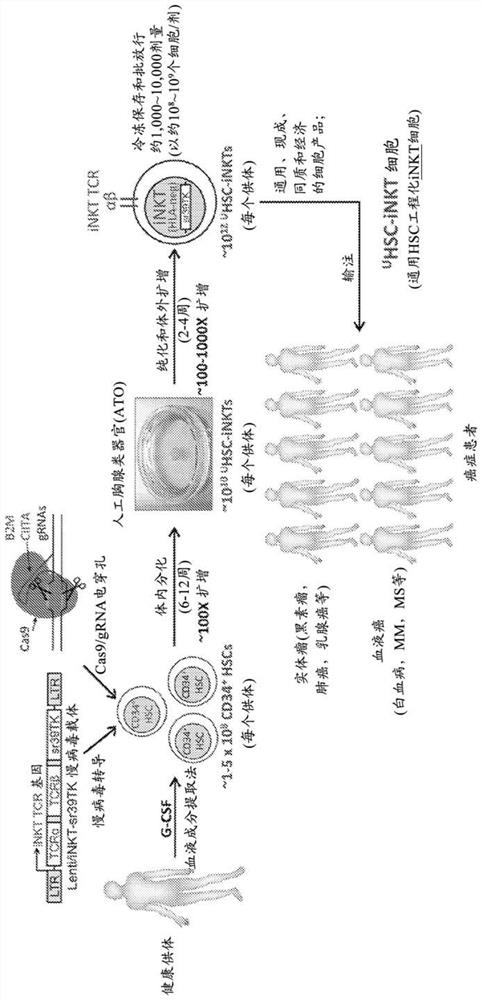
[0363] Overview of CMC In certain embodiments, manufacturing U HSC-iNKT involves: 1) collection of G-CSF-mobilized leukopak; 2) purification of G-CSF-leukopak to CD34 + 3) Transduction of HSCs with lentiviral vector Lenti / iNKT-sr39TK; 4) Gene editing of B2M and CIITA by CRISPR / Cas9; 5) in vitro differentiation into iNKT cells by ATO; 6) purification of iNKT cells; 7) In vitro cell expansion; 8) Cell collection, preparation and cryopreservation ( Figure 14 ). As an example, there are two drug substances (Lenti / iNKT-sr39TK vector and U HSC-iNKT cells), and at least in some cases the final drug product is formulated and cryopreserved in an infusion bag U HSC-iNKTs.
[0364] 1. Vector manufacturing
[0365] Vector structure One vector used to genetically engineer HSCs into iNKT cells is the HIV-1-derived lentiviral vector Lenti / iNKT-sr39TK, which encodes the human iNKT TCR gene and the sr39TK PET imaging / suicide gene ( Figure 13 ). The key components of this third-generat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com