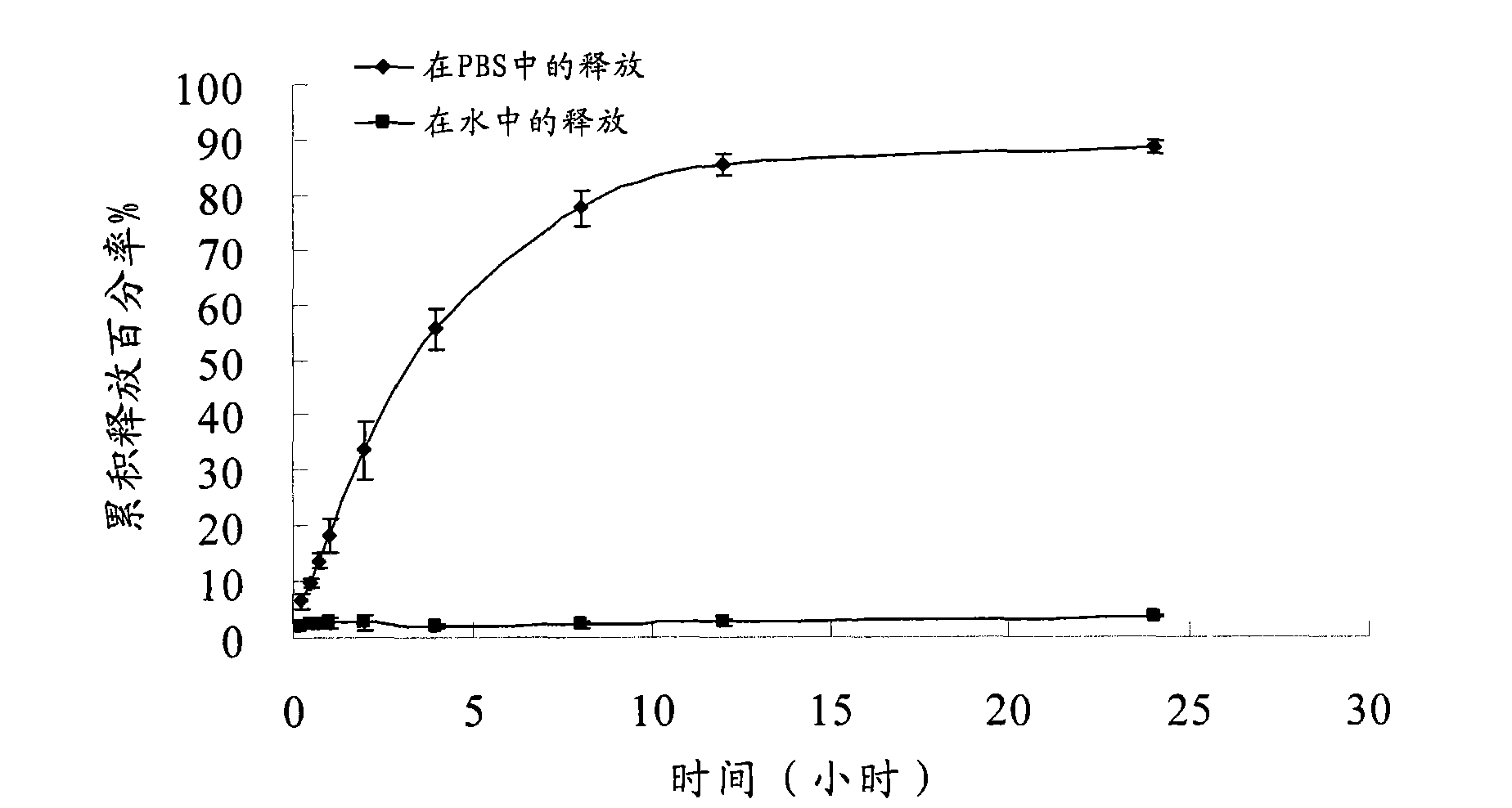
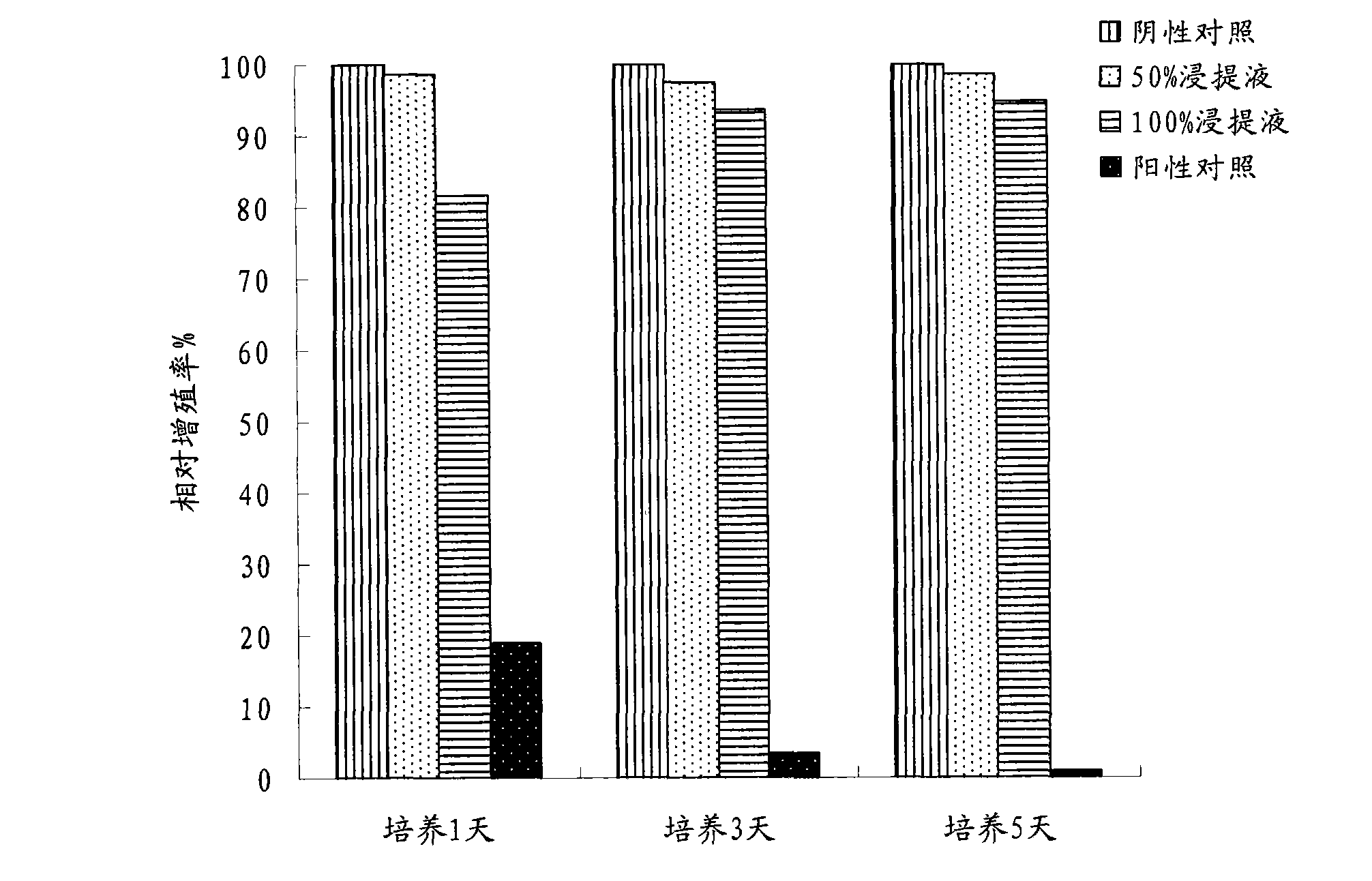
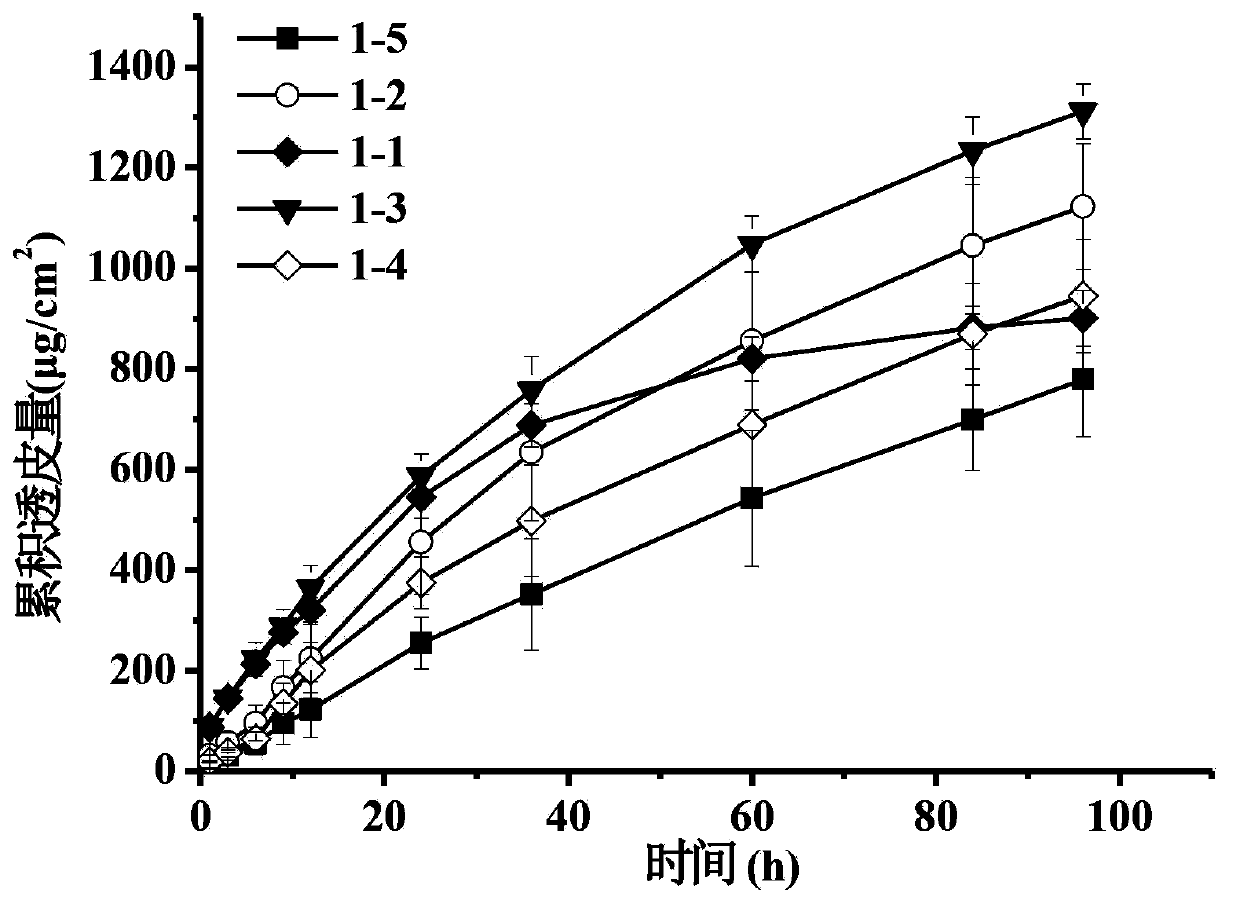
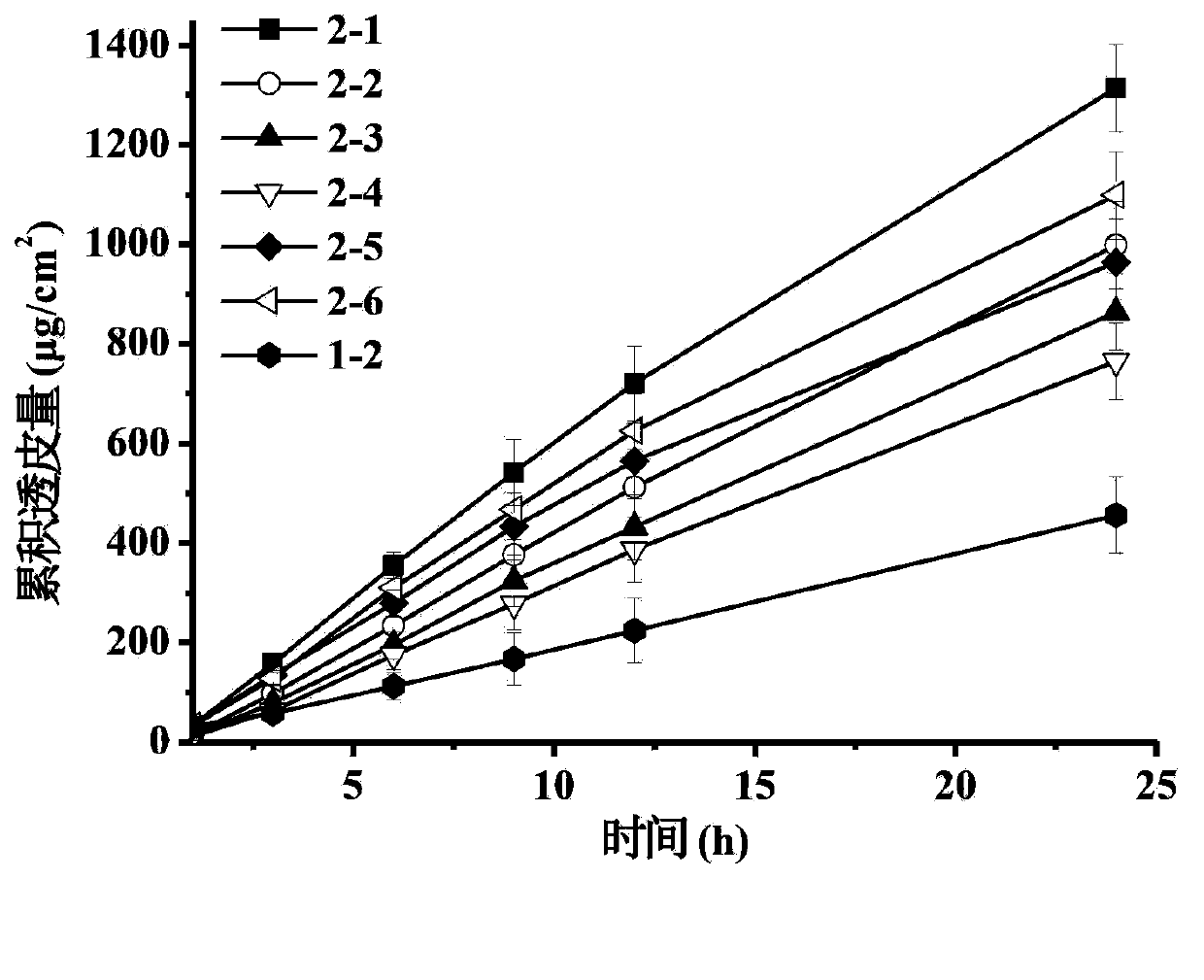
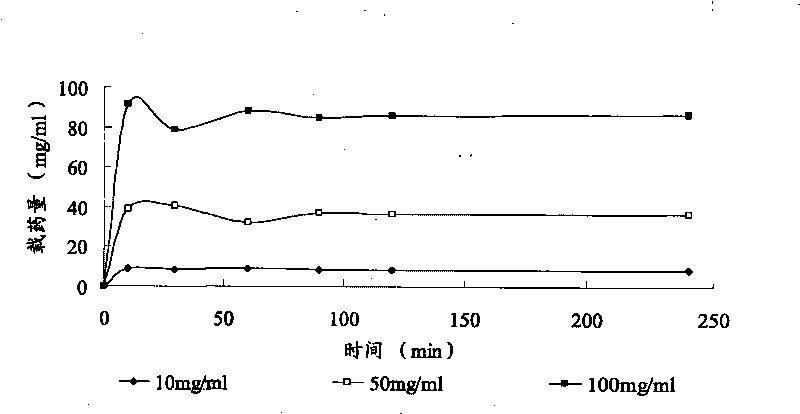
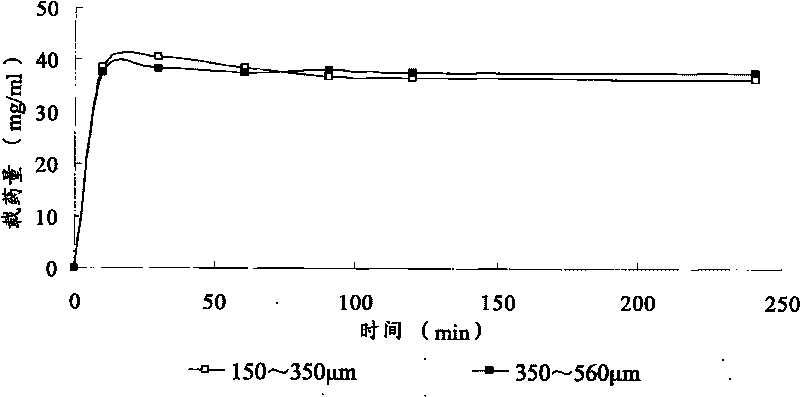
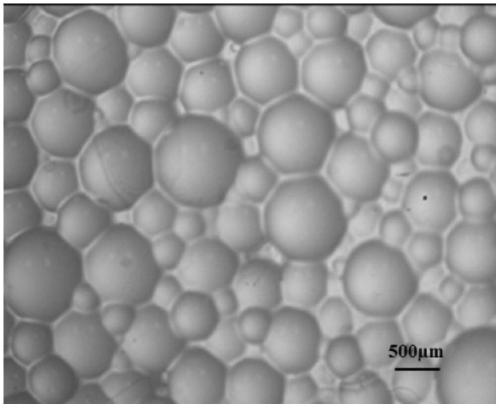
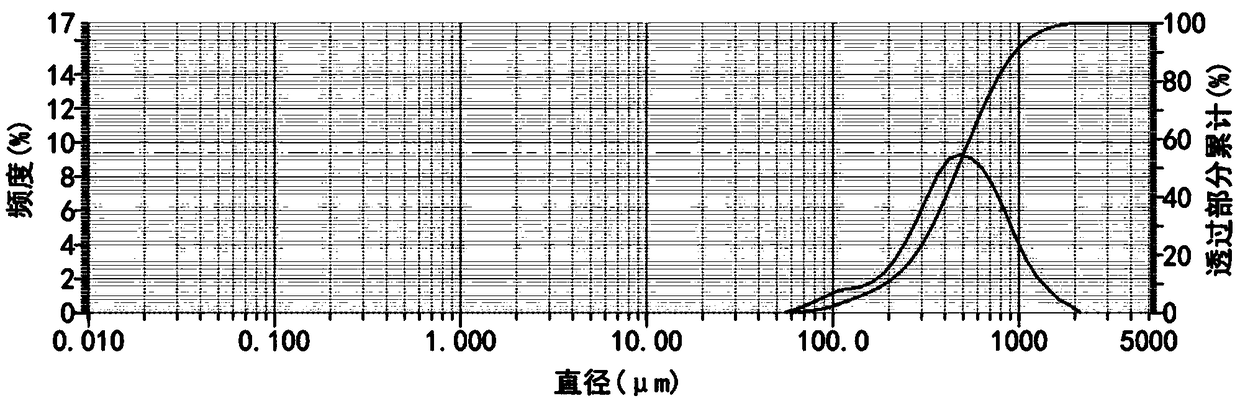
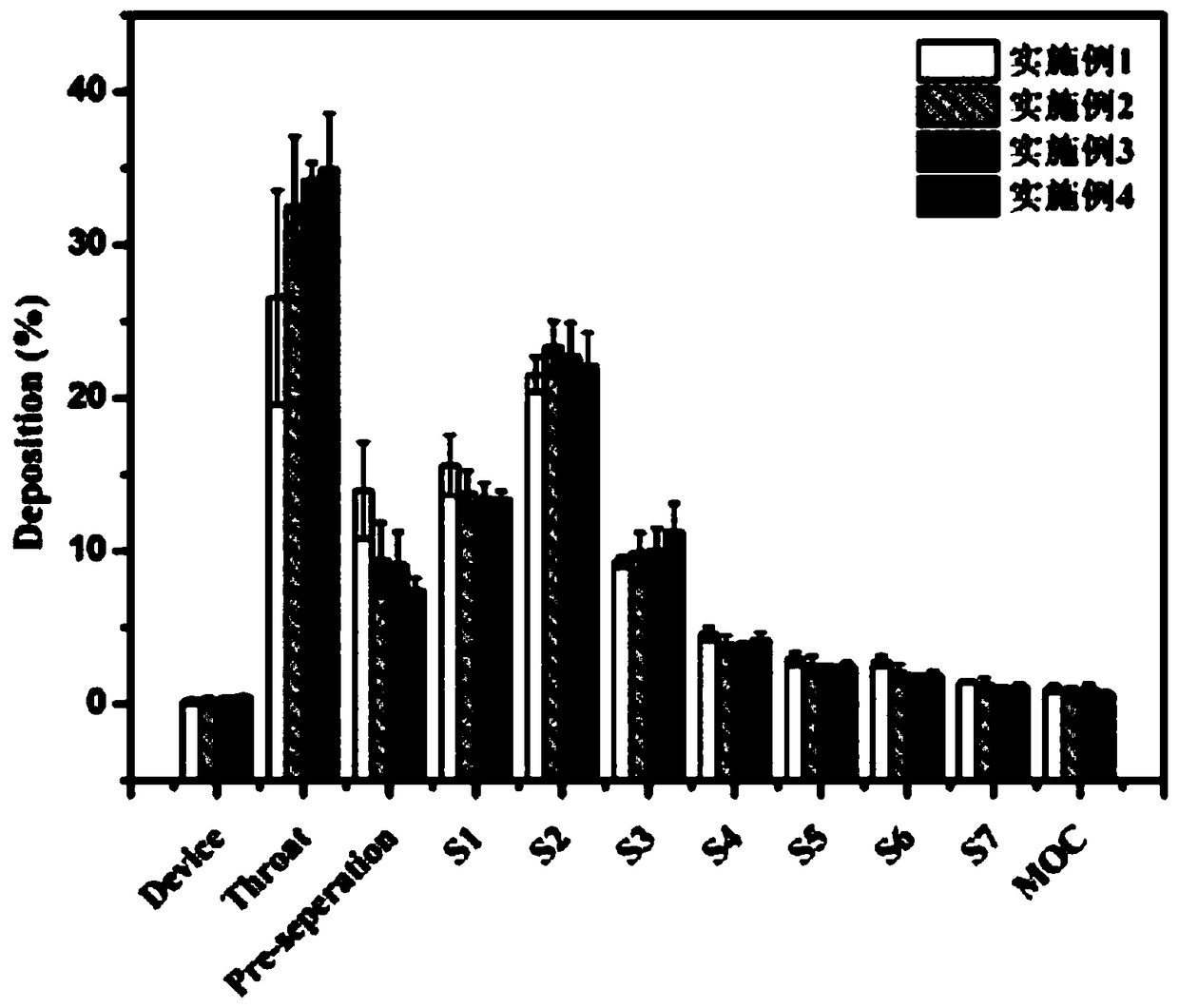
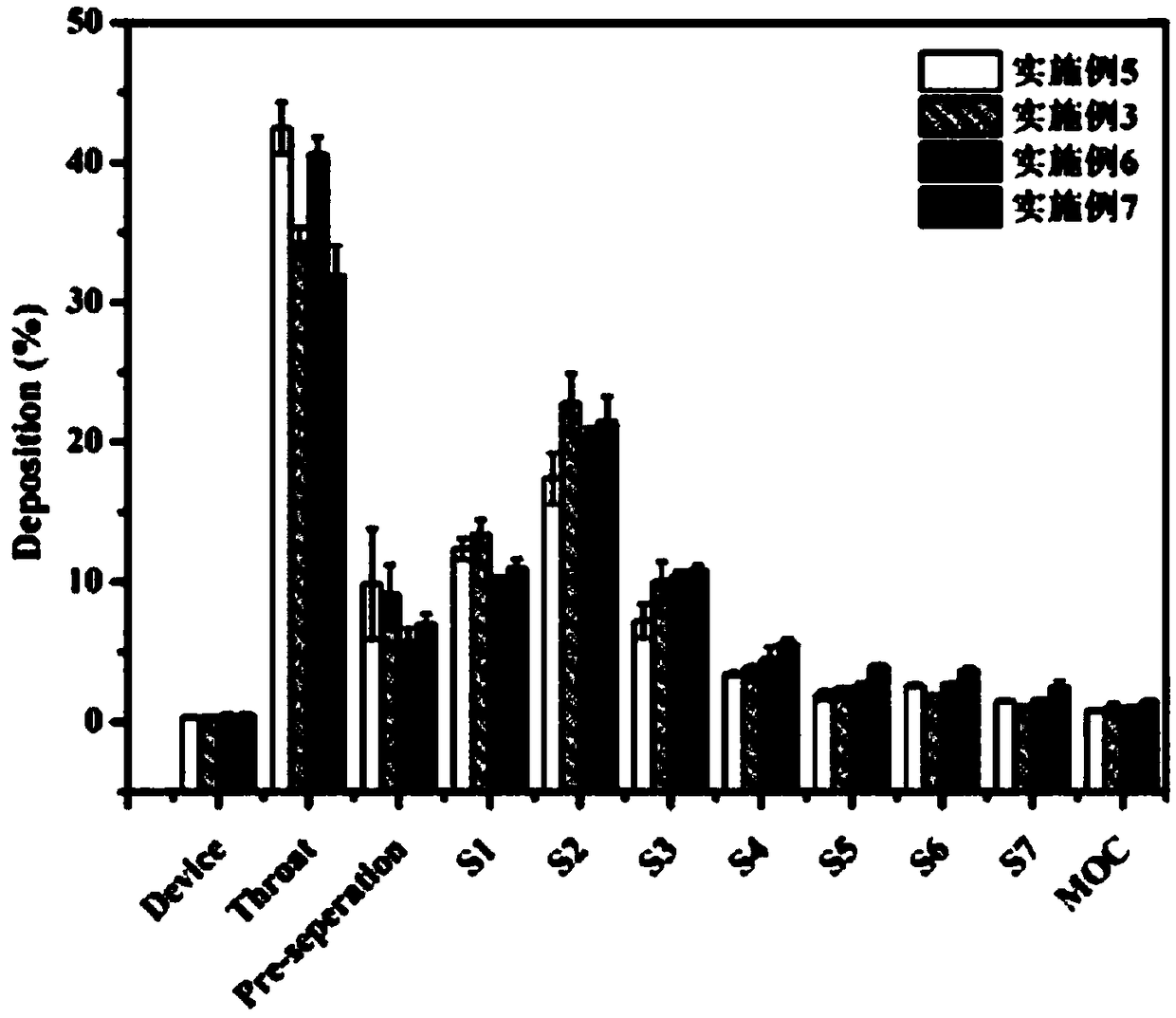
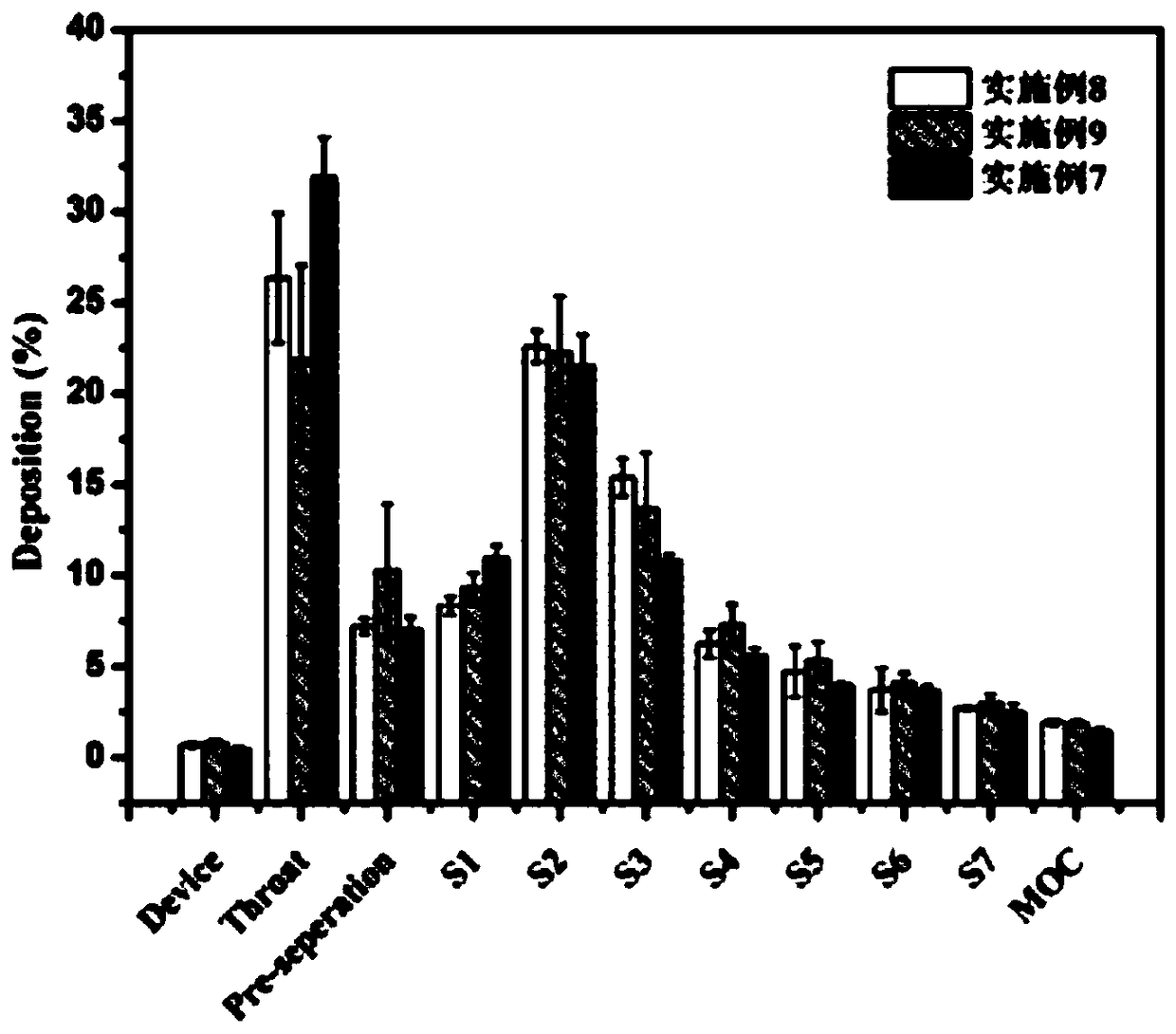
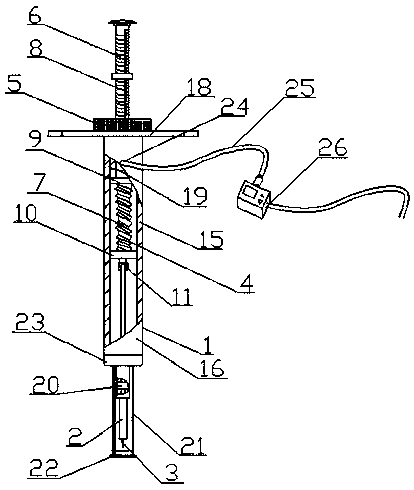
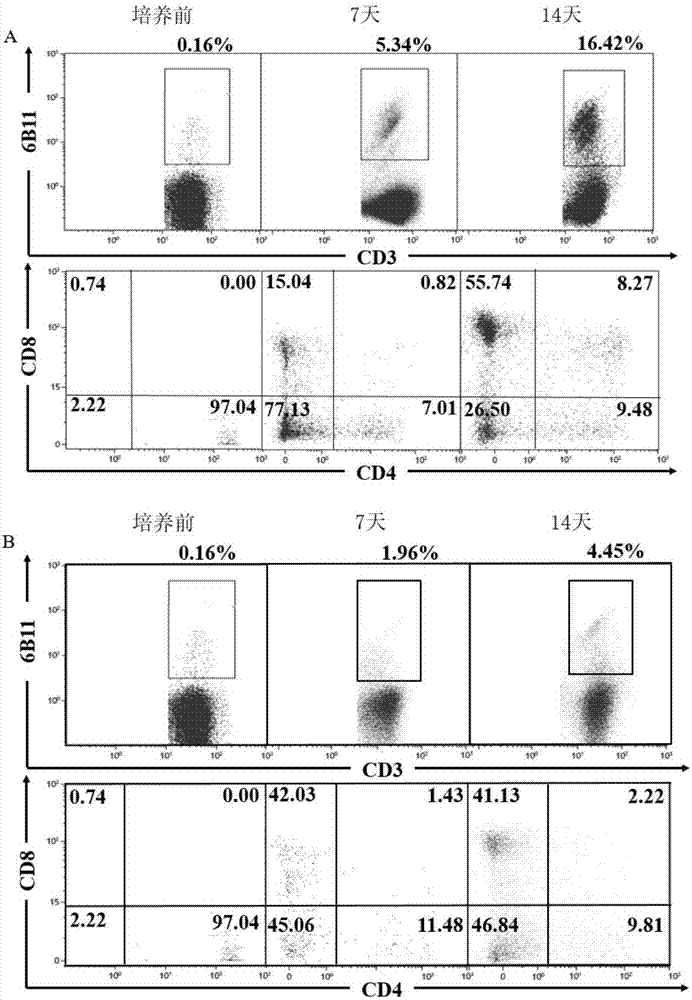
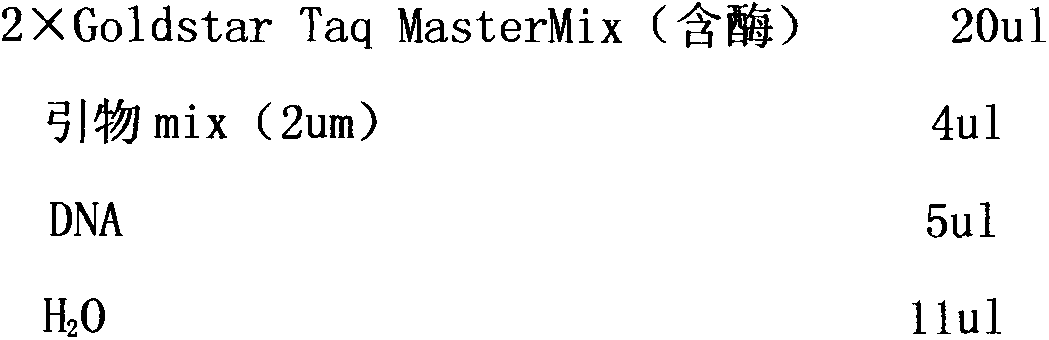Patents
Literature
61results about How to "Meet the needs of clinical treatment" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pharmaceutical composition for treating embolism and preparation method thereof
ActiveCN101670095AIncrease concentrationReduce concentrationSurgerySaccharide peptide ingredientsEmbolization AgentDouble bond
The invention provides a pharmaceutical composition for treating embolism and a preparation method thereof, the pharmaceutical composition comprises a hydroxyl-contained biocompatible polymer materialand a monomer containing unsaturated double bonds and anion groups, as well as a polymer generated by polymerization reaction of an optional vinyl monomer, wherein the polymerization is initiated through free radicles, and bleomycin or pingyangmycin is combined on the anion groups of the generated polymer. The preparation method combines the bleomycin or the pingyangmycin on a carrier of the polymer, thereby being capable of fully playing the dual effects of an anti-tumor antibiotic and a hardening agent owned by the bleomycin or the pingyangmycin during the embolism treatment. The anion partof the polymer can be properly combined with the bleomycin or the pingyangmycin which is rich in amino groups, thereby not only realizing the higher drug loading, but also leading the drug in an emboliaztion agent to be exchanged by cation in human body, and further realizing the slow release. In addition, the embolic carrier of the polymer has the advantages of simple prepration technology andlow cost, thereby being applicable to large-scale industrial production.
Owner:HYGEA MEDICAL TECH CO LTD
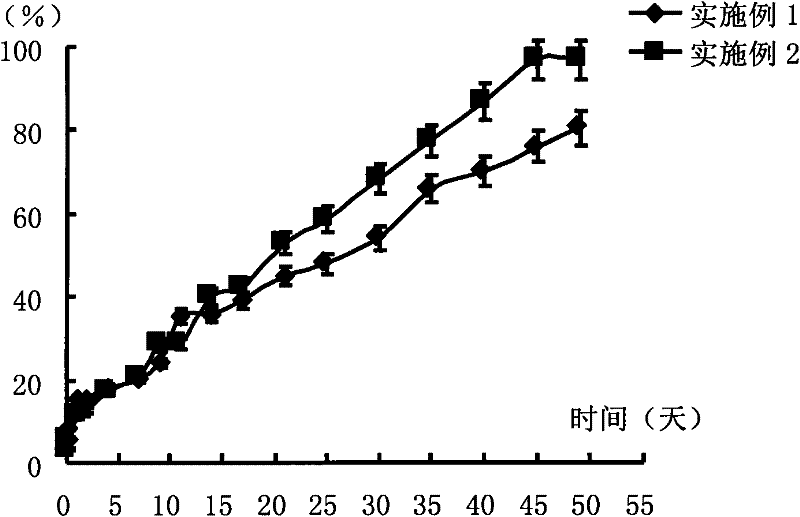
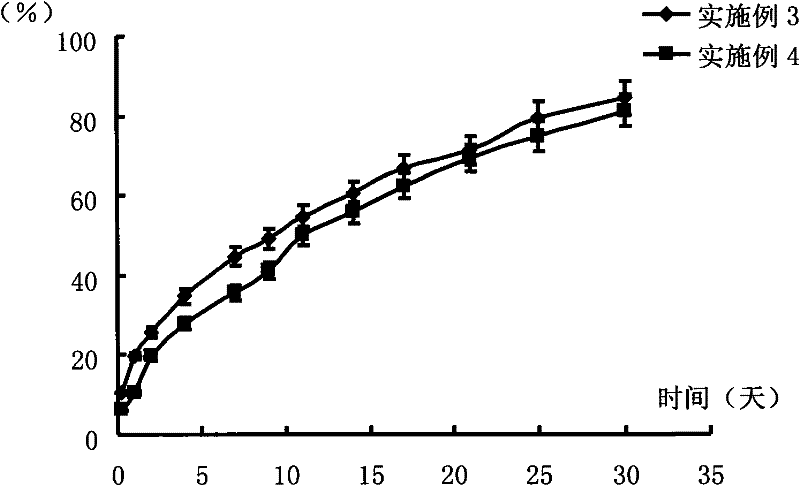
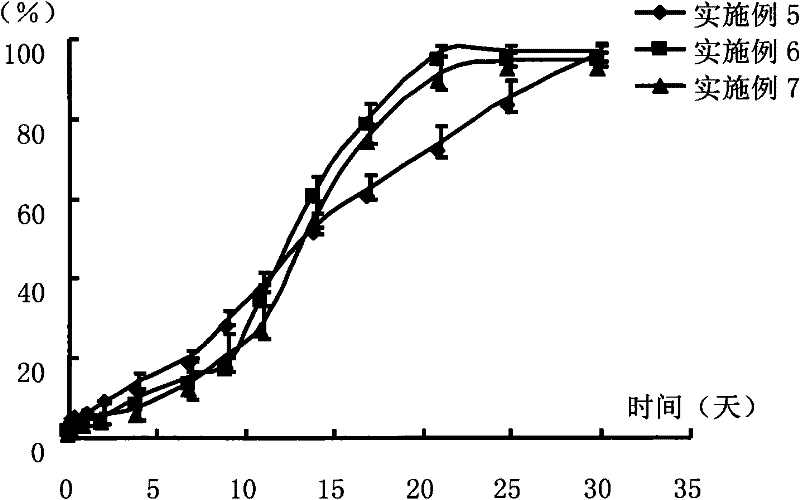
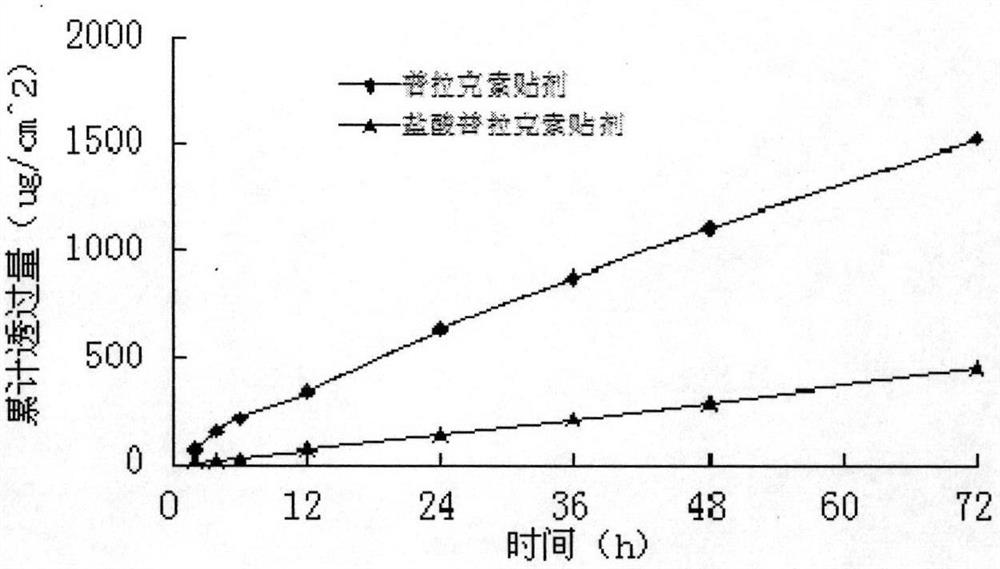
Transdermal patch containing pramipexole
ActiveCN103432104AGood solubilityImprove uniformityOrganic active ingredientsNervous disorderSolventMicrogram
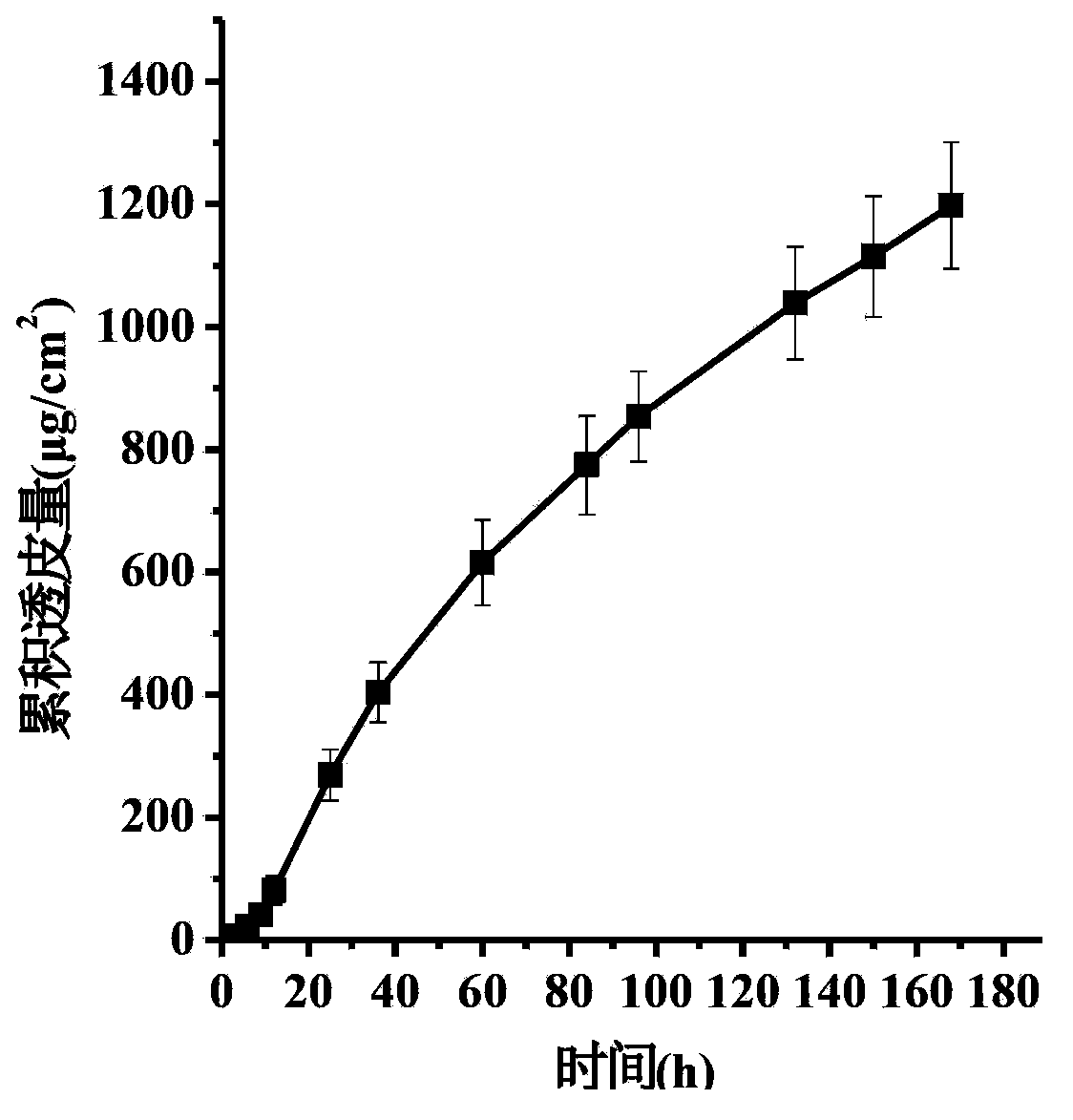
The invention discloses a transdermal patch containing pramipexole. The transdermal patch comprises a medicine carrying pressure-sensitive adhesive layer, the pramipexole, solvent and penetration enhancer, wherein the medicine carrying pressure-sensitive adhesive layer comprises acrylate pressure-sensitive adhesive containing carboxyl base groups and acrylate pressure-sensitive adhesive containing hydroxyl base groups, which form mixed pressure-sensitive adhesive; the pramipexole is dissolved in the acrylate mixed pressure-sensitive adhesive, with the content being 10 to 30 weight percent; the content of the mixed pressure-sensitive adhesive is 50 to 80 weight percent; the content of the solvent is 5 to 20 weight percent; the content of the penetration enhancer is 2 to 15 weight percent. The pramipexole can be dissolved in a mixed pressure-sensitive adhesive patch in a high concentration way and is not crystallized, the medicine availability is high, the pramipexole has good stability in pressure-sensitive adhesive matrix, and can be continuously administrated for 5 to 7 days in a transdermal way at a relatively stable permeation rate of being larger than 5.0 micrograms / cm2 / h, and the application area of a pramipexole patch is smaller than 40cm2.
Owner:GUANGDONG HONGSHANHU PHARM CO LTD
Liquid medicine filtering membrane, and preparation method and application thereof
ActiveCN102527255ASimple structureAvoid cloggingSemi-permeable membranesFiltering accessoriesPore diameterSilicon dioxide
The invention discloses a liquid medicine filtering membrane. The liquid medicine filtering membrane is a silicon dioxide ceramic membrane, wherein the pore diameter of the silicon dioxide ceramic membrane is 1.8-2.8 microns, and the porosity of the silicon dioxide ceramic membrane is 70-75 percent. The liquid medicine filtering membrane is low in price, high and stable in filtering rate, stable in flow rate, safe and reliable. The invention also discloses a preparation method of the liquid medicine filtering membrane, a liquid medicine filter using the liquid medicine filtering membrane, and a precise transfusion device using the liquid medicine filter.
Owner:シャンハイジュミンバイオテックサイエンスカンパニーリミテッド
Nanometer vauqueline liposome and preparation method thereof
InactiveCN1698611AMeet the needs of clinical treatmentImprove the safety of useOrganic active ingredientsAntipyreticDiseaseCholesterol
The invention discloses a nanometer vauqueline liposome and preparation method which consists of dissolving strychnine, lecithin and cholestrin into organic solvent with a predetermined volume, removing organic solvent from the solution through depressed evaporation for film forming, charging microcosmic salt cushioning solution containing a predetermined concentration of surface active agent, and carrying out swelling, free assembling, ultrasonic dispersing. The prepared strychnine liposome suspending liquid can be used for treating various diseases including gout, arthritis and traumatic injury.
Owner:EAST CHINA UNIV OF SCI & TECH
Medicine composite used for embolotherapy and acesodyne and preparation method thereof
ActiveCN101716349ALower drug concentrationSmall side effectsAntipyreticDigestive systemLidocaine HydrochlorideDouble bond
The invention provides a medicine composite used for embolotherapy and acesodyne and a preparation method thereof. The medicine composite comprises a biocompatibility macromolecular compound containing hydroxy, a monomer containing unsaturated double bond and anion group, a polymer, and local anesthetic containing amino group, wherein the polymer is generated through a polymerization reaction of an optional vinyl monomer and the polymerization reaction is initiated by free radicals, and the local anesthetic is combined to an anion group of the generated polymer. In the invention, lidocaine hydrochloride is combined to a polymer carrier; which can give full play to the acesodyne effect of the local anesthetic in the embolotherapy; the anion part of the polymer can properly combine with the local anesthetic containing the amino group, which can both realize higher medicine loading capacity and enable the medicine in an emboliaztion agent to be exchanged by cations in vivo and then slowly released. Moreover, the polymer emboliaztion carrier has simple technology, low cost, and suitability for large scale industrial production.
Owner:HYGEA MEDICAL TECH CO LTD
Embolization polymer, novel blood vessel embolization chemotherapy composite as well as preparation and application of novel blood vessel embolization chemotherapy composite
InactiveCN109053953AImprove solubilityThe polymerization process is easy to controlOrganic active ingredientsSurgical adhesivesDiseaseCross-link
The invention provides an embolization polymer. The embolization polymer is prepared by performing polymerization reaction initiated by free radicals on micromolecule monomer containing unsaturated double bonds, monomer containing unsaturated double bonds and an optional cross-linking agent; the cross-linking agent is polyfunctionality water-soluble acrylate or acrylamide. The embolization polymeris an ion exchange microsphere carrier and has higher deformation ability and higher drug trapping efficiency and drug loading capacity; meanwhile, a slow release effect is better. The invention further provides a novel blood vessel embolization chemotherapy composite. The embolization chemotherapy composite disclosed by the invention can jointly deliver an embolization agent and chemotherapy drug to a target blood vessel part through a catheter, so that a curative effect of the chemotherapy drug is fully played, peripheral normal tissues are prevented from being damaged, and disease relapsing is reduced.
Owner:深圳市比德泰克生物医药科技有限公司
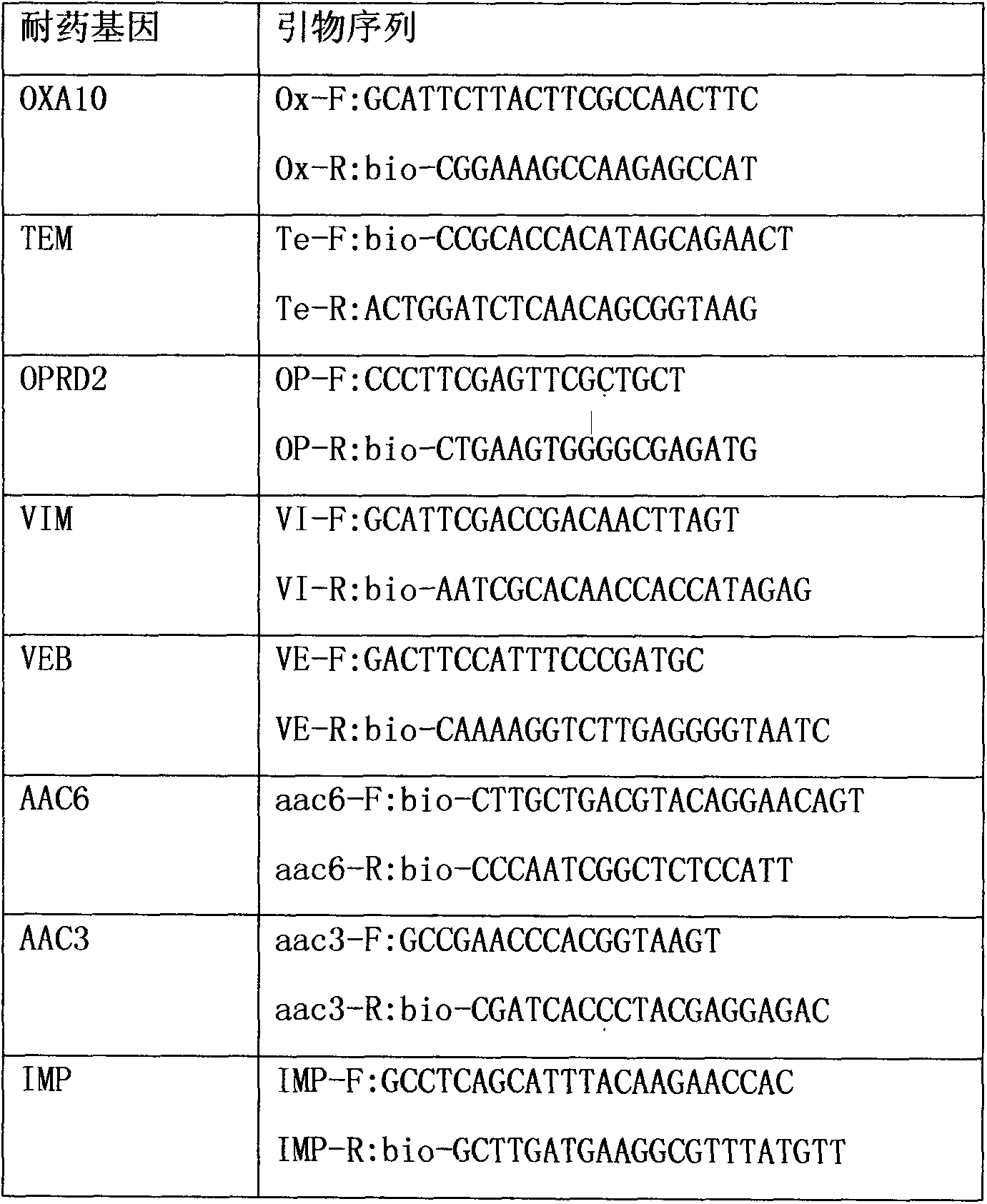
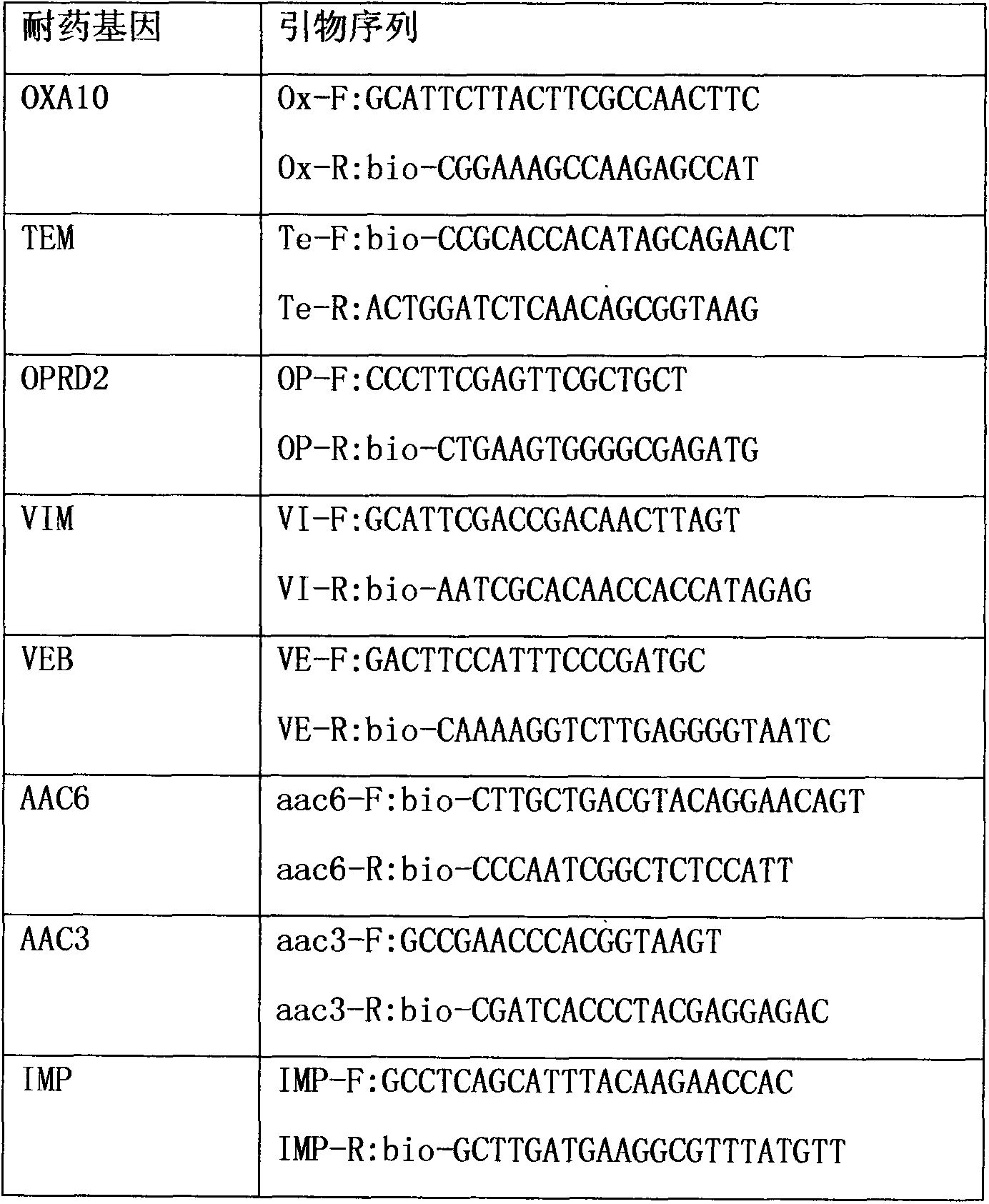
Liquid-chip detection kit for multiple drug-resistant genes of pseudomonas aeruginosa
InactiveCN104313166AStrong specificityHigh detection throughputMicrobiological testing/measurementBiotin-streptavidin complexMicrosphere
The invention discloses a liquid-chip detection kit for multiple drug-resistant genes of pseudomonas aeruginosa. The eight multiple drug-resistant genes of the pseudomonas aeruginosa are OPRD2, TEM, OXA10, AAC3, AAC6, VIM, VEB and IMP. The kit comprises a nucleic-acid amplification component and a hybridization component, wherein the nucleic-acid amplification component comprises the following specific components: premixed liquid, primer mixed liquid, polymerase, a positive quality control product and a negative quality control product; and the hybridization component comprises the following specific components: microsphere hybridization liquid, quality control microspheres and streptavidin-phycoerythrin. The liquid-chip detection kit disclosed by the invention has the advantages that the multiple drug-resistant genes of the pseudomonas aeruginosa can be detected simultaneously, and the drug resistance of the pseudomonas aeruginosa can be rapidly and accurately detected, so that the need of clinical treatment is met; and simultaneously, the kit has the beneficial effects of high specificity, combined screening, high detection flux and more convenience in promotion and application.
Owner:中国人民解放军济南军区第四〇一医院
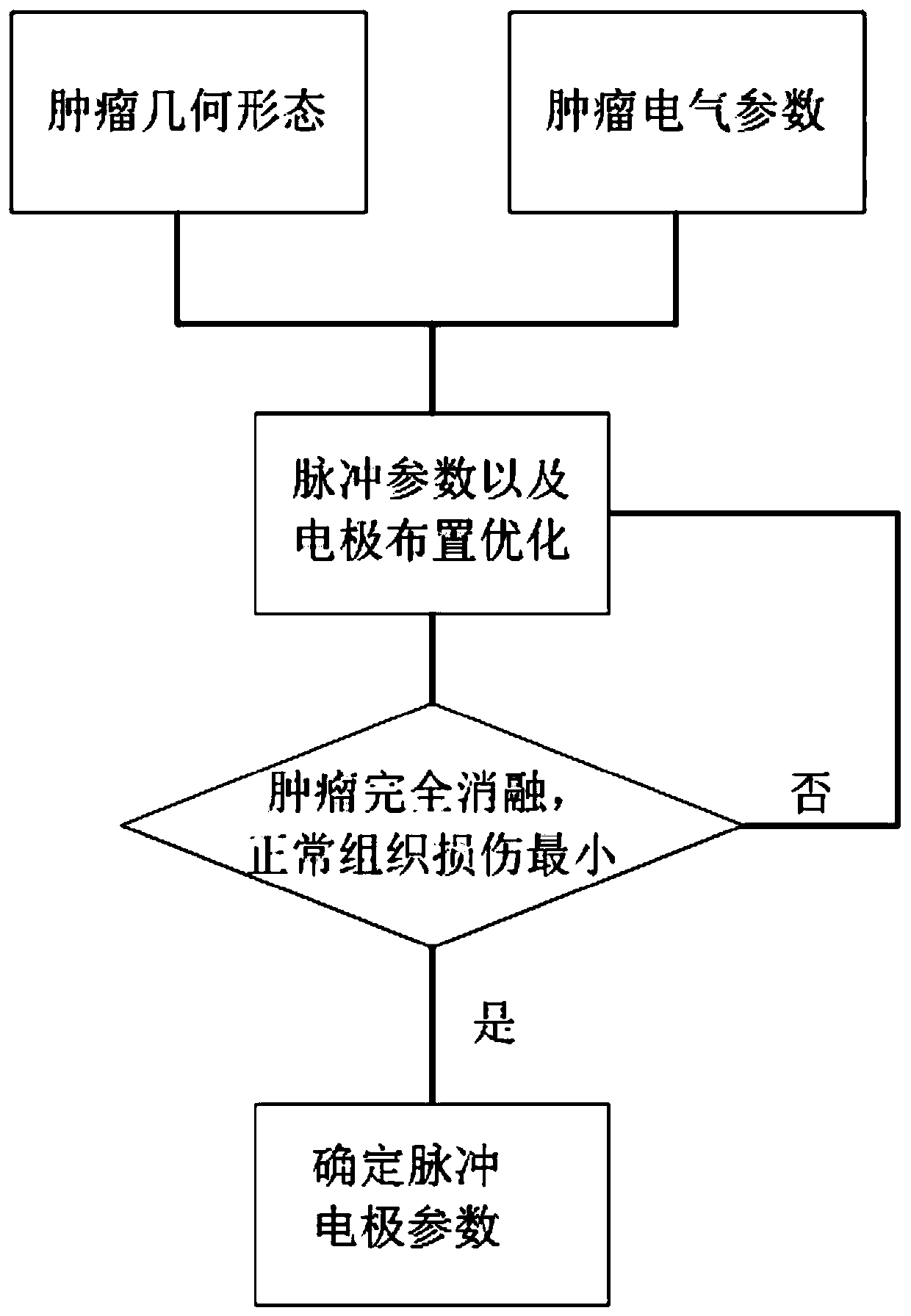
Pulsed electric field tumour ablation parameter optimization system
ActiveCN110755148AReduce harmAchieve complete ablationControlling energy of instrumentSurgical instruments for heatingEngineeringPulse sequence
The invention discloses a pulsed electric field tumour ablation parameter optimization system. The pulsed electric field tumour ablation parameter optimization system mainly comprises an image processing module, a physical parameter measuring module, a pulse electrode parameter setting module, a pulse sequence forming module, a plurality of pulse electrodes and a database, wherein the image processing model is used for obtaining biological tissue images of a user, and performing optimization processing on the biological tissue images of the user to obtain a three-dimensional structure of the biological tissue; the pulse electrode parameter setting module is used for processing received physical parameters, performing calculation to obtain pulse voltage, pulse number, pulse electrode position parameters and the depth that each pulse electrode is inserted into the biological tissue of the user, and transmitting the calculated pulse voltage, the calculated pulse number, the calculated pulse electrode position parameters and the calculated depth that each pulse electrode is inserted into the biological tissue of the user to the pulse sequence forming module; and the pulse electrodes can be used for performing pulse stimulation on the biological tissue of the user. According to the pulsed electric field tumour ablation parameter optimization system disclosed by the invention, optimal electrode arrangement and pulse parameters are arranged, so that the purposes that the tumor tissue is completely melted, and normal tissue damage and heat damage are minimum can be realized.
Owner:HANGZHOU WKNIFE MEDICAL TECH CO LTD
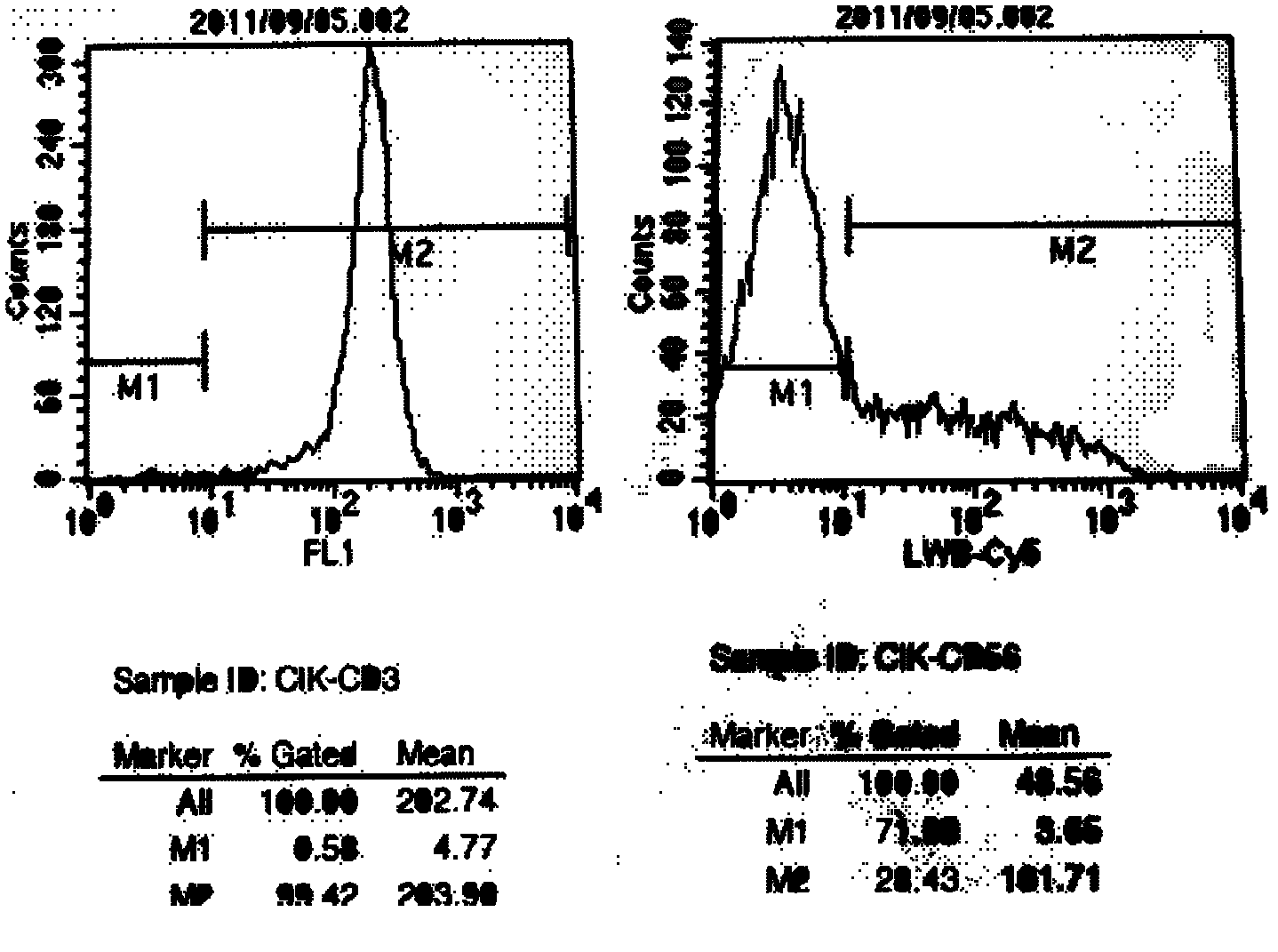
Method for preparing CIK cell with killing effect on tumor cell
InactiveCN103184192ASatisfy the separation effectEasy to separateBlood/immune system cellsFicollLymphocyte culture
The invention discloses a method for preparing CIK cells with a killing effect on tumor cells. The CIK cells are obtained through induced culture of 17-20 days by using a ficoll mononuclear cell separation medium with a density of 1.084 in the PBS system to separate mononuclear cells in peripheral blood or umbilical cord blood. According to the present invention, mononuclear cells are separated and obtained efficiently by using the ficoll density gradient centrifugation method, and a sufficient amount of CIK is obtained by using cell culture bags and a CIK cell culture system to meet the needs of clinical treatment. The Takara lymphocyte culture medium and the autologous serum and cytokine co-culture technique are used in the method, thereby preventing application of fetal bovine serum, reducing contamination risks of exogenous pyrogen and sensitinogen, and while maintaining the advantage of CIK cell efficient proliferation. The cell culture bag technology is used to reduce risk of cell contamination, and is suitable for clinical therapeutic application.
Owner:UNION STEMCELL & GENE ENG
Kit for treating human bone marrow, umbilical cord blood, and peripheral blood cells, and cell treatment method
InactiveCN102965339AAvoid interferenceAvoid the risk of rejectionBlood/immune system cellsArtificially induced pluripotent cellsHuman bodySeparation technology
The invention discloses a kit for treating human bone marrow, umbilical cord blood, and peripheral blood cells, and provides a kit for treating human bone marrow, umbilical cord blood, and peripheral blood cells and a cell treatment method which are strong in operationality, high in clinical safety, and convenient for clinical popularization. The kit comprises four reagents: No. A liquid is a diluent; No. B liquid is density fluid; No. C liquid is washing liquid; No. D liquid is erythrocyte-removing liquid. The invention fundamentally solves the problems of high cost, low cell activity, undefined human body influence for markers entering human body, pain for patients due to mobilization agent injection, cumbersome and inapplicable operations, and the like for current cell separation technology.
Owner:WUHAN HAMILTON BIOTECH
A transdermal patch containing pramipexole
ActiveCN103432104BImprove uniformityImprove solubilityOrganic active ingredientsNervous disorderHigh concentrationTransdermal patch
The invention discloses a transdermal patch containing pramipexole. The transdermal patch comprises a medicine carrying pressure-sensitive adhesive layer, the pramipexole, solvent and penetration enhancer, wherein the medicine carrying pressure-sensitive adhesive layer comprises acrylate pressure-sensitive adhesive containing carboxyl base groups and acrylate pressure-sensitive adhesive containing hydroxyl base groups, which form mixed pressure-sensitive adhesive; the pramipexole is dissolved in the acrylate mixed pressure-sensitive adhesive, with the content being 10 to 30 weight percent; the content of the mixed pressure-sensitive adhesive is 50 to 80 weight percent; the content of the solvent is 5 to 20 weight percent; the content of the penetration enhancer is 2 to 15 weight percent. The pramipexole can be dissolved in a mixed pressure-sensitive adhesive patch in a high concentration way and is not crystallized, the medicine availability is high, the pramipexole has good stability in pressure-sensitive adhesive matrix, and can be continuously administrated for 5 to 7 days in a transdermal way at a relatively stable permeation rate of being larger than 5.0 micrograms / cm2 / h, and the application area of a pramipexole patch is smaller than 40cm2.
Owner:GUANGDONG HONGSHANHU PHARM CO LTD
Zedoary oil product of virus safe medicine and its preparing method
InactiveCN1430981ARelieve painGood social benefitsAntiviralsUnknown materialsPolyethylene glycolPrimary component
An antivirus medicine in the form of capsule is prepared from zedoary, oil as primary component, semisynthetic glycerine ester of fatty acid, polyethanediol and surfactant through proportioning, heating zedonary oil to 30-150 deg.c, sieving by 40-100 meshes, mixing with polyethanediol and surfactant, sirring for 30-60 min, and loading in capsule at 10-60 deg.C. Its advantages are high curative effect and no toxic by-effect.
Owner:张忠义
High-efficiency induction culture method for preparing natural killer NK cells
PendingCN111690610AIncrease the number ofImprove survival rateCell dissociation methodsCulture processPeripheral blood mononuclear cellCD16
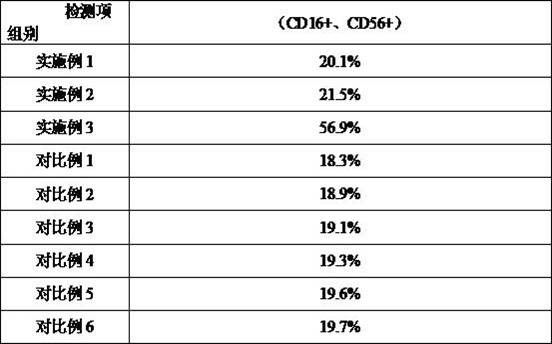
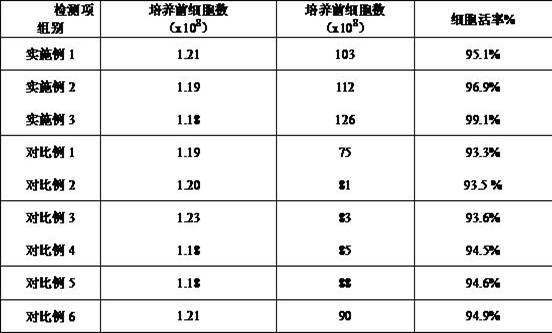
The present invention belongs to the technical field of cells and specifically relates to a high-efficiency induction culture method for preparing natural killer NK cells. The high-efficiency induction culture method for preparing natural killer NK cells mainly includes the following steps: S1, during a preparation stage, using a cell culture bottle coated with recombinant human fibronectin and CD16 antibody; S2, conducting peripheral blood mononuclear cell induction culture; S3, conducting expansion culture; and S4, using a trypan blue staining method and counting cells with a cell counting plate to calculate cell number and cell viability. The provided method can enable the NK cells to proliferate in large quantities, obtains the natural killer NK cells with high quantity, high viabilityand high quality, at the same time, has high safety and can meet needs of clinical treatment.
Owner:暨赛国际再生医学科技有限公司
Correction device membrane and correction device
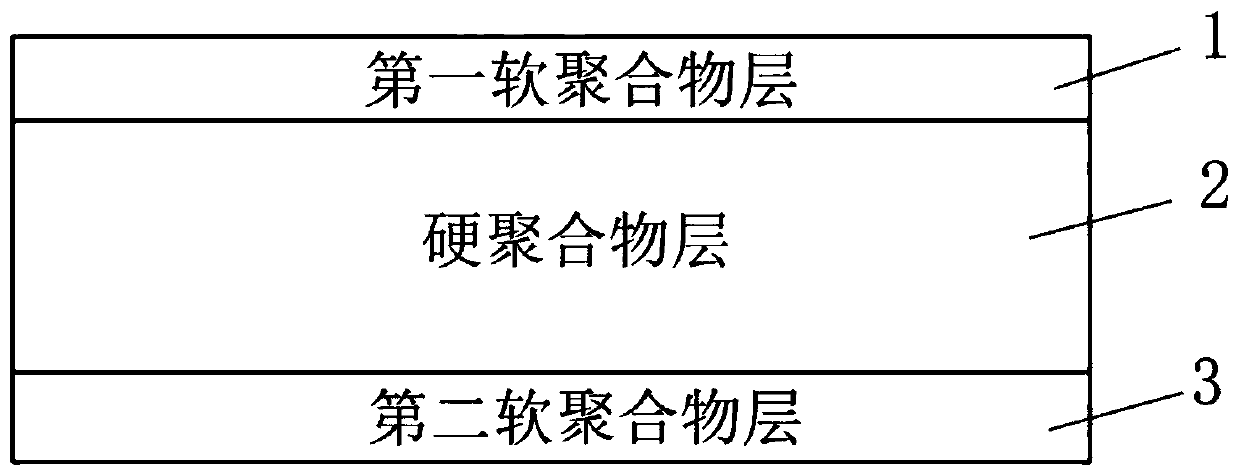
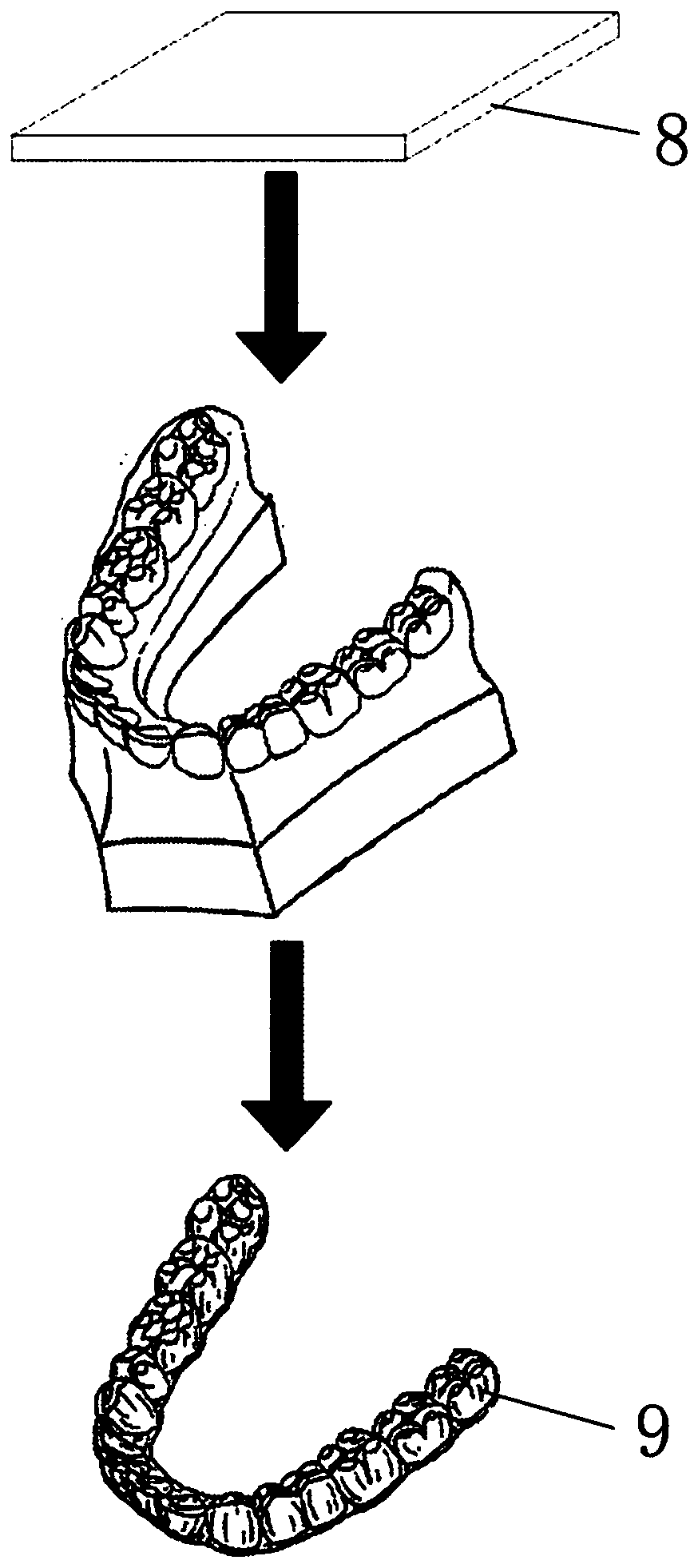
InactiveCN110215300AImprove durabilityImprove wear resistanceOthrodonticsPolymer scienceFlexural modulus
The invention discloses a correction device membrane and a correction device. The correction device membrane comprises a first soft polymer layer, a hard polymer layer and a second soft polymer layerwhich are combined from top to bottom in sequence; the thicknesses of the first soft polymer layer and the second soft polymer layer are smaller than the thickness of the hard polymer layer; for the hard polymer layer, the yield elongation is larger than 4%, the breaking elongation is larger than 70%, the tensile modulus is larger than 150,000 psi, and the flexural modulus is larger than 150,000 psi; for the first soft polymer layer and the second soft polymer layer, the hardness is 60 A-85 D, the ultimate tensile strength is larger than 5,000 psi, the breaking elongation is 180-220%, and theflexural modulus is larger than 35,000 psi. Through structural design of the correction device membrane, the orthodontics membrane generates more continuous, stable and effective correction force, teeth of a patient can be moved to the expected positions, the balance, stability and attractiveness of the mouth-jaw system are achieved, and the satisfied orthodontics effect is achieved.
Owner:SHENZHEN TECH UNIV
Human bone marrow cell processing kit and cell processing method
InactiveCN105670991AWeight increaseSave raw materialsSkeletal/connective tissue cellsHydroxyethyl starchSodium Chloride Injection
The invention relates to a human bone marrow cell processing kit and a cell processing method. The human bone marrow cell processing kit is characterized by consisting of the following three reagents: No.1 reagent which, as a thinner, is 0.5-20% of a sodium chloride injection or PBS (poly butylenes succinate) liquid, No.2 reagent which, as a precipitator, is 0.5-20% of hydroxyethyl starch or methylcellulose, and No.3 reagent which, as layering liquid, is prepared from ficoll and meglumine diatrizoate and is 1.0-1.2 in density. The cell processing method comprises the following steps: adding marrow containing sodium citrate anti-coagulation liquid to a high-capacity nutrient solution bottle which contains the No.1 reagent, and then adding the No.2 reagent and uniformly shaking; standing by, sucking upper cell liquid after layering and centrifuging for 1-20min, thinning the cell liquid by virtue of normal saline after the cell liquid is centrifuged and concentrated and paving the normal saline on the upper layer of No.3 liquid, then centrifuging so that a stem cell layer is separated, collecting the stem cell layer, and cleaning cells by virtue of normal saline for later use. The cell processing method is strong in operability, high in clinical safety and convenient for clinical popularization; and the kit is easy for storage and transportation, applicable to industrial production, and convenient and rapid to use.
Owner:杨淑芬
Recombination adeno-associated virus of expression human antisense phospho lamban gene and its preparation method
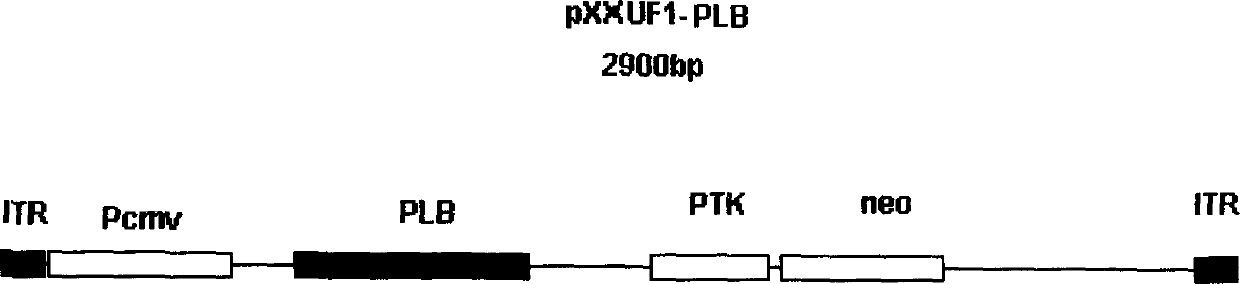
InactiveCN1657631AAddressing Contaminated In Vitro Mass Replication IssuesEasy to shrinkGenetic material ingredientsViruses/bacteriophagesDiseaseManagement of heart failure
A recombinant adeno-associated virus (AAV) able to express human antisense phospholamban gene for treating heart failure, cardial infaction and other associated diseases is prepared from natural AAV and adenovirus through artificial shearing, modifying and processing. Its carrier system includes packing plasmid pXX2, auxiliary plasmid pXX6, and eukaryotic expression carriers pXXUF1, and pXXUF3. The coding sequence of human phospholamban gene is inserted in pXXUF1 reversely.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
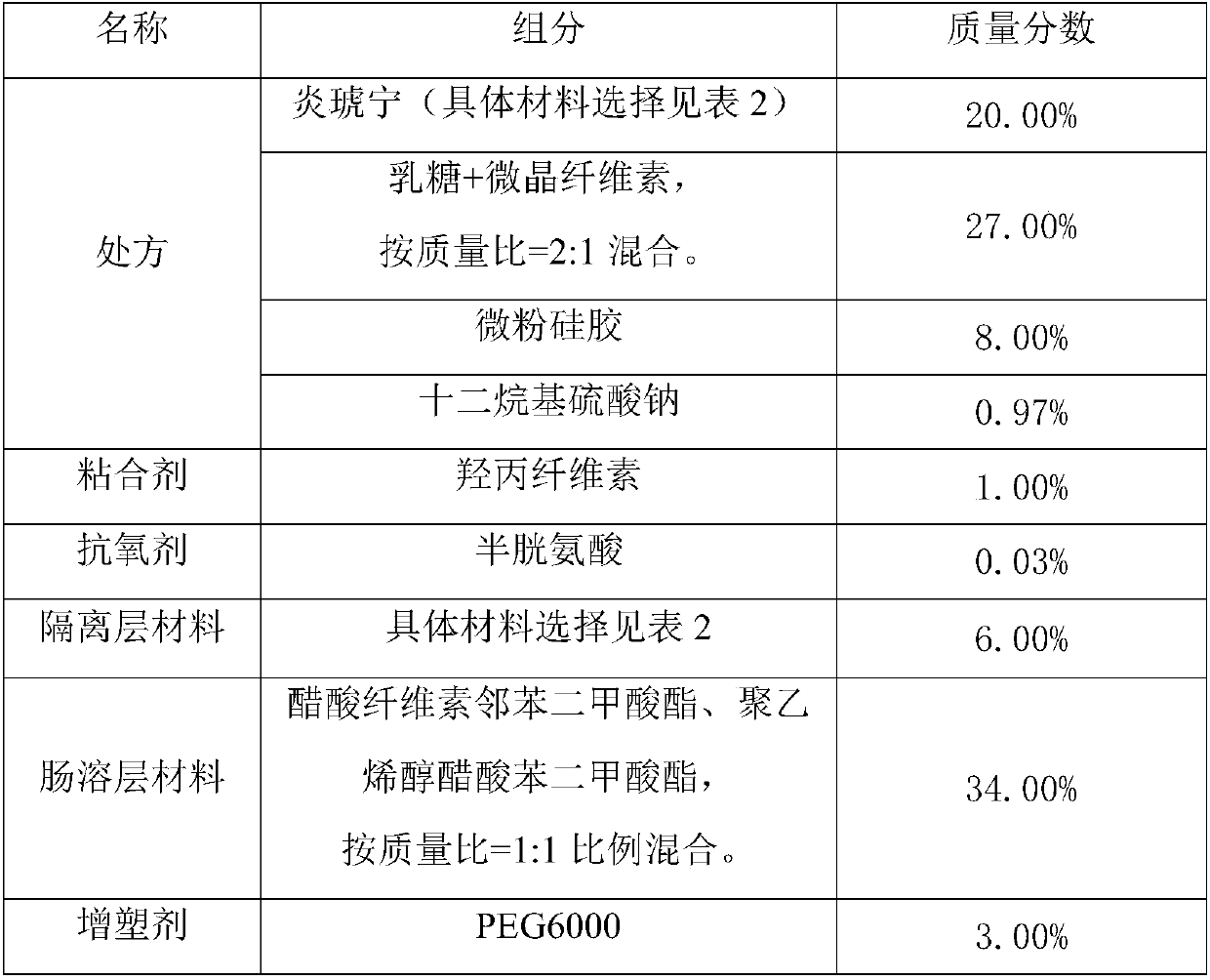
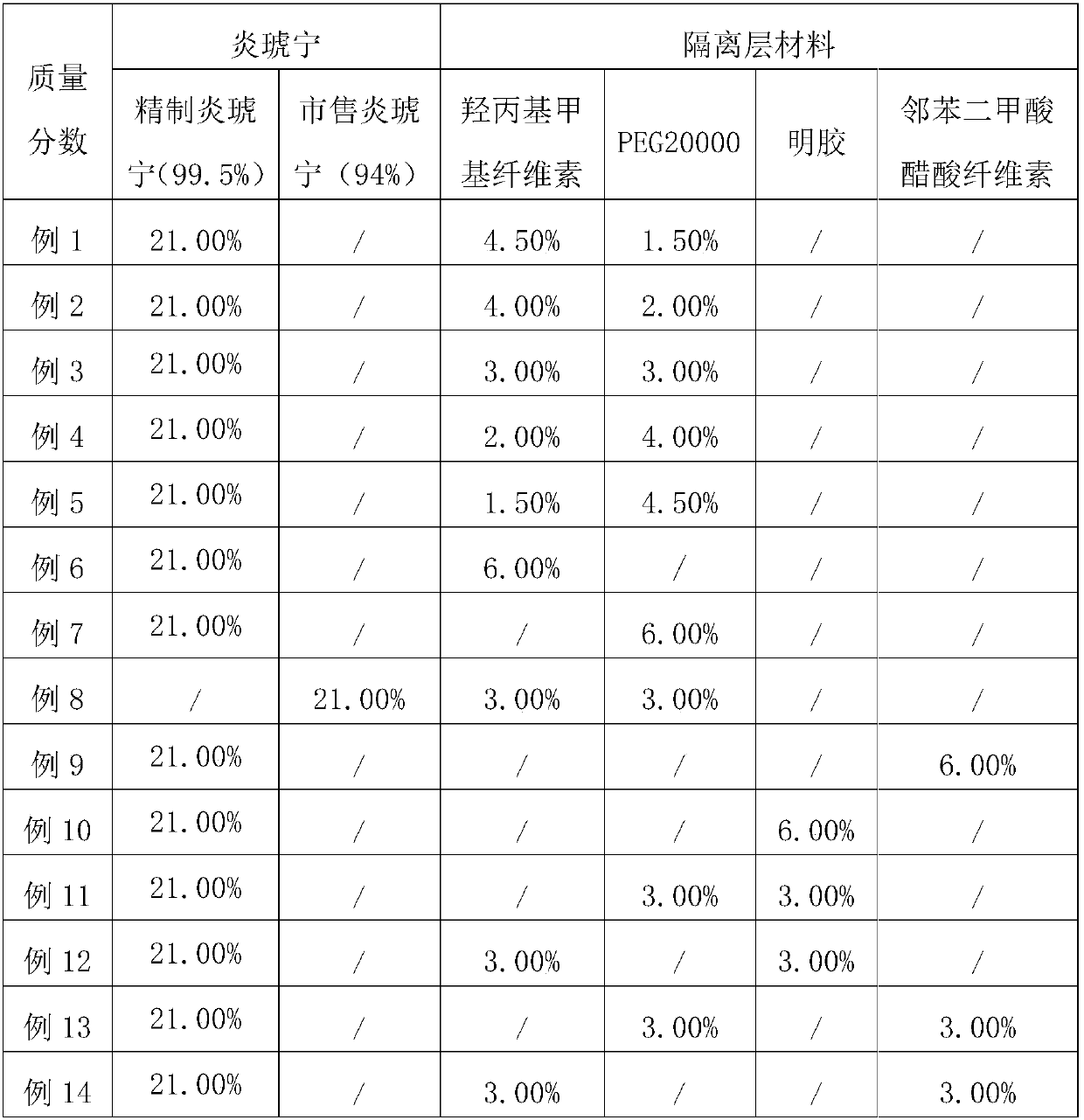
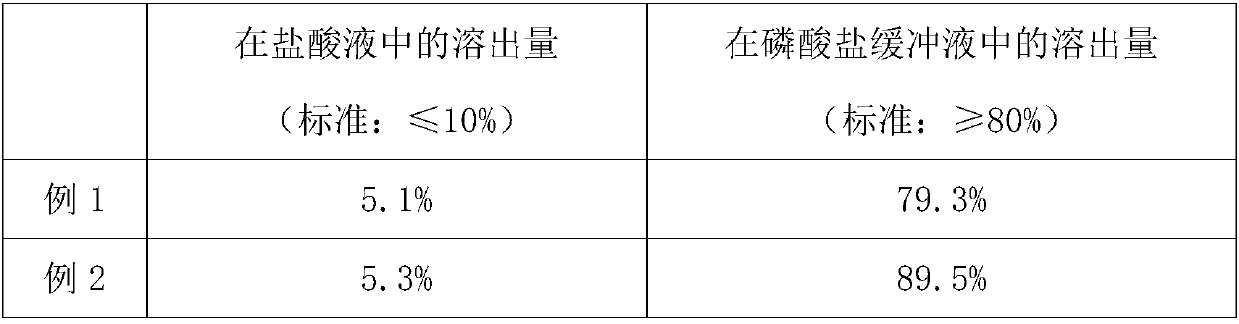
Efficient PSDS (potassium sodium dehydroandrographolide succinate) enteric-coated tablets and preparation method
ActiveCN108042503ASignificant effectMeet the needs of clinical treatmentOrganic active ingredientsAntipyreticSide effectAdditive ingredient
The invention belongs to the field of pharmaceutical processing and particularly relates to efficient PSDS (potassium sodium dehydroandrographolide succinate) enteric-coated tablets and a preparationmethod. The preparation method comprises specific steps as follows: PSDS is taken as an effective component, pharmaceutically acceptable auxiliary materials are added, a specific prescription is obtained, isolation layer coating and enteric-coated layer coating are performed with a specific processing technology, then appropriate auxiliary materials are added, and the enteric-coated tablets are prepared. According to the refined PSDS preparation method, generation of impurities is greatly reduced, and the occurrence rate of side effects is reduced; according to the special preparation processand parameters for production of the PSDS enteric-coated tablets, the active ingredients of PSDS can be protected effectively, pharmaceutical effect of a finished product is guaranteed, and bioavailability of oral administration of PSDS is improved; the production technology has a simple process, is easy to operate and facilitates industrial production.
Owner:HUANGSHAN C KING PHARMA
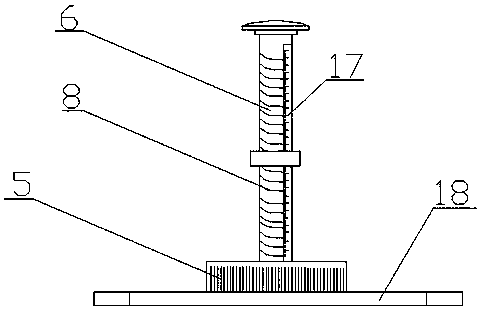
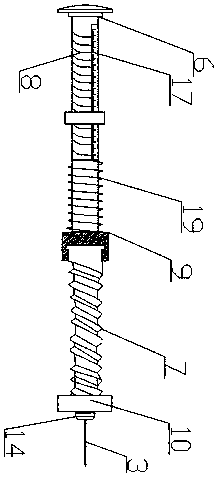
Bone traction device
PendingCN109620508AContinuous and precise adjustmentEasy to operatePneumatic massageChiropractic devicesLocking mechanismTraction frame
The invention discloses a bone traction device, and relates to the technical field of medical instruments. The bone traction device comprises a base, a support component for supporting a limb of a patient and a traction component for pulling the limb. The traction component comprises a traction frame mounted on the base and a traction rope respectively connected to the traction frame and the limb,and the traction rope is provided with an adjustment assembly for adjusting the length of the traction rope. The traction frame comprises a lifting rod hinged on the base and a traction rod connectedto the lifting rod through a locking mechanism. The locking mechanism includes a gear I disposed on the side end portion of the lifting rod, a gear II disposed on the side end portion of the tractionrod and matched with the gear I and a control component for controlling whether the gear I and the gear II are meshed. The gear I and the gear II include a plurality of racks arranged in a circular shape uniformly, and the rack mounting direction is consistent with the circular radial direction. The bone traction device has the advantages of being simple in structure, convenient to operate, efficient and practical, good in treatment rehabilitation efficacy and capable of adjusting the direction, the angle and the size of the therapeutic traction force.
Owner:THE SECOND XIANGYA HOSPITAL OF CENT SOUTH UNIV
Traditional Chinese medicine mixture for removing urinary calculus and preparation method thereof
InactiveCN113633724AImprove physical functionThe formula is scientific and reasonableUrinary disorderPteridophyta/filicophyta medical ingredientsWolfiporia extensaBiology
The invention discloses a traditional Chinese medicine mixture for removing urinary calculus and a preparation method thereof. The mixture is prepared from, by weight, 16 g of herba pyrrosiae, 20 g of fried endothelium corneum gigeriae galli, 12 g of lysimachia christinae hance, 10 g of radix rehmanniae, 12 g of chingma abutilon seeds, 6 g of akebiaquinata, 10 g of peach kernels, 10 g of poria cocos, 10 g of radix achyranthis bidentatae, 16 g of raw liquorice, 12 g of talc, 12 g of lygodium japonicum, 10 g of rhizoma alismatis, 10 g of lalang grass rhizome, 6 g of radix scutellariae, 6 g of radix linderae and 9 g of cortex moutan. The traditional Chinese medicine composition is scientific and reasonable in formula, novel and reasonable in preparation method, high in process parameter precision, less in component loss and stable in medicine effect, is suitable for various urinary calculus diseases such as kidney calculus and urethral calculus, is not easy to relapse after being cured, and meets clinical treatment requirements.
Owner:王晓兵
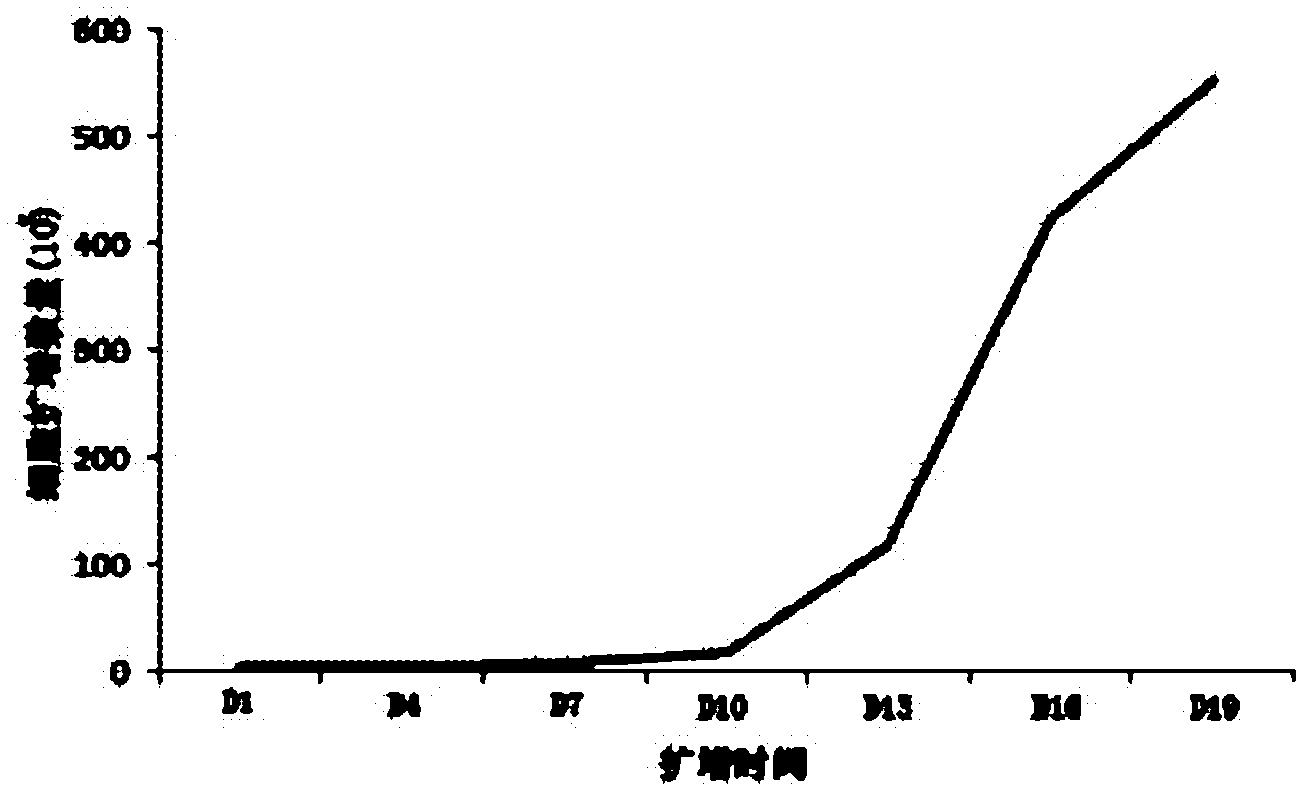
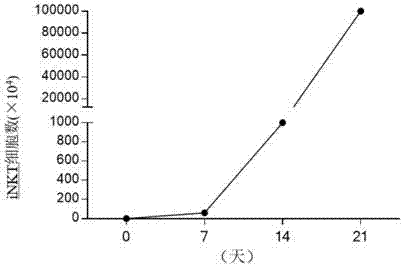
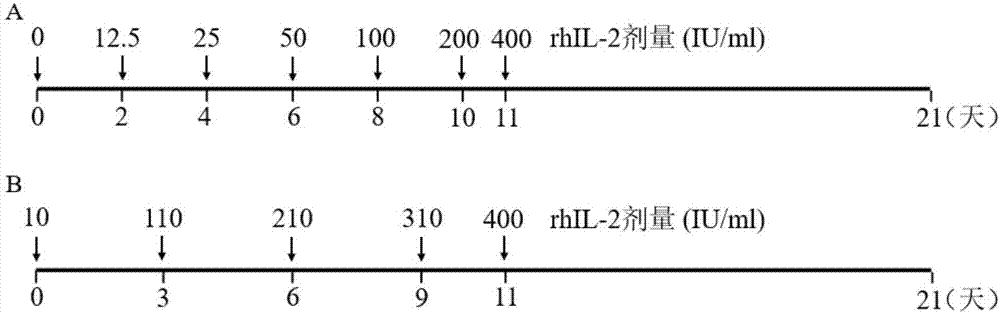
Concentration gradient rhIL-2 dependent iNKT cell amplification method and application thereof
InactiveCN106566807AHigh amplification efficiencyStrengthening the immune enhancement and tumor immune monitoring of iNKT cells expanded in vitroMammal material medical ingredientsCell culture supports/coatingCulture cellPeripheral blood mononuclear cell
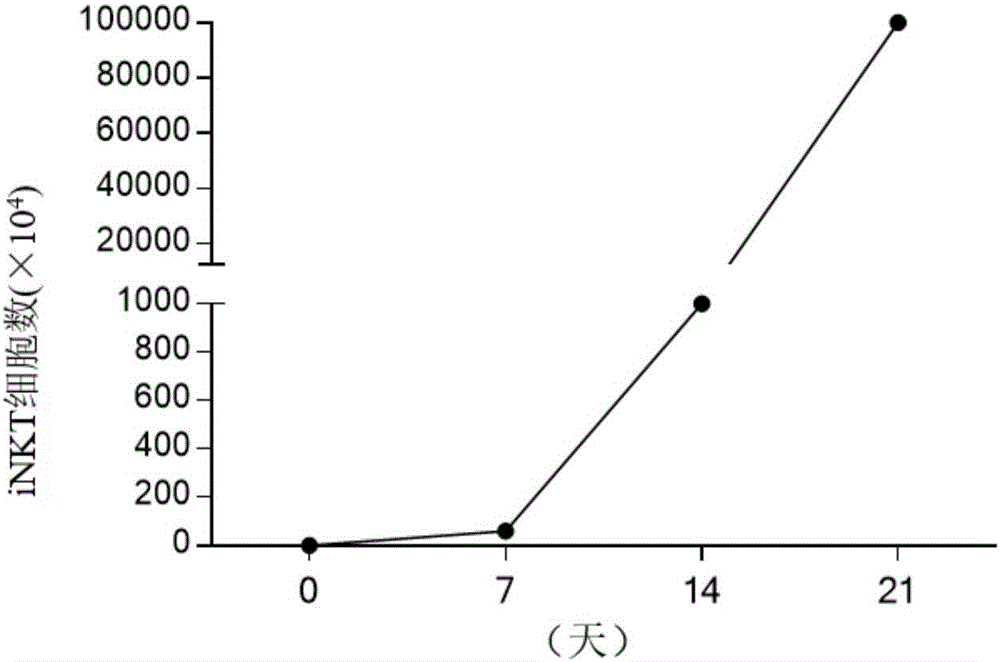
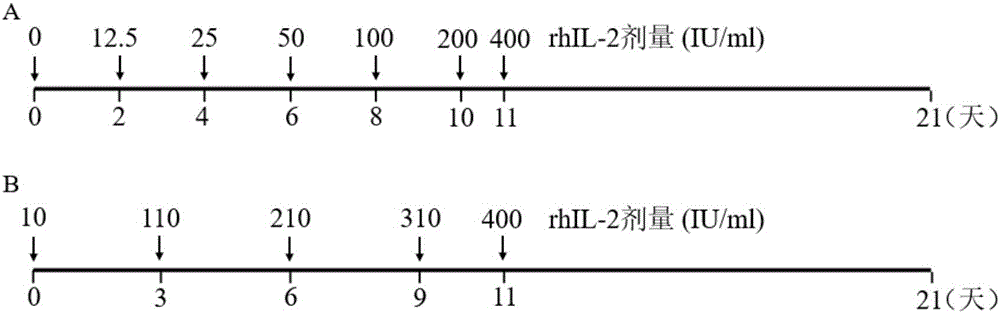
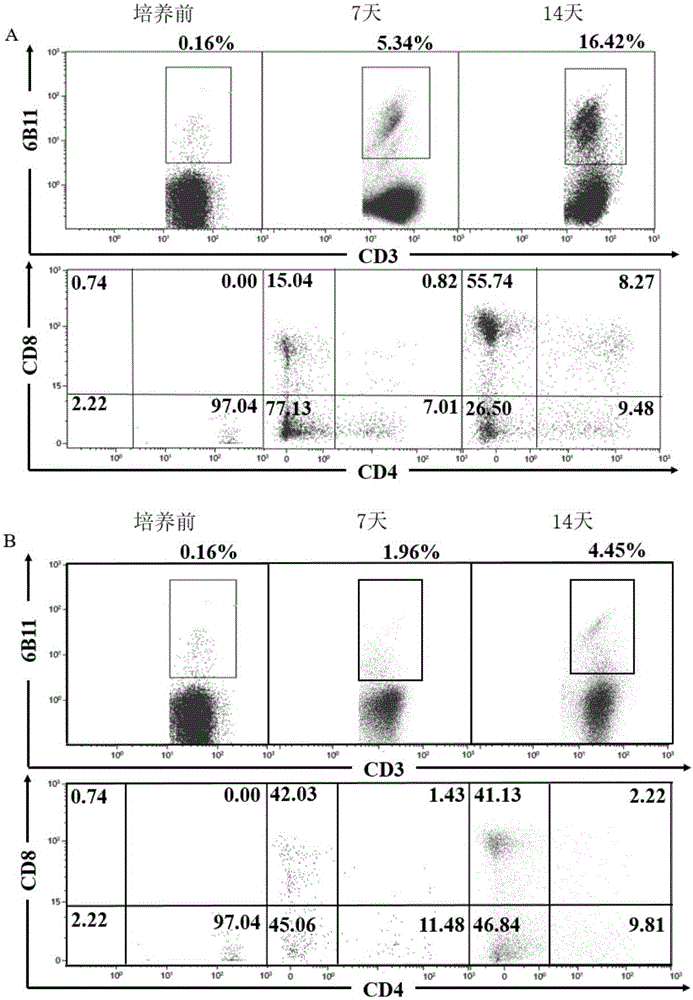
The invention discloses a concentration gradient rhIL-2 dependent iNKT cell amplification method and an application thereof. The method comprises the following steps: (1) extracting peripheral blood mononuclear cells PBMC; (2) adding [alpha]-GalCer, which is 100ng / ml in final concentration, to the PBMC cells which are extracted in the step (1), and conducting cultivation for 48h; and (3) gradually increasing an rhIL-2 concentration in a culture medium in the cells which are cultivated in the step (2) in a gradient mode. According to the cell amplification method provided by the invention, when the iNKT cells are collected on the 21st day of cultivation, the quantity of the iNKT cells is increased by 100,000 times, including more than 90% of CD4-iNKT cells which have effects of up-regulating immune reaction and directly killing tumors. The iNKT cells, which are activated and amplified by virtue of the method provided by the invention, are higher in amplification efficiency, and the method can selectively amplify the Th1-like iNKT cells and enhance functions of in vitro amplification iNKT cell immunity as well as tumor immunity surveillance and killing.
Owner:闾军 +1
Risperidone sustained-release gel injection and preparation method thereof
ActiveCN101584652BImprove quality controlOvercoming clogging defectsOrganic active ingredientsNervous disorderIn vivoMentally ill
The invention discloses a risperidone sustained-release gel injection and preparation method thereof. The risperidone sustained-release gel injection is composed of risperidone or analogue thereof, biological degradable polymer and biocompatible dissolvent, wherein the mass ratio between risperidone or analogue thereof and sum of biological degradable polymer and biocompatible dissolvant is 1:3-67. Continuous and constant-speed release of risperidone is up to several weeks immediately after the injection is injected into appropriate part in vivo, thereby improving compliance therapy in psychotic with risperidone analogue.
Owner:SHANGAI PHARMA GRP CO LTD +1
A kind of pramipexole weekly effect transdermal patch and preparation method thereof
ActiveCN104510725BMeet the needs of clinical treatmentIncrease penetration rateOrganic active ingredientsNervous disorderTransdermal patchDrug reservoir
The invention specifically relates to a pramipexole weekly transdermal patch and a preparation method thereof, belonging to the field of pharmaceutical preparations. The pramipexole weekly transdermal patch comprises three parts: a drug reservoir, an anti-adhesive layer and a backing layer. The invention adopts the hydrophilic substrate mixed penetration enhancer and the lamination pouring method to prepare the double-layer patch, which effectively solves the problem in the preparation of the multi-layer film, and can ensure the constant-speed release of the drug within seven days. Animal experiments show that it is non-irritating and sensitizing to the skin. The patch of the invention has the advantages of clear curative effect, stable quality, good safety and convenient use, and is used for the treatment of Parkinson's disease.
Owner:CHINA PHARM UNIV
Pharmaceutical composition for treating embolism and preparation method thereof
ActiveCN101670095BPlay a hardening roleHigh drug loadingSurgeryX-ray constrast preparationsDual effectDouble bond
The invention provides a pharmaceutical composition for treating embolism and a preparation method thereof, the pharmaceutical composition comprises a hydroxyl-contained biocompatible polymer material and a monomer containing unsaturated double bonds and anion groups, as well as a polymer generated by polymerization reaction of an optional vinyl monomer, wherein the polymerization is initiated through free radicles, and bleomycin or pingyangmycin is combined on the anion groups of the generated polymer. The preparation method combines the bleomycin or the pingyangmycin on a carrier of the polymer, thereby being capable of fully playing the dual effects of an anti-tumor antibiotic and a hardening agent owned by the bleomycin or the pingyangmycin during the embolism treatment. The anion part of the polymer can be properly combined with the bleomycin or the pingyangmycin which is rich in amino groups, thereby not only realizing the higher drug loading, but also leading the drug in an emboliaztion agent to be exchanged by cation in human body, and further realizing the slow release. In addition, the embolic carrier of the polymer has the advantages of simple prepration technology andlow cost, thereby being applicable to large-scale industrial production.
Owner:HYGEA MEDICAL TECH CO LTD
Ciprofloxacin dry powder inhaler and preparation method thereof
ActiveCN108771660AFluffy cotton-like structureHigh emptying rateAntibacterial agentsOrganic active ingredientsSpidroinMannitol
The invention discloses a ciprofloxacin dry powder inhaler and a preparation method thereof. The dry powder inhaler is prepared from a drug and a binary auxiliary material, wherein the drug accounts for 80 to 20 percent by mass of the dry powder inhaler, the binary auxiliary material accounts for 20 to 80 percent by mass of the dry powder inhaler, and the binary auxiliary material comprises a mucus diluting agent and biological adhesive protein; the mucus diluting agent is at least one of mannitol, ambroxol hydrochloride, aminothiopropionic acid, and bromhexine hydrochloride; and the biological adhesive protein is at least one of silk fibroin, albumin or spidroin. The dry powder inhaler prepared by the invention is good in mobility, good in aerodynamic performance, capable of targeting thedrug to lung and capable of effectively increasing the local concentration of the drug, has mucus diluting and biological mucosa adsorption characteristics, and is particularly suitable for a patientwith bronchiectasia caused by the chronic infection of the lung.
Owner:SUN YAT SEN MEMORIAL HOSPITAL SUN YAT SEN UNIV
Traditional Chinese medicine electrical fire needle
InactiveCN108066148AReasonable structural designNovel ideaDevices for heating/cooling reflex pointsAcupuncturePrecessionEngineering
The invention relates to the technical field of traditional Chinese medicine physical therapy equipment, and in particular relates to a traditional Chinese medicine electrical fire needle which includes a shell, an electric heating sleeve, a fire needle and a propulsion mechanism arranged in the shell. The propulsion mechanism comprises a straight pressure rod and a spiral push rod; the upper partof the spiral push rod is connected with straight spiral connection part in an annular clamping mode, the direction of the axis of the spiral push rod is the same as the direction of the rotating center axis; the spiral push rod is sleeved with a rotation limit ring, the lower portion of the spiral push rod is provided with a fire needle fastening structure, and the tail end of the fire needle isconnected with the spiral push rod. According to the traditional Chinese medicine electrical fire needle, by arrangement o the spiral push rod, an original needle input method is overturned. Under the action of the spiral push rod and the rotation limit ring, the fire needle is fed in a precession mode, the needle feeding and discharge are quick, and the fire needle is prevented from bending.
Owner:陈为密
Concentration gradient rhIL-2 dependent iNKT (invariant natural killer) cell amplification method and application thereof
ActiveCN107502591AAddressing Purity IssuesMeet the needs of clinical treatmentMammal material medical ingredientsCell culture supports/coatingAbnormal tissue growthPeripheral blood mononuclear cell
The invention discloses a concentration gradient rhIL-2 dependent iNKT (invariant natural killer) cell amplification method and an application thereof. The method includes: 1), extracting PBMCs (peripheral blood mononuclear cell); 2), adding alpha-GalCer 100ng / ml in final concentration to the extracted PBMCs in the step 1) for culture for 48 hours; gradually increasing concentration of rhIL-2 in a culture medium in a gradient manner in the cultured cells in the step 2). The iNKT cells are obtained on the 21 day of culture, the number of the iNKT cells is increased by 100,000 times, more than 90% of the cells are CD4-iNKT cells, and functions of immune reaction up-regulation and direct tumor killing are achieved; the method is higher in amplification efficiency in stimulating and amplifying the iNKT cells, Th1 sampled iNKT cells are selectively amplified, and immune enhancement of the in vitro amplified iNKT cells and tumor immune surveillance and killing functions are strengthened.
Owner:BEIJING GENE KEY LIFE TECH CO LTD
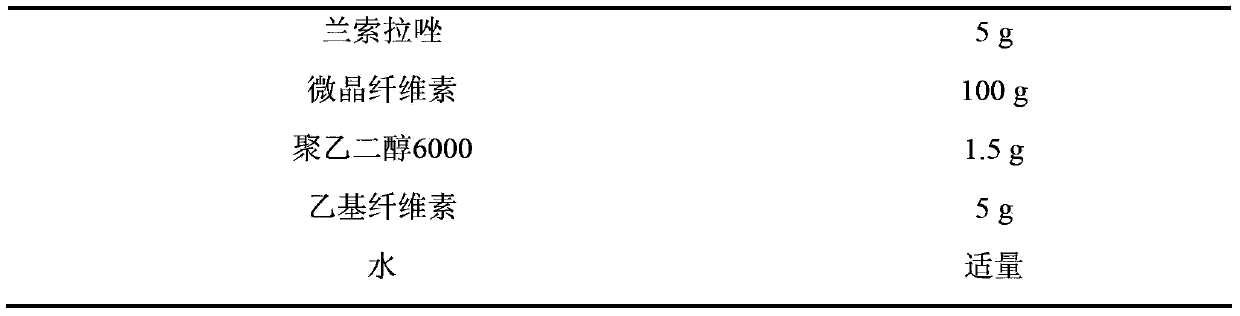
Ketoprofen lansoprazole sustained-release pellets, as well as preparation method and preparation thereof
ActiveCN103432130AImprove stabilityImprove bioavailabilityOrganic active ingredientsAntipyreticSustained release pelletsIrritation
The invention discloses ketoprofen lansoprazole sustained-release pellets. The pellets comprise the following components in parts by weight: 30-50 parts of ketoprofen, 3-5 parts of lansoprazole, 60-100 parts of filler, 3-5 parts of retardant, and 0-2 parts of lubricant, wherein the filler is one or more of starch, saccharose, lactose and microcrystalline cellulose, the retardant is ethyl cellulose, and the lubricant is one or more of magnesium stearate, talcum powder and polyethylene glycol 6000. Compound sustained-release pellets prepared from ketoprofen and lansoprazole have the advantages of high bioavailability, small local irritation, uniform medicine absorption speed and the like; the sustained-release pellets are coated, so that the medicament stability can be improved, the bitter taste of the pellets can be effectively covered, and the compliance of patient administration can be strengthened; sustained-release pellet tablets or sustained-release pellet capsules which are further prepared from the sustained-release pellets or coated pellets are convenient to carry and take; ketoprofen lansoprazole sustained-release pellets prepared by adopting an extrusion spheronization method or centrifugal granulation method are simple in operation, easy to control quality, narrow in size distribution range, good in roundness and smooth in surface, and are suitable for further coating, subpackaging or preparing.
Owner:SOUTHWEST UNIV
Antibiotic//TCP composite nano-material and preparation method thereof
InactiveCN101318033AWide range of clinical applicationEvenly distributedProsthesisNanomaterialsAnti infectives
The invention pertains to the technical field of a new material of medicines, relating to antibiotic / TCP nanocomposite and a method thereof. The antibiotic / TCP nanocomposite provided by the invention is a ball-type porous material which consists of antibiotic and TCP and the matter motes with nanometer size are absorbed on the supporting framework of organic matter. The adjustable range of the ball-type size is from a plurality of hundred nanometers to dozens of microns. The surface of the ball is provided with a portiforium structure. The antibiotic / TCP nanocomposite is a new-type anti-infective bone implanting material which has high drug loading and good re-absorption rate and can control slow releasing rate of drugs.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
Traditional Chinese medicine mixture for removing urinary calculus and preparation method thereof
InactiveCN113350456AHas excretionPromote excretionDispersion deliveryFungi medical ingredientsWolfiporia extensaBiology
The invention discloses a traditional Chinese medicine mixture for removing urinary calculus and a preparation method thereof. The mixture is prepared from following raw materials in parts by weight, 16 g of herba pyrrosiae, 16 g of radix rehmanniae, 12 g of chingma abutilon seeds, 10 g of poria cocos, 6 g of raw liquorice, 12 g of lygodium japonicum, 20 g of fried endothelium corneum gigeriae galli, 6 g of radix scutellariae, 12 g of talc, 10 g of rhizoma alismatis, 10 g of polyporus umbellatus, 9 g of plantain herb, 15 g of herba patriniae, 10 g of lalang grass rhizome, 20 g of lysimachia christinae hance and 10 g of gardenia jasminoides. The traditional Chinese medicine composition is scientific and reasonable in formula, novel and reasonable in preparation method, high precision of process parameter, less in component loss and stable in medicine effect. The traditional Chinese medicine composition is suitable for various urinary calculus diseases such as kidney calculus and urethral calculus, is not easy to relapse after being cured, and meets clinical treatment requirements.
Owner:王晓兵
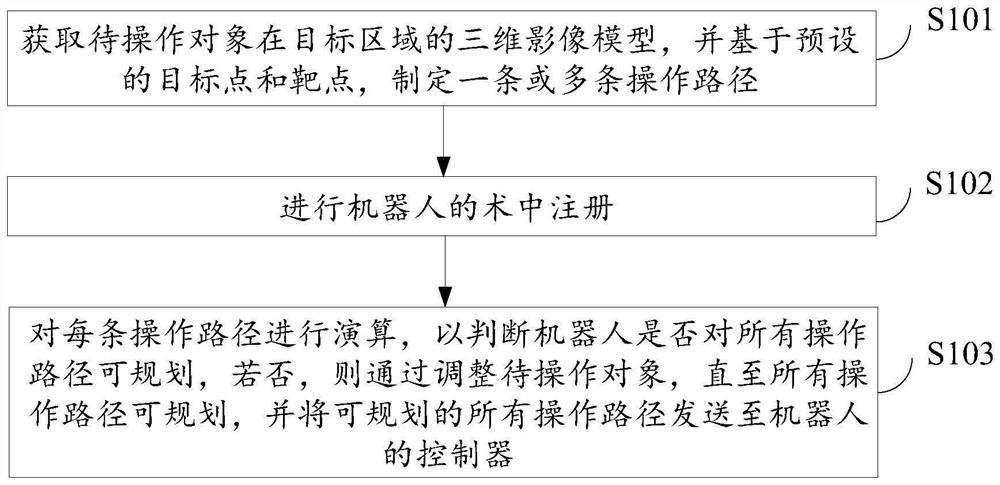
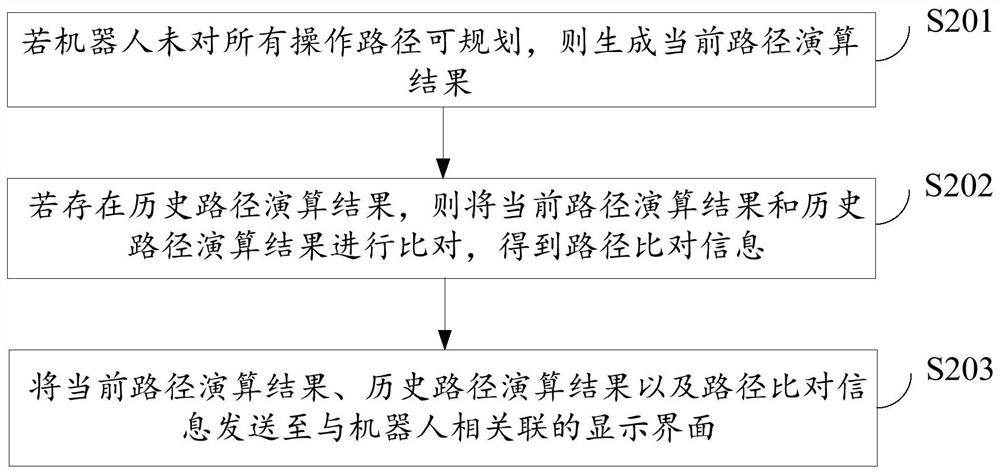
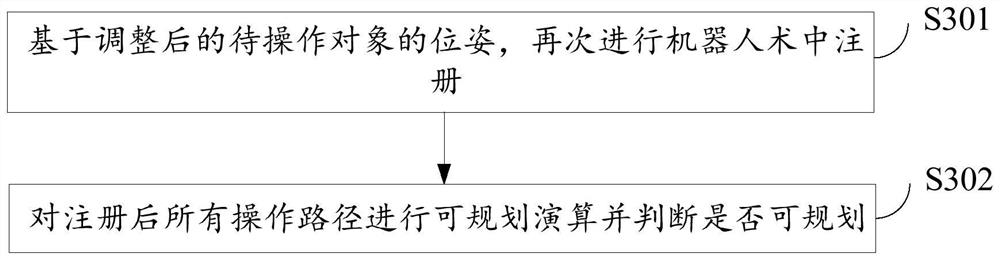
Surgical robot path planning method, system and equipment and storage medium
ActiveCN114129263AEasy to convertEasy to navigateSurgical navigation systemsTotal factory controlSimulationReoperative surgery
The invention relates to a surgical robot path planning method, system and device and a storage medium, and the method comprises the steps: obtaining a three-dimensional image model of a to-be-operated object in a target region, and formulating one or more operation paths based on a preset target point and a target point; performing intraoperative registration of the robot; and calculating each operation path to judge whether the robot can plan all the operation paths, if not, adjusting the object to be operated until all the operation paths can be planned, and sending all the plannable operation paths to a controller of the robot. The operation efficiency of a patient can be effectively improved, and the operation risk is reduced.
Owner:WUHAN UNITED IMAGING HEALTHCARE SURGICAL TECH CO LTD
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com