Risperidone sustained-release gel injection and preparation method thereof
A technology of sustained-release gel injection and risperidone, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the complicated quality control of microsphere products and the loss of drugs and excipients , Syringe blockage and other problems, achieve good pharmacoeconomic value, good biocompatibility, and improve compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
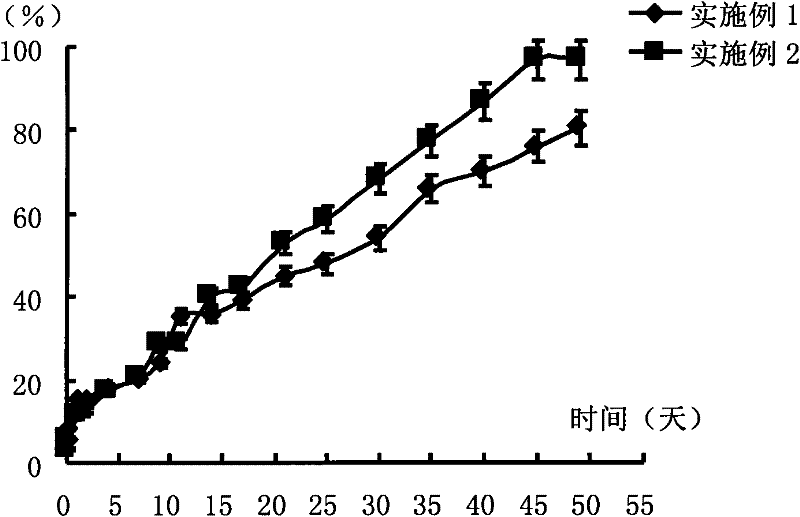
[0034] Weigh 0.05 g of PLA (molecular weight 20,000 Daltons) and dissolve it in 0.2 g of NMP to form a blank polymer solution, then weigh 25.0 mg of risperidone and suspend and disperse it in the above solution to prepare a drug-loading solution. Put the prepared drug-loading solution in a 10ml vial, add a few drops of phosphate buffer solution with pH=7.4 to it to make it turn into a gel, and then soak the entire vial into phosphoric acid with pH=7.4 Release it in a reagent bottle with 2000ml of salt buffer. The release conditions were a constant temperature water bath at 37°C and a shaking speed of 100 rpm. 6 hours, 1, 2, 4, 7, 9, 11, 14, 17, 21, 25, 30, 35, 40, 45, and 49 days after the start of the release, take 2ml of the release medium and measure the content of risperidone in it , calculate the cumulative release percentage, and operate 3 copies in parallel, the results are shown in figure 1 .
Embodiment 2
[0036] Weigh 0.035g of PLA (molecular weight 20,000 Daltons) and dissolve it in 0.2g Glycofurol to form a blank polymer solution, then weigh 25.0mg of risperidone and suspend and disperse it in the above solution to make a drug-loading solution. Put the prepared drug-loading solution in a 10ml vial, add a few drops of phosphate buffer solution with pH=7.4 to it to make it turn into a gel, and then soak the entire vial into phosphoric acid with pH=7.4 Release it in a reagent bottle with 2000ml of salt buffer. The release conditions were a constant temperature water bath at 37°C and a shaking speed of 100 rpm. 6 hours after the start of release, 1, 2, 4, 7, 9, 11, 14, 17, 21, 25, 30, 40, 45, and 49 days, take 2ml of the release medium, measure the content of risperidone in it, and calculate Cumulative release percentage, 3 parallel operations, see the results figure 1 .
Embodiment 3
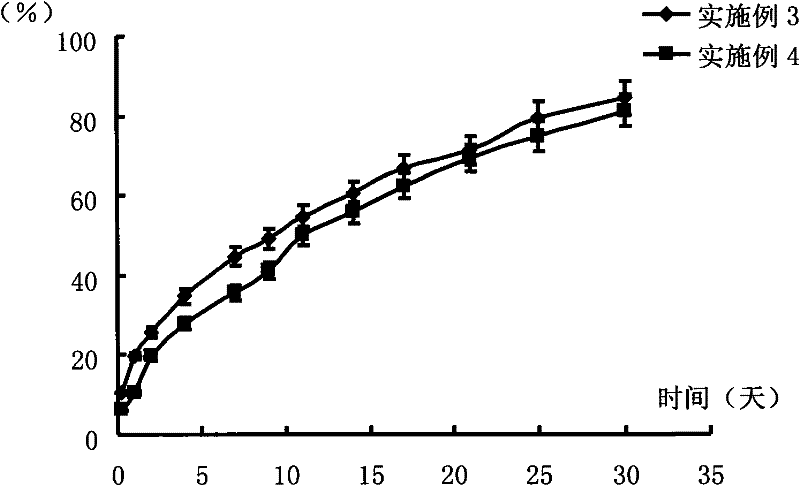
[0038] Weigh 0.05 g of PLGA (molecular weight 50,000 Daltons, polymer monomer mass ratio lactic acid: glycolic acid = 75:25) and dissolve it in 0.2 g TEC to form a blank polymer solution, then weigh 37.5 mg risperidone and mix it Suspended and dispersed in the above solution to make a drug-loading solution. Put the prepared drug-loading solution in a 10ml vial, add a few drops of phosphate buffer solution with pH=7.4 to it to make it turn into a gel, and then soak the entire vial into phosphoric acid with pH=7.4 Release it in a reagent bottle with 2000ml of salt buffer. The release conditions were a constant temperature water bath at 37°C and a shaking speed of 100 rpm. 6 hours after the start of the release, 1, 2, 4, 7, 9, 11, 14, 17, 21, 25, and 30 days, take 2ml of the release medium respectively, measure the content of risperidone in it, calculate the cumulative release percentage, and operate in parallel 3 copies, see results figure 2 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com