Transdermal patch containing pramipexole
The technology of a transdermal patch and pramipexole, which is applied in the field of medicine, can solve the problems of difficult control of the preparation process and complex preparation process of the storage type transdermal patch, so as to improve uniformity, facilitate dissolution and release, The effect of good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
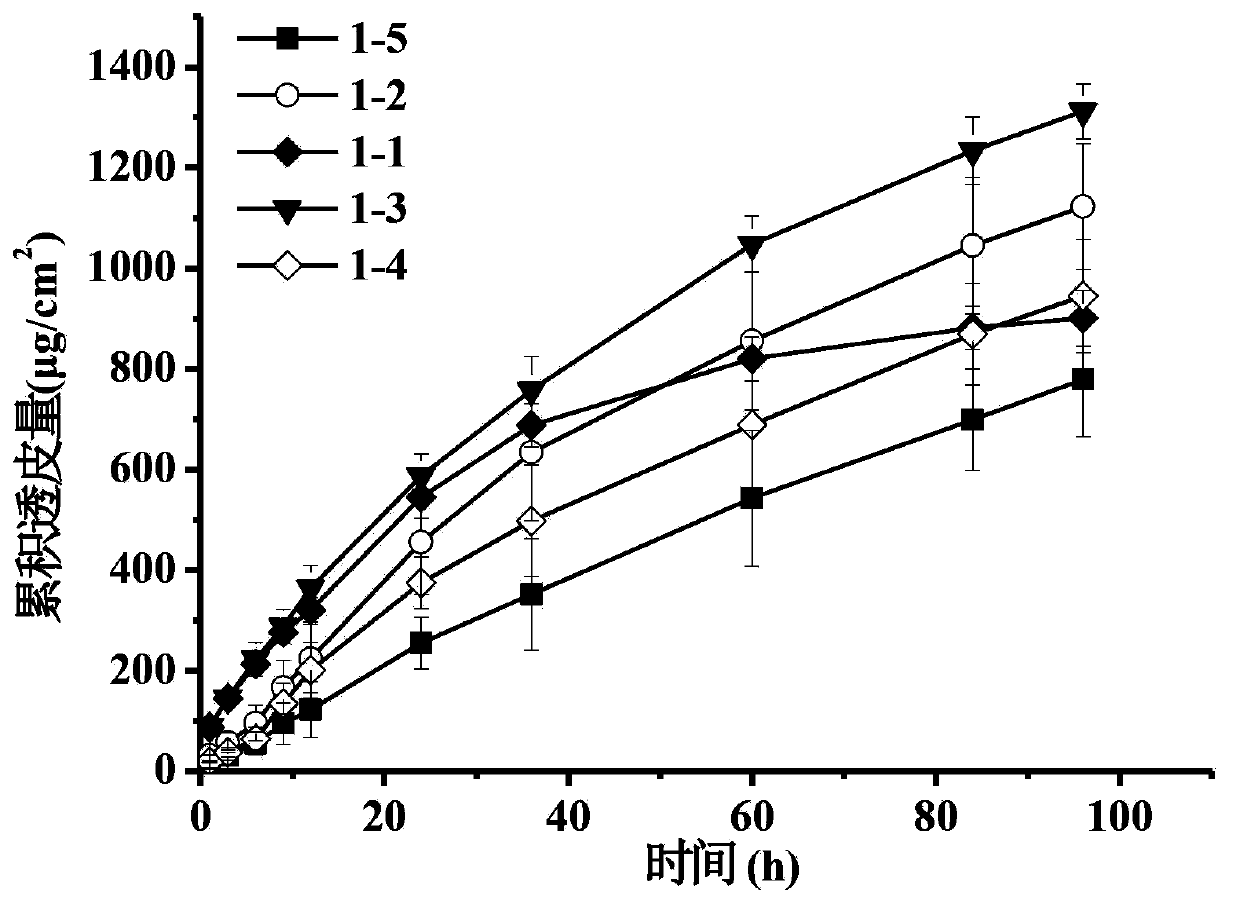
[0038] Prepare the pramipexole transdermal patch according to the following prescription, conduct the transdermal test, and obtain the cumulative transdermal amount in 24 hours.
[0039]
Embodiment 2
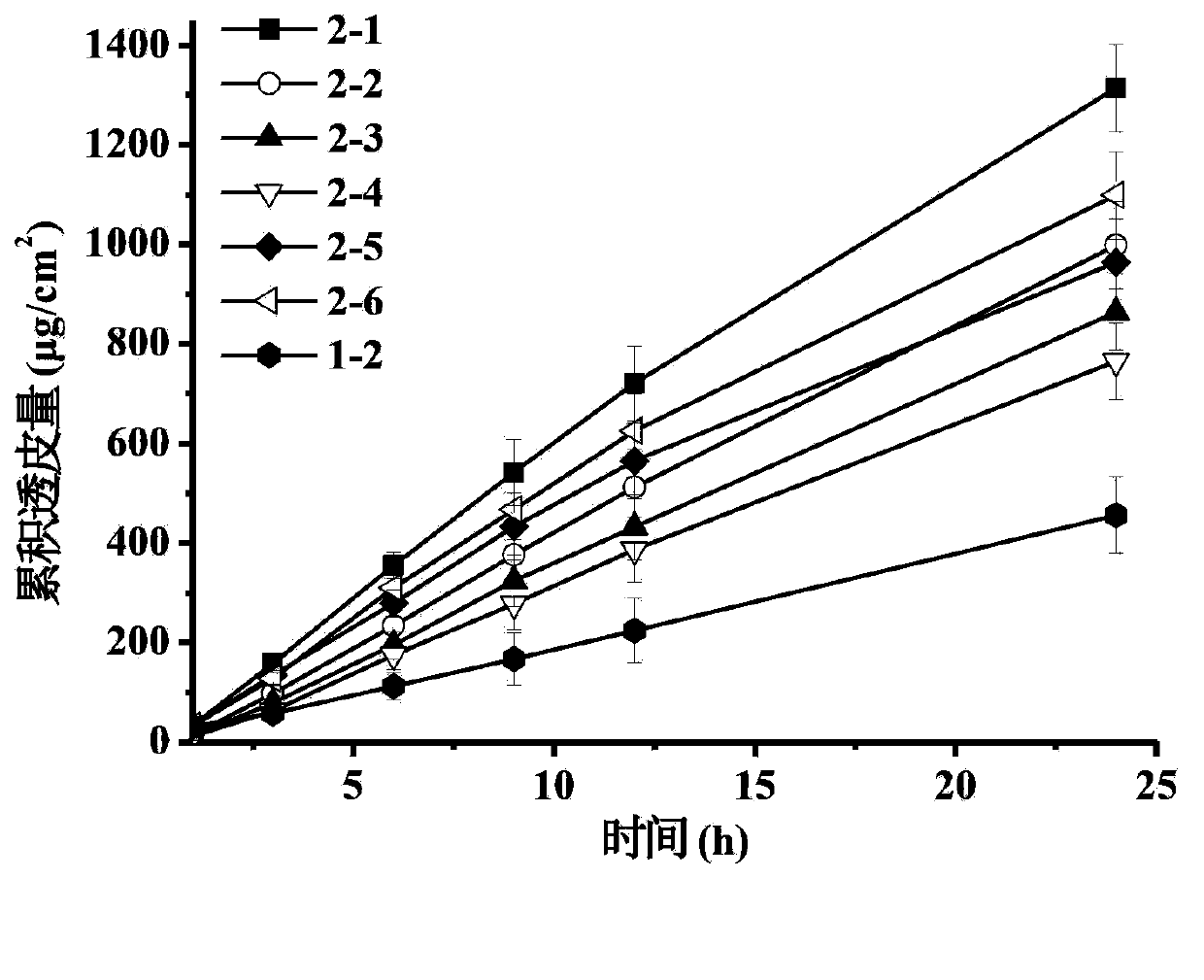
[0041] The pramipexole transdermal patch was prepared by using pressure-sensitive adhesives containing different groups, placed in an accelerated test box (40°C, 75%RH), and the crystallization of the drug was observed under a microscope to investigate the solubility of pramipexole in the pressure-sensitive adhesive .
[0042]
[0043]
[0044] "—": no crystallization (the same below); "√": crystallization (the same below).
Embodiment 3
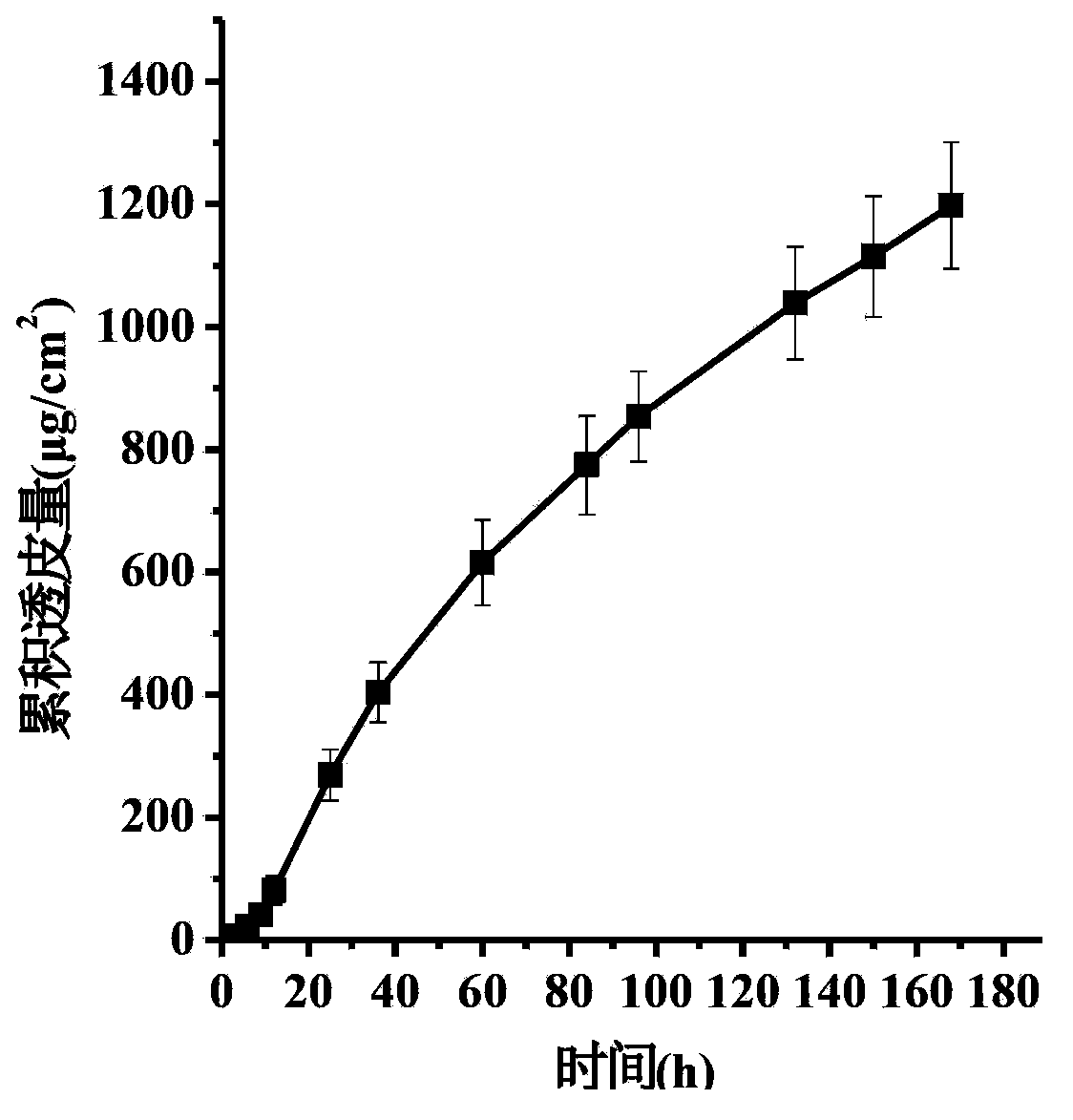
[0046] Dissolving pramipexole transdermal patches (containing 3%wt pramipexole) were prepared, and the stability of pramipexole in pressure-sensitive adhesives was investigated under accelerated conditions (40°C, 75%RH).
[0047]
[0048] "N.D.": Not detected (same below).
[0049] Comprehensive embodiment 1~3 result can be found:
[0050] 1. Pramipexole has higher percutaneous permeability in pressure-sensitive adhesives containing hydroxyl groups;
[0051] 2. Pramipexole has high solubility and stability in pressure-sensitive adhesives containing carboxyl functional groups;
[0052] Based on the advantages of carboxyl group-containing pressure-sensitive adhesives over pramipexole in terms of solubility and stability, and the advantages of hydroxyl-group-containing pressure-sensitive adhesives in drug release and transdermal properties, the two types of pressure-sensitive adhesives were used in different ways. Proportions were mixed, and the percutaneous permeability, so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com