A kind of pramipexole weekly effect transdermal patch and preparation method thereof
A technology of pramipexole and transdermal patch, which is applied to medical preparations with no active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc. It can solve the problems of poor fat solubility and poor penetration through the skin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 1.5 g of polyvinyl alcohol (PVA17-88), 0.3 g of polyvinylpyrrolidone (PVP), 0.2 g of propylene glycol, 0.2 g of glycerin, 0.1 g of disodium edetate, and 40 mg of pramipexole were used to prepare pramipexole patches.
[0043] Polyvinyl alcohol (PVA17-88) 1.5g, polyvinylpyrrolidone (PVP) 0.3g, propylene glycol 0.2g, glycerin 0.2g, edetate disodium 0.1g, pramipexole hydrochloride 40mg, prepare pramipexole hydrochloride paste piece.
[0044] Preparation method: fully swell polyvinyl alcohol and polyvinylpyrrolidone with 60% ethanol, add propylene glycol and glycerin, stir magnetically in a water bath at 70°C to fully dissolve and mix, add the drug solution when the temperature drops to 40°C, mix well, and let stand Until the air bubbles disappear, the film is laid by casting method, dried, formed and cooled, then compounded, covered with polyethylene-aluminum-polyethylene composite film and polyethylene anti-adhesive layer, and prepared in divided doses to contain pramipexo...
Embodiment 2
[0047] Polyvinyl alcohol (PVA17-88) 1.6g, polyvinylpyrrolidone (PVP) 0.4g, eucalyptus oil 0.2g, glycerol 0.1g, edetate disodium 0.1g, pramipexole 100mg, prepare pramipexole paste agent.
[0048] Preparation method: fully swell polyvinyl alcohol and polyvinylpyrrolidone with 60% ethanol, add glycerin and penetration enhancer, stir magnetically in a water bath at 70°C to fully dissolve and mix, add the drug solution when the temperature drops to 40°C, and mix evenly. Let it stand until the bubbles disappear, lay the film by casting method, dry and form it and then compound after cooling, cover the polyethylene-aluminum-polyethylene composite film and polyethylene anti-adhesive layer, and divide the dose to prepare pramipexole containing 10.0mg / cm 2 patch.
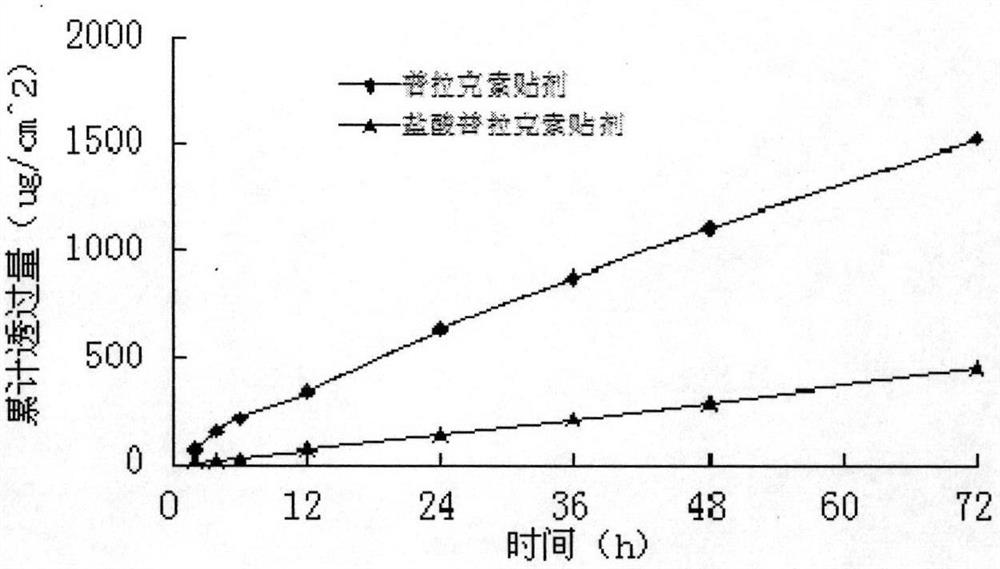
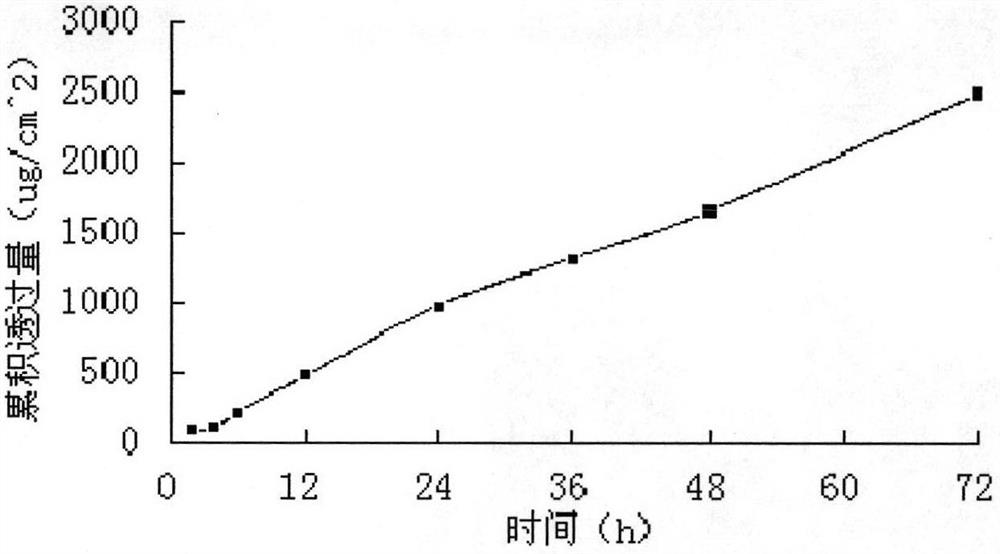
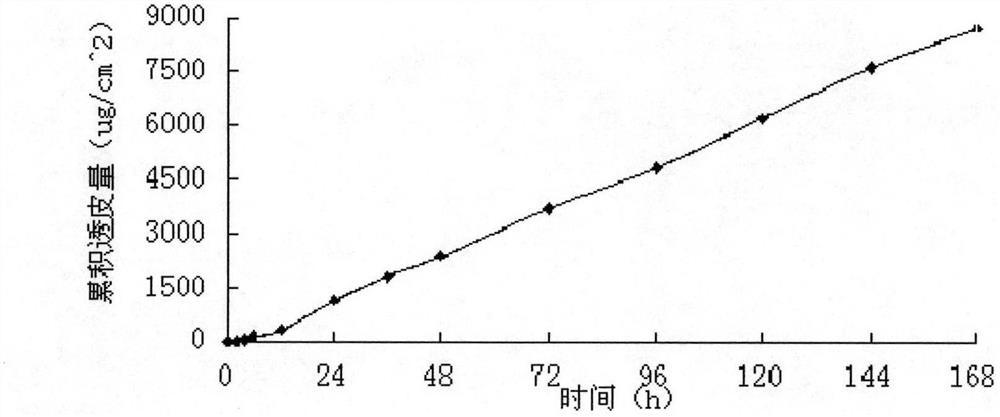
[0049] A modified Franz diffusion cell was used to measure the permeability of the transdermal patch on the inner skin of the rabbit ear, and the area of the patch used in the transdermal diffusion experiment was 2.92cm 2...
Embodiment 3
[0051] The composition of the first layer (close to the release layer): 2.8g polyvinyl alcohol (PVA17-88), 0.4g polyvinylpyrrolidone (PVP), 0.3g eucalyptus oil, 0.2g propylene glycol, 0.3g glycerin, ethylene glycol Disodium amine tetraacetate 0.2g, pramipexole free base 40mg.
[0052] The composition of the second layer (close to the backing layer): polyvinyl alcohol (PVA17-88) 1.6g, polyvinylpyrrolidone (PVP) 0.5g, eucalyptus oil 0.4g, propylene glycol 0.1g, glycerin 0.2g, ethylenediaminetetra Disodium acetate 0.1g, pramipexole free base 120mg.
[0053] Preparation method: fully swell polyvinyl alcohol and polyvinylpyrrolidone with 60% ethanol, add propylene glycol and glycerin, and magnetically stir in a water bath at 70°C to fully dissolve and mix. When the temperature drops to 40°C, add the drug and penetration enhancer solution, and mix well After that, let it stand until the air bubbles disappear, lay the film by casting method, dry and form it and then compound after c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| depth | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com