Ciprofloxacin dry powder inhaler and preparation method thereof
A technology of ciprofloxacin hydrochloride and dry powder inhaler, which is applied in the field of medicine and achieves the effects of increasing drug loading, meeting clinical treatment needs, and high emptying rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
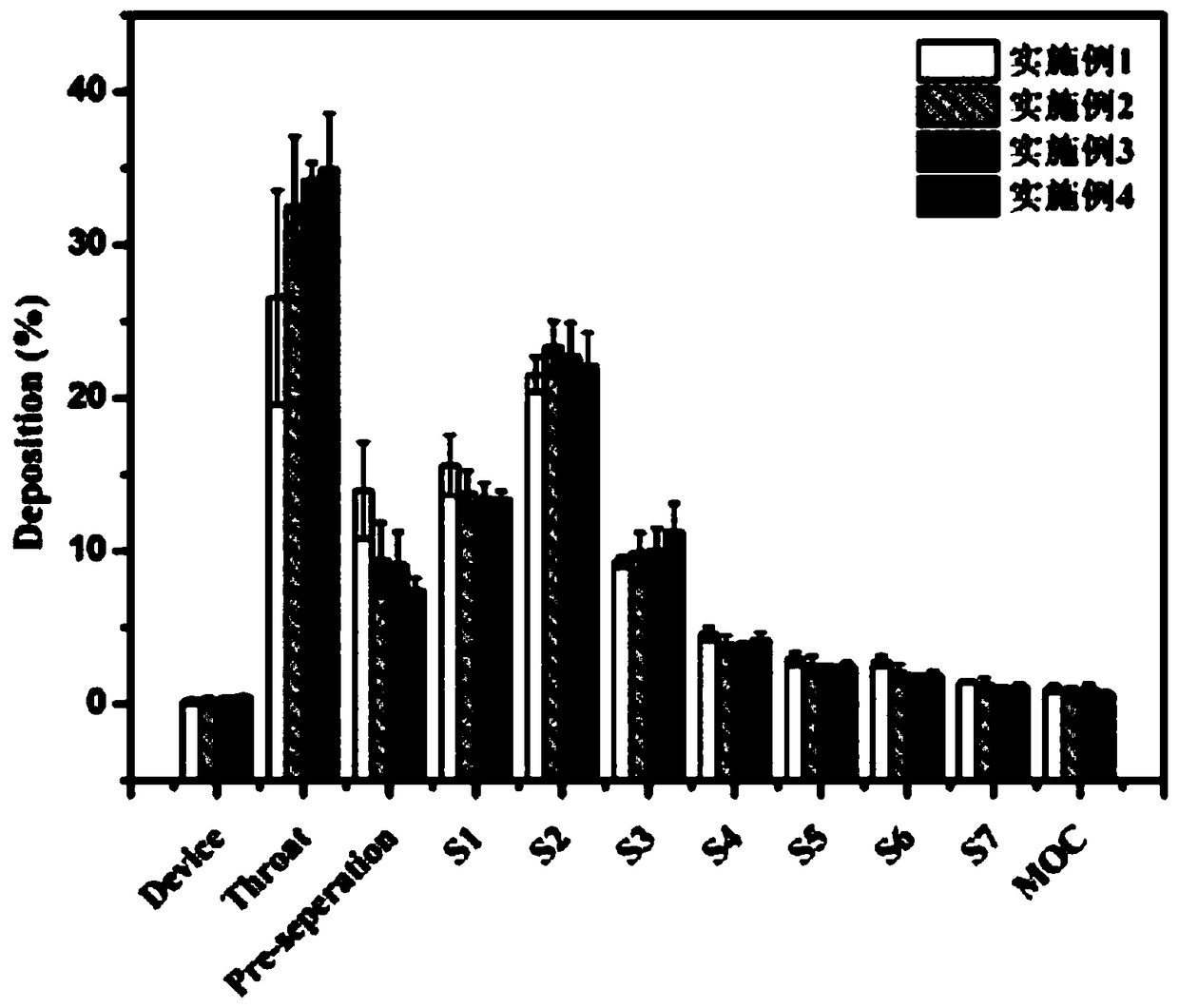
[0046] This embodiment prepares a kind of ciprofloxacin hydrochloride dry powder inhalation, which is composed of ciprofloxacin hydrochloride as the active ingredient, carrier mannitol and silk fibroin, and the mass percentages of the three are 20%, 40%, and 40% respectively. Drugs accounted for 20%.
[0047] The preparation method of above-mentioned ciprofloxacin hydrochloride dry powder inhalation comprises the steps:
[0048] (1) Dissolving silk fibroin in deionized water, adding ciprofloxacin hydrochloride and mannitol, and stirring to form a uniform solution;
[0049] (2) adopt spray drying technology to carry out spray drying to above-mentioned solution, obtain ciprofloxacin hydrochloride dry powder inhalation; Wherein the technological condition of spray drying is: inlet temperature (being drying temperature): 110 ℃; Atomization pressure: 15kPa; Liquid velocity: 8.30mL / min; solid-liquid content: 25mg / mL; gas flow: 0.60~0.65m 3 / min, the nozzle diameter is 0.71mm;
[...
Embodiment 2
[0052] This example prepares a ciprofloxacin hydrochloride dry powder inhaler, the components of which are basically the same as those in Example 1, the difference being that in the process conditions of spray drying, the inlet temperature is 120°C.
Embodiment 3
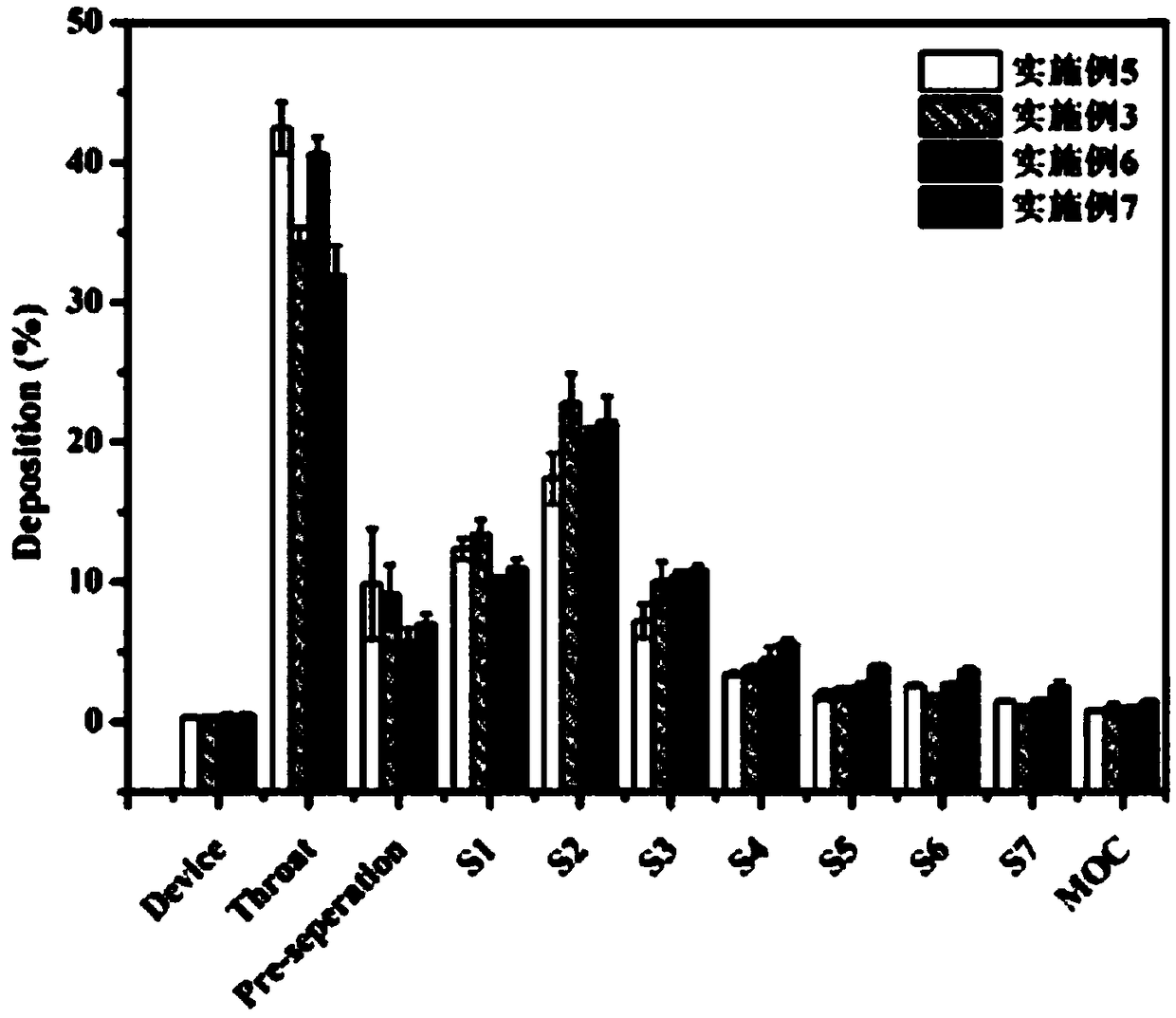
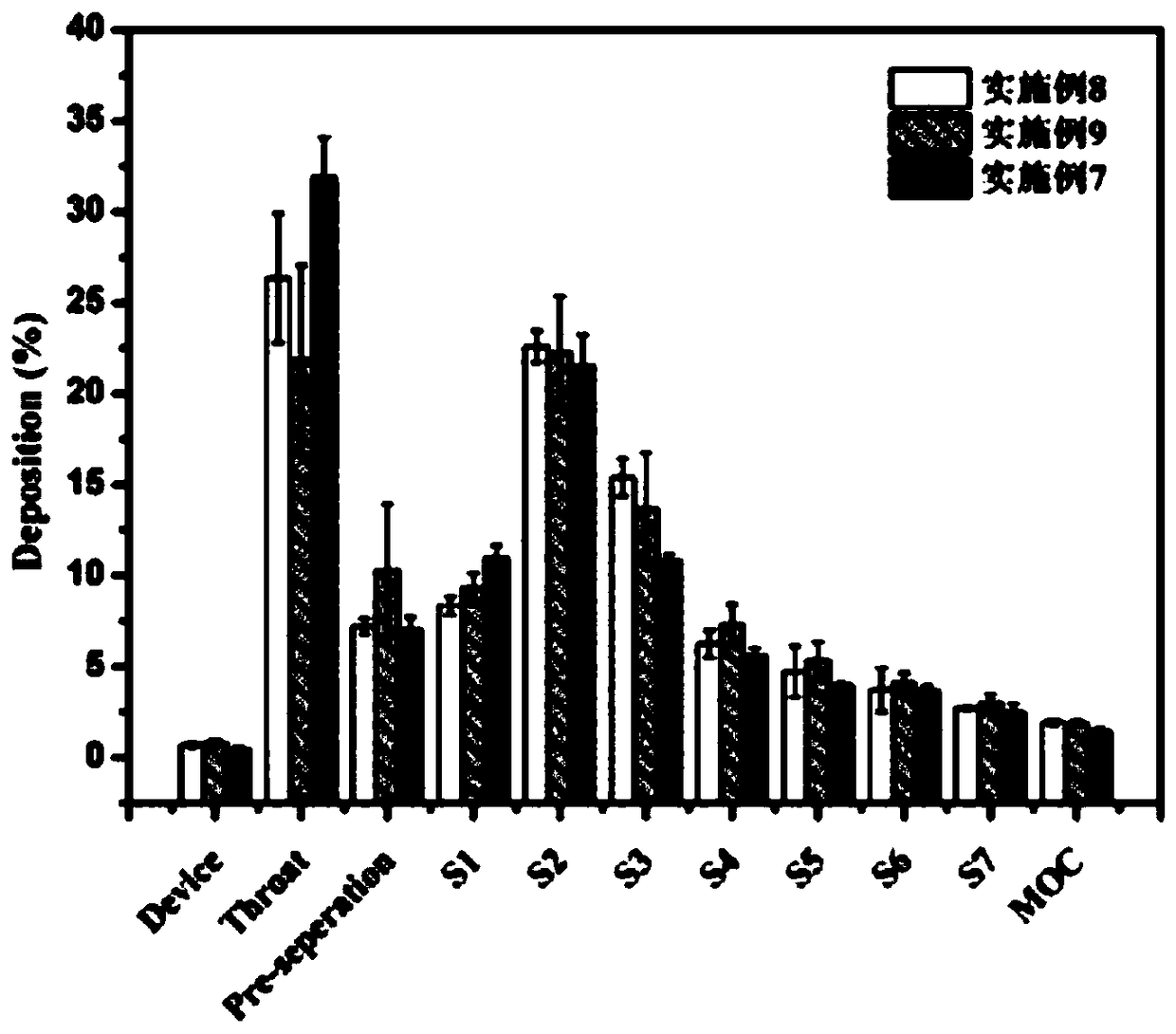
[0054] In this example, a ciprofloxacin hydrochloride dry powder inhaler was prepared, the components of which were basically the same as those in Example 1, the difference being that in the process conditions of spray drying, the inlet temperature was 130°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com